The Association of Residential Altitude on the Molecular Profile and Survival of Melanoma: Results of an Interreg Study
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients’ Features
2.2. Mutational Characterization
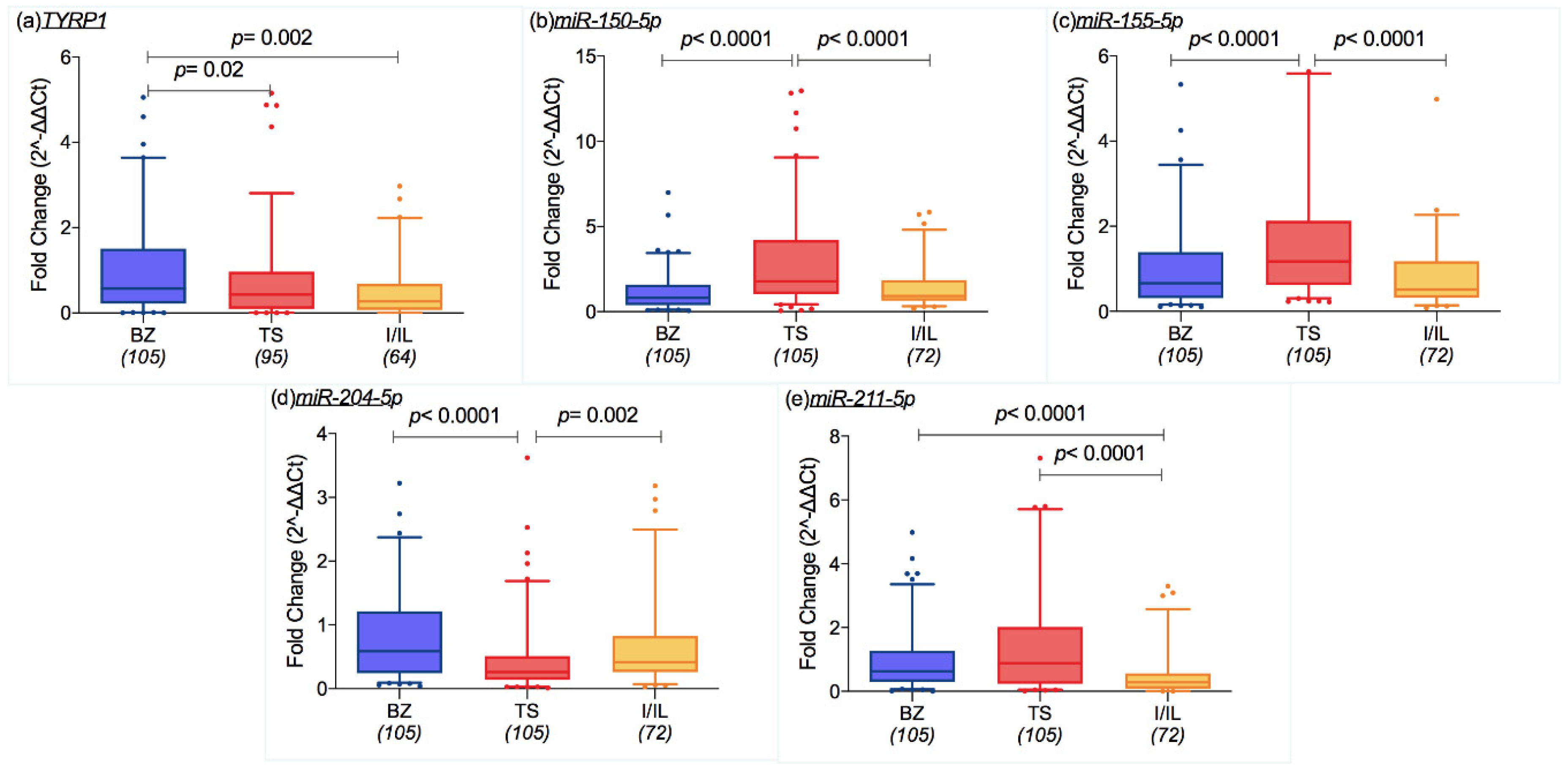
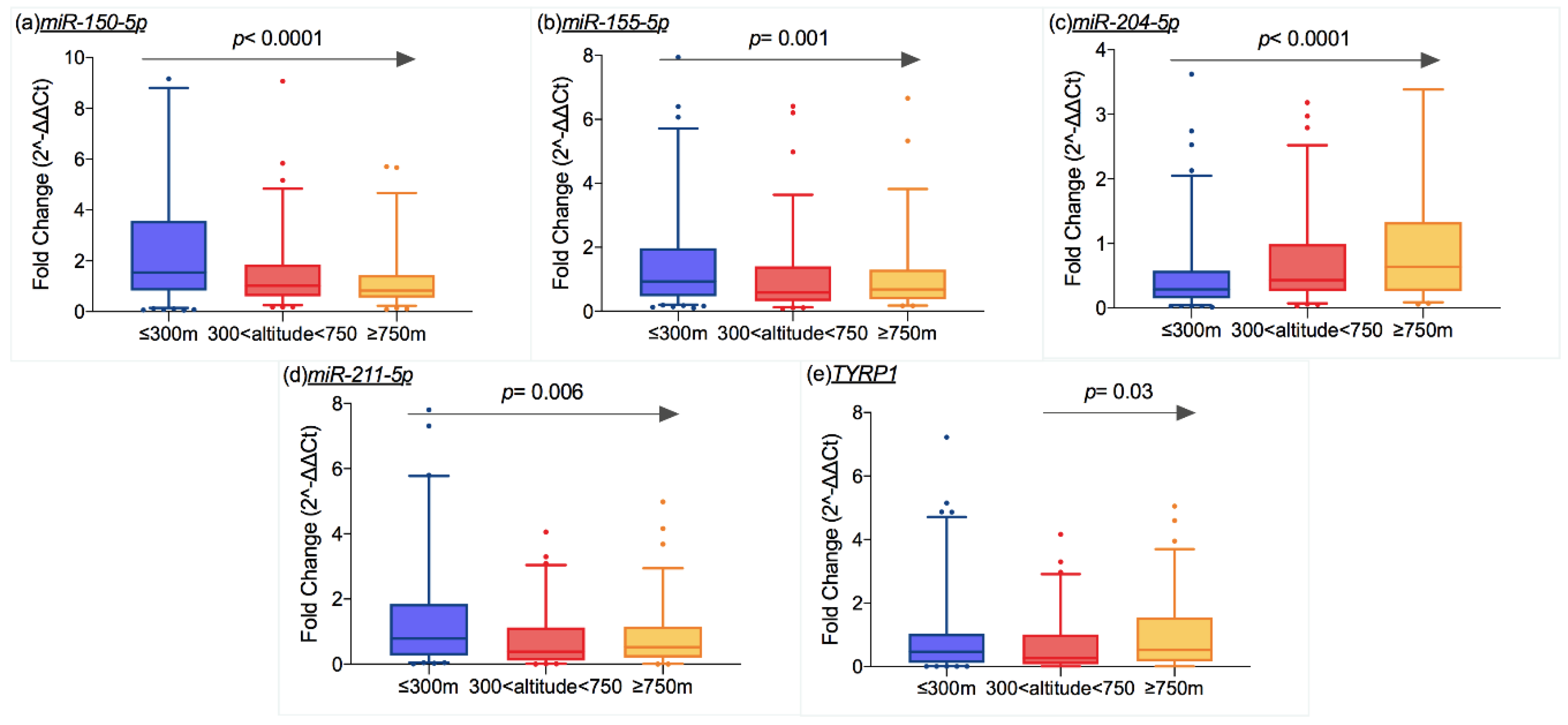
2.3. Molecular Results and Geographic Features
2.4. Transcriptomic Profile and Demography
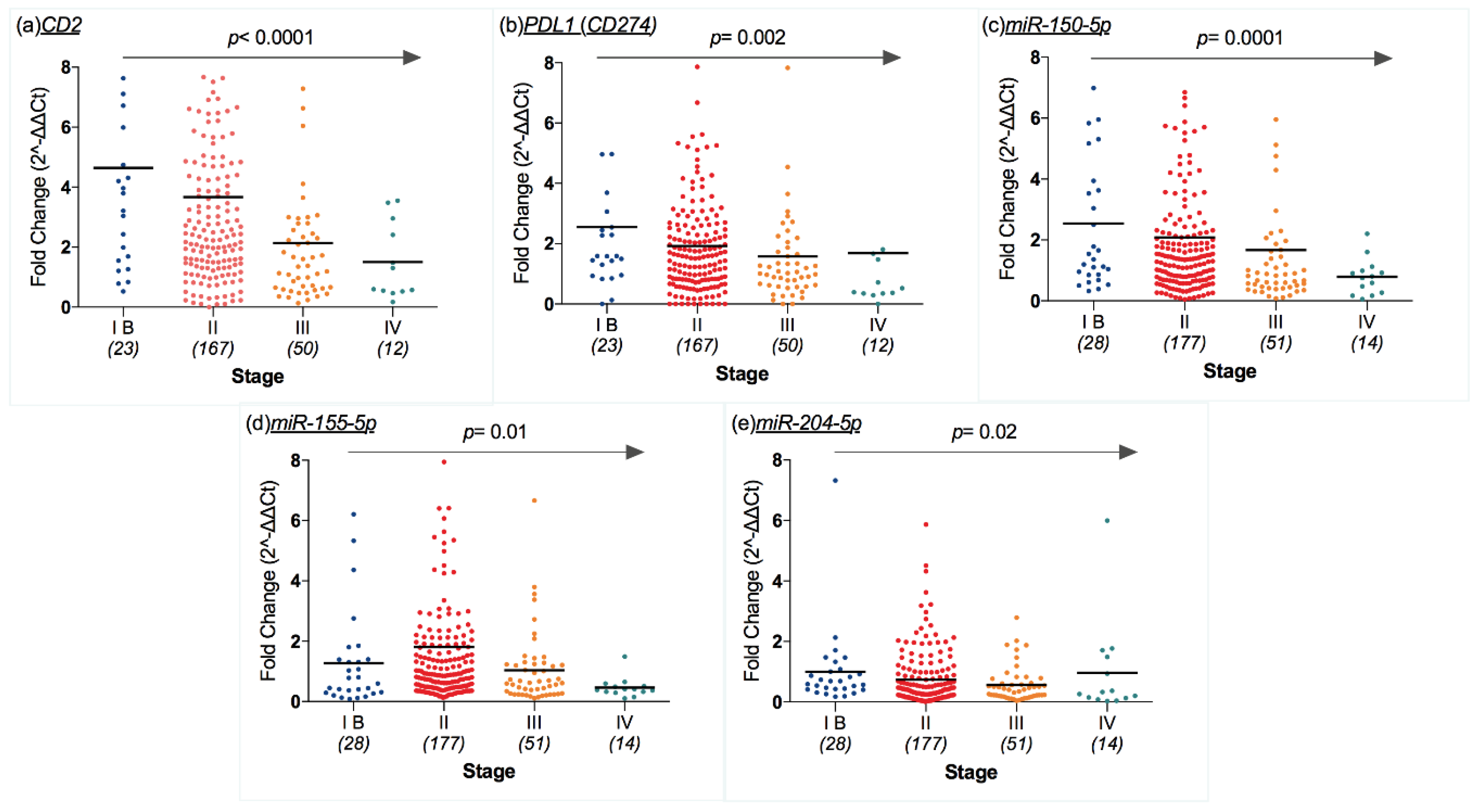
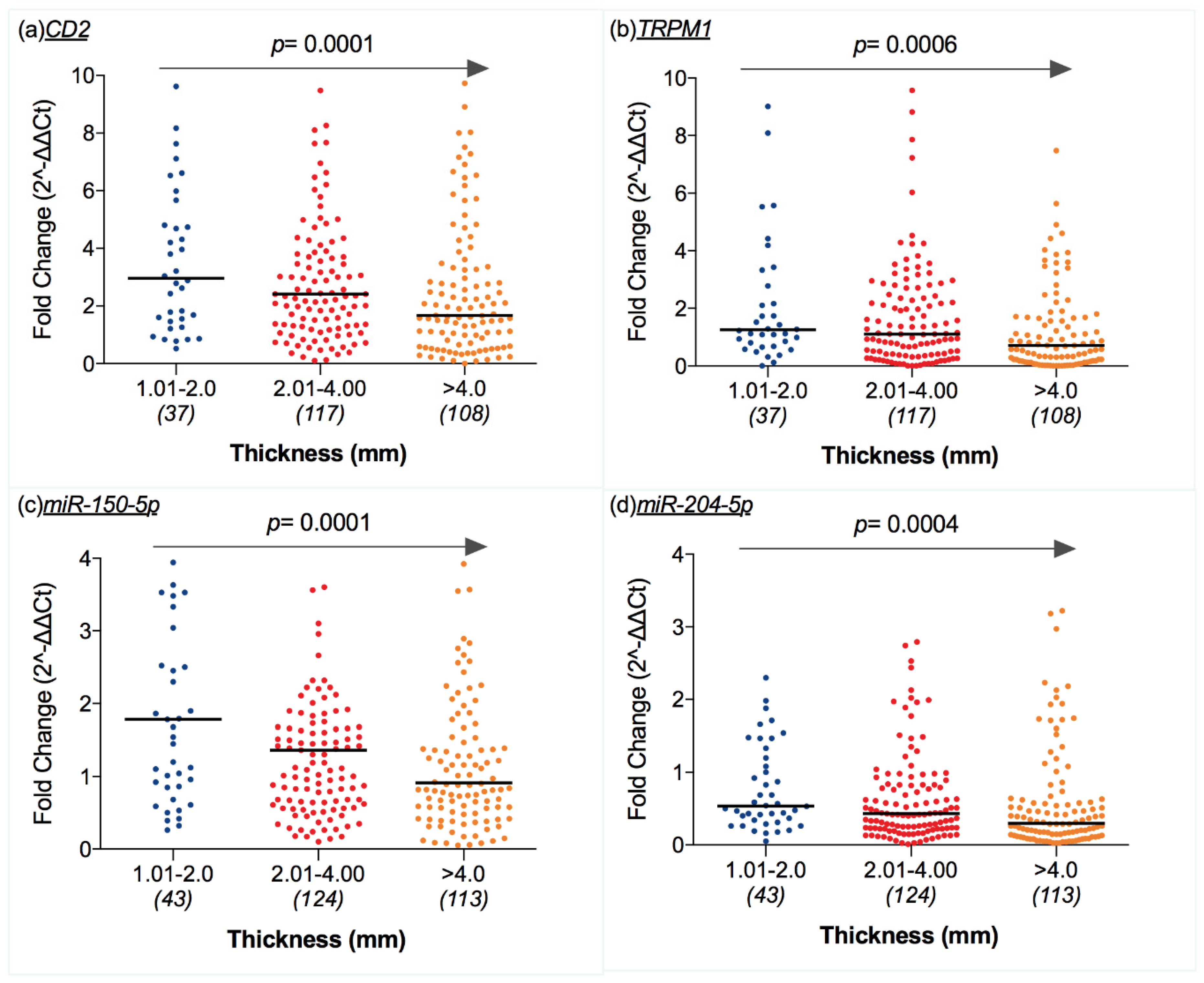
2.5. Transcriptomic Profile and CM Features
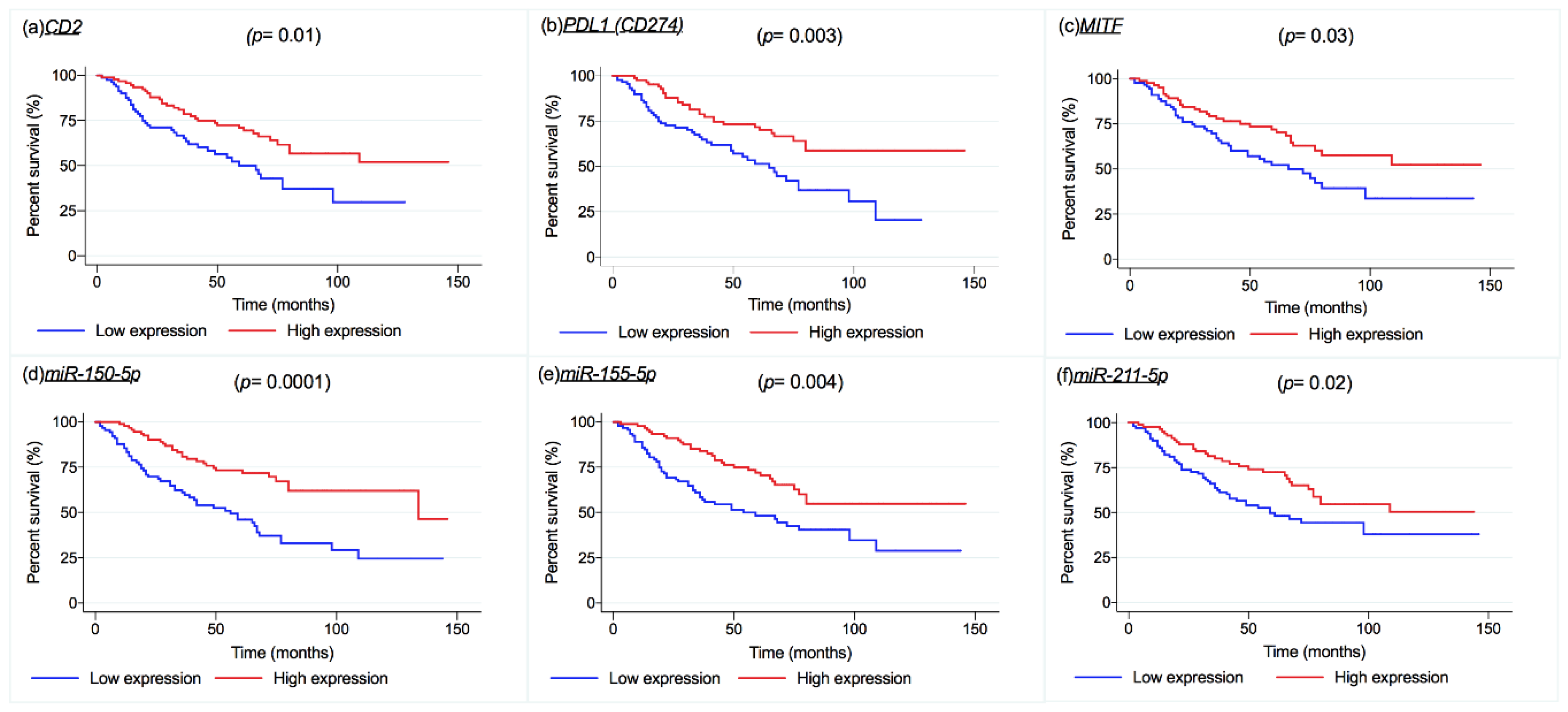
2.6. Melanoma-Specific Survival and Overall Survival
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Immunohistochemistry
4.3. DNA Extraction and Digital Droplet PCR (ddPCR)
4.4. RNA Analysis
4.4.1. Total RNA extraction
4.4.2. Reverse Transcription and Real-Time PCR Assays of mRNAs
4.4.3. Reverse Transcription and Real-Time PCR Assays of MicroRNAs
4.5. Molecular Subtypes
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, C.; Murzaku, E.C.; Penn, L.; Abbasi, N.R.; Davis, P.D.; Berwick, M.; Polsky, D. More skin, more sun, more tan, more melanoma. Am. J. Public Health 2014, 104, e92–e99. [Google Scholar] [CrossRef]
- Emri, G.; Paragh, G.; Tosaki, A.; Janka, E.; Kollár, S.; Hegedûs, C.; Gellén, E.; Horkay, I.; Koncz, G.; Remenyik, É. Ultraviolet radiation-mediated development of cutaneous melanoma: An update. J. Photochem. Photobiol. B Boil. 2018, 185, 169–175. [Google Scholar] [CrossRef]
- Roider, E.M.; Fisher, D.E. Red Hair, Light Skin, and UV-Independent Risk for Melanoma Development in Humans. JAMA Dermatol. 2016, 152, 751–753. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Sensi, M.L.; Nicolini, G.; Petti, C.; Bersani, I.; Lozupone, F.; Molla, A.; Vegetti, C.; Nonaka, D.; Mortarini, R.; Parmiani, G.; et al. Mutually exclusive NRASQ61R and BRAFV600E mutations at the single-cell level in the same human melanoma. Oncogene 2006, 25, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Galasso, M.; Morrison, C.; Minotti, L.; Corrà, F.; Zerbinati, C.; Agnoletto, C.; Baldassari, F.; Fassan, M.; Bartolazzi, A.; Vecchione, A.; et al. Loss of miR-204 expression is a key event in melanoma. Mol. Cancer 2018, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Hanniford, D.; Zhong, J.; Koetz, L.; Gaziel-Sovran, A.; Lackaye, D.J.; Shang, S.; Pavlick, A.; Shapiro, R.; Berman, R.S.; Darvishian, F.; et al. A miRNA-Based Signature Detected in Primary Melanoma Tissue Predicts Development of Brain Metastasis. Clin. Cancer Res. 2015, 21, 4903–4912. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, K.; Schramm, S.-J.; Tembe, V.; Müller, S.; Thompson, J.F.; Scolyer, R.A.; Mann, G.J.; Yang, J. Identification, Review, and Systematic Cross-Validation of microRNA Prognostic Signatures in Metastatic Melanoma. J. Investig. Dermatol. 2016, 136, 245–254. [Google Scholar] [CrossRef]
- Lu, T.; Chen, S.; Qu, L.; Wang, Y.; Chen, H.-D.; He, C. Identification of a five-miRNA signature predicting survival in cutaneous melanoma cancer patients. PeerJ 2019, 7, e7831. [Google Scholar] [CrossRef]
- Segura, M.F.; Belitskaya-Lévy, I.; Rose, A.E.; Zakrzewski, J.; Gaziel, A.; Hanniford, D.; Darvishian, F.; Berman, R.S.; Shapiro, R.L.; Pavlick, A.C.; et al. Melanoma MicroRNA Signature Predicts Post-Recurrence Survival. Clin. Cancer Res. 2010, 16, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Boyle, G.M.; Woods, S.L.; Bonazzi, V.; Stark, M.S.; Hacker, E.; Aoude, L.G.; Dutton-Regester, K.; Cook, A.L.; Sturm, R.; Hayward, N.K. Melanoma cell invasiveness is regulated by miR-211 suppression of the BRN2 transcription factor. Pigment. Cell Melanoma Res. 2011, 24, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martínez, M.; Benito-Jardón, L.; Alonso, L.; Koetz-Ploch, L.; Hernando, E.; Teixidó, J. miR-204-5p and miR-211-5p Contribute to BRAF Inhibitor Resistance in Melanoma. Cancer Res. 2017, 78, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Qian, Y.; Ni, X.; Bu, X.; Xia, Y.; Wang, J.; Ruan, H.; Ma, S.; Xu, B. miR-204-5p acts as a tumor suppressor by targeting matrix metalloproteinases-9 and B-cell lymphoma-2 in malignant melanoma. OncoTargets Ther. 2017, 10, 1237–1246. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, C.; Cao, Y.; Liu, E. miR-150 Suppresses Tumor Growth in Melanoma through Downregulation of MYB. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 317–323. [Google Scholar] [CrossRef]
- Soengas, M.S.; Hernando, E. TYRP1 mRNA goes fishing for miRNAs in melanoma. Nat. Cell Biol. 2017, 19, 1311–1312. [Google Scholar] [CrossRef]
- Mazar, J.; Deyoung, K.; Khaitan, D.; Meister, E.; Almodovar, A.; Goydos, J.; Ray, A.; Perera, R.J. The Regulation of miRNA-211 Expression and Its Role in Melanoma Cell Invasiveness. PLoS ONE 2010, 5, e13779. [Google Scholar] [CrossRef]
- Orimo, H.; Ito, H.; Suzuki, T.; Araki, A.; Hosoi, T.; Sawabe, M. Reviewing the definition of “elderly”. Geriatr. Gerontol. Int. 2006, 6, 149–158. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2017; p. 272. [Google Scholar]
- Singh, G.; Singh, L.C.G.K. High altitude dermatology. Indian J. Dermatol. 2017, 62, 59–65. [Google Scholar] [CrossRef]
- Galibert, M.-D.; Carreira, S.; Goding, C.R. The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced Tyrosinase expression. EMBO J. 2001, 20, 5022–5031. [Google Scholar] [CrossRef]
- Bell, A.; Bell, D.; Chakravarti, N.; Ma, J.; Henton, N.; Prieto, V.G. Detection of a MicroRNA molecular signature of ultraviolet radiation in the superficial regions of melanocytic nevi on sun-exposed skin. Mod. Pathol. 2018, 31, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H. MicroRNA Networks Modulate Oxidative Stress in Cancer. Int. J. Mol. Sci. 2019, 20, 4497. [Google Scholar] [CrossRef] [PubMed]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Hufbauer, M.; Lazic, D.; Reinartz, M.; Akgül, B.; Pfister, H.; Weissenborn, S.J. Skin tumor formation in human papillomavirus 8 transgenic mice is associated with a deregulation of oncogenic miRNAs and their tumor suppressive targets. J. Dermatol. Sci. 2011, 64, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Khan, M.I.; Shabbir, M.; Mukhtar, H. MicroRNAs in skin response to UV radiation. Curr. Drug Targets 2013, 14, 1128–1134. [Google Scholar] [CrossRef]
- Sun, J.-S.; Zhang, X.-L.; Yang, Y.-J.; Nie, Z.-G.; Zhang, Y. Hypoxia promotes C-X-C chemokine receptor type 4 expression through microRNA-150 in pancreatic cancer cells. Oncol. Lett. 2015, 10, 835–840. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.; Yang, J.; Ma, Y.; Liu, Z.; Li, C.; Wang, T.; Yan, Z.; Du, N. MiR-150-5p regulates melanoma proliferation, invasion and metastasis via SIX1-mediated Warburg Effect. Biochem. Biophys. Res. Commun. 2019, 515, 85–91. [Google Scholar] [CrossRef]
- Santoni, G.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Marinelli, O.; Amantini, C. Targeting Transient Receptor Potential Channels by MicroRNAs Drives Tumor Development and Progression. Adv. Exp. Med. Biol. 2020, 1131, 605–623. [Google Scholar] [CrossRef]
- Thiersch, M.; Swenson, E.R. High Altitude and Cancer Mortality. High Alt. Med. Boil. 2018, 19, 116–123. [Google Scholar] [CrossRef]
- Haluza, D.; Simic, S.; Moshammer, H. Temporal and Spatial Melanoma Trends in Austria: An Ecological Study. Int. J. Environ. Res. Public Heal. 2014, 11, 734–748. [Google Scholar] [CrossRef]
- Aceituno-Madera, P.; Buendía-Eisman, A.; Olmo, F.; Jiménez-Moleón, J.-J.; Serrano-Ortega, S.; Olmo-Reyes, F.J. Melanoma, altitud y radiación UVB. Actas Dermo-Sifiliográficas 2011, 102, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Tembe, V.; Schramm, S.-J.; Stark, M.S.; Patrick, E.; Jayaswal, V.; Tang, Y.H.; Barbour, A.P.; Hayward, N.K.; Thompson, J.F.; Scolyer, R.A.; et al. MicroRNA and mRNA expression profiling in metastatic melanoma reveal associations with BRAF mutation and patient prognosis. Pigment. Cell Melanoma Res. 2015, 28, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Gilot, D.; Migault, M.; Bachelot, L.; Journé, F.; Rogiers, A.; Donnou-Fournet, E.; Mogha, A.; Mouchet, N.; Pinel-Marie, M.-L.; Mari, B.; et al. A non-coding function of TYRP1 mRNA promotes melanoma growth. Nat. Cell Biol. 2017, 19, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Guo, H.; Yang, S.-E.; Cornelius, L.; Linette, G.; Murphy, M.; Sheehan, C.; Ross, J.; Slominski, A.T.; Carlson, J.A. TRPM1 (melastatin) expression is an independent predictor of overall survival in clinical AJCC stage I and II melanoma patients. J. Cutan. Pathol. 2017, 44, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Carlson, J.A.; Slominski, A.T. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef]
- Harcharik, S.; Bernardo, S.; Moskalenko, M.; Pan, M.; Sivendran, M.; Bell, H.; Hall, L.D.; Castillo-Martin, M.; Fox, K.; Cordon-Cardo, C.; et al. Defining the role of CD2 in disease progression and overall survival among patients with completely resected stage-II to -III cutaneous melanoma. J. Am. Acad. Dermatol. 2014, 70, 1036–1044.e3. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Young, G.D.; McMiller, T.L.; Chen, S.; Salas, J.T.; Pritchard, T.S.; Xu, H.; Meeker, A.K.; Fan, J.; Cheadle, C.; et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin. Cancer Res. 2015, 21, 3969–3976. [Google Scholar] [CrossRef]
- Bauer, J.; Büttner, P.; Murali, R.; Okamoto, I.; Kolaitis, N.A.; Landi, M.T.; Scolyer, R.A.; Bastian, B.C. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment. Cell Melanoma Res. 2011, 24, 345–351. [Google Scholar] [CrossRef]
- Bonin, S.; Albano, A.; Di Meo, N.; Gatti, A.; Stinco, G.; Zanconati, F.; Trevisan, G. Cutaneous melanoma frequencies and seasonal trend in 20 years of observation of a population characterised by excessive sun exposure. Radiol. Oncol. 2015, 49, 379–385. [Google Scholar] [CrossRef]
- Pracht, M.; Mogha, A.; Lespagnol, A.; Fautrel, A.; Mouchet, N.; Le Gall, F.; Paumier, V.; Lefeuvre-Plesse, C.; Rioux-Leclerc, N.; Mosser, J.; et al. Prognostic and predictive values of oncogenicBRAF, NRAS, c-KITandMITFin cutaneous and mucous melanoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1530–1538. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, J.-W.; Kim, Y.-S. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: A meta-analysis. Br. J. Dermatol. 2011, 164, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, G.; Potter, L.; DaForno, P.; Pringle, J.H. Cutaneous Melanoma Subtypes Show Different BRAF and NRAS Mutation Frequencies. Clin. Cancer Res. 2006, 12, 4499–4505. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hahn, H.J.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J.; Kim, S.-N. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J. Am. Acad. Dermatol. 2015, 72, 1036–1046.e2. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Maldonado, J.L.; Fridlyand, J.; Patel, H.; Jain, A.N.; Busam, K.; Kageshita, T.; Ono, T.; Albertson, N.G.; Pinkel, D.; Bastian, B.C. Determinants of BRAF mutations in primary melanomas. J. Natl. Cancer Inst. 2003, 95, 1878–1890. [Google Scholar] [CrossRef]
- Thomas, N.E.; Edmiston, S.N.; Alexander, A.; Groben, P.A.; Parrish, E.; Kricker, A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; From, L.; et al. Association Between NRAS and BRAF Mutational Status and Melanoma-Specific Survival Among Patients With Higher Risk Primary Melanoma. JAMA Oncol. 2015, 1, 359–368. [Google Scholar] [CrossRef]
- Johnson, D.B.; Puzanov, I. Treatment of NRAS-mutant melanoma. Curr. Treat. Options Oncol. 2015, 16, 15. [Google Scholar] [CrossRef]
- Mandalà, M.; Merelli, B.; Massi, D. Nras in melanoma: Targeting the undruggable target. Crit. Rev. Oncol. 2014, 92, 107–122. [Google Scholar] [CrossRef]
- Nardon, E.; Donada, M.; Bonin, S.; Dotti, I.; Stanta, G. Higher random oligo concentration improves reverse transcription yield of cDNA from bioptic tissues and quantitative RT-PCR reliability. Exp. Mol. Pathol. 2009, 87, 146–151. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef]
- University of California. Genome Browser-BLAT. Available online: https://genome.ucsc.edu (accessed on 21 August 2018).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J. A wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef] [PubMed]

| Dataset Characteristics | ||||||
|---|---|---|---|---|---|---|
| Variable and its description | BZ n = 107 (35%) | TS n = 125 (41%) | I/IL n = 74 (24%) | Total n = 306 (100%) | ||
| Geographical characteristics | ||||||
| Altitude of residence | Mean in meter (range) | 884 (214–2153) | 53 (0–253) | 699 (564–1513) | p < 0.0001† | 279 (0–2153) |
| Demographic characteristics | ||||||
| Gender | Male | 59 of 107 (55%) | 66 of 125 (52.8%) | 41 of 74 (55.4%) | p = 0.9 ‡ | 166 of 306 (54%) |
| Female | 48 of 107 (45%) | 59 of 125 (47.2%) | 33 of 74 (44.6%) | 140 of 306 (46%) | ||
| Mean age | Year (range) | 67 (19–101) | 69 (16–98) | 70 (21–100) | p = 0.5 † | 69 (16–101) |
| Pathological characteristics | ||||||
| Localization § | Head&Neck&face a | 25 of 107 (23.4%) | 29 of 125 (23.2%) | 18 of 74 (24.3%) | p = 0.9 ‡ | 72 of 306 (23.5%) |
| Trunk b | 35 of 107 (32.7%) | 41 of 125 (32.8%) | 25 of 74 (33.8%) | 101 of 306 (33%) | ||
| Upper Limb c | 16 of 107 (15%) | 16 of 125 (12.8%) | 12 of 74 (16.2%) | 44 of 306 (14.4%) | ||
| Lower Limb d | 30 of 107 (28%) | 35 of 125 (28%) | 18 of 74 (24.3%) | 83 of 306 (27.1%) | ||
| Other sites e | 1 of 107 (0.9%) | 4 of 125 (3.2%) | 1 of 74 (1.4%) | 6 of 306 (2%) | ||
| Stage | I B | 10 of 107 (9.3%) | 9 of 125 (7.2%) | 12 of 74 (16.2%) | p = 0.003 ‡ | 31 of 306 (10%). |
| II | 68 of 107 (63.6%) | 89 of 125 (71.2 %) | 34 of 74 (45.9%) | 191 of 306 (62%) | ||
| III | 21 of 107 (19.6%) | 25 of 125 (20%) | 9 of 74 (12.2%) | 55 of 306 (18%) | ||
| IV | 6 of 107 (5.6%) | 2 of 125 (1.6%) | 9 of 74 (12.2%) | 17 of 306 (6%) | ||
| unknown | 2 of 107 (1.9%) | 0 of 125 (0%) | 10 of 74 (13.5%) | 12 of 306 (4%) | ||
| Lymph nodes | Positive | 23 of 107 (21.5%) | 22 of 125 (17.6%) | 13 of 74 (17.6%) | p = 0.7 ‡ | 58 of 306 (19%) |
| Negative | 82 of 107 (76.6%) | 103 of 125 (82.4%) | 51 of 74 (68.9%) | 236 of 306 (77%) | ||
| unknown | 2 of 107 (1.9%) | 0 of 125 (0%) | 10 of 74 (13.5%) | 12 of 306 (4%) | ||
| Breslow’s thickness | Mean in mm (range) | 4.5 (1.8−27.6) | 4.6 (1.8−30) | 4.6 (1.8−30) | p = 0.9 † | 4.6 mm (1.8−30) |
| Ulceration status | yes | 52 of 107 (48.6%) | 65 of 125 (52%) | 23 of 74 (31%) | p = 0.3 ‡ | 140 of 306 (46%) |
| no | 54 of 107 (50.5%) | 57 of 125 (45.6%) | 34 of 74 (46%) | 145 of 306 (47%) | ||
| unknown | 1 of 107 (0.9%) | 3 of 125 (2.4%) | 17 of 74 (23%) | 21 of 306 (7%) | ||
| Histotype ¶ | NOS f | 2 of 107 (1.9%) | 46 of 125 (36.8%) | 37 of 74 (50%) | p < 0.0001‡ | 85 of 306 (27.7%) |
| Nodular g | 57 of 107 (53.3%) | 61 of 125 (48.8%) | 22 of 74 (29.7%) | 140 of 306 (46%) | ||
| Superficial spreading h | 32 of 107 (29.9%) | 1 of 125 (0.8%) | 7 of 74 (9.5%) | 40 of 306 (13%) | ||
| Acral i | 5 of 107 (4.7%) | 0 of 125 (0%) | 3 of 74 (4.1%) | 8 of 306 (3%) | ||
| Others l, m, n, o, p, q, r | 10 of 107 (9.3%) | 17 of 125 (13.6%) | 5 of 74 (6.8%) | 32 of 306 (10%) | ||
| Unknown | 1 of 107 (0.9%) | 0 of 125 (0%) | 0 of 74 (0%) | 1 of 306 (0.3%) | ||
| Mutation Status | n = 288 | (100%) |
|---|---|---|
| BRAF a | n = 96 | (33.3%) |
| NRAS b | n = 62 | (21.5%) |
| Focal positivity for NRAS b c | n = 5 | (1.8%) |
| BRAF and NRAS negative | n = 124 | (43%) |
| Positive IHC for BRAF and NRAS | n = 1 | (0.4%) |
| Characteristics | BRAF Mutated | NRAS Mutated | No Mutated | Total | p Value | p Value BRAF Mut vs. No Mutated | p Value NRAS Mut vs. No Mutated | p Value BRAF Mut vs. NRAS Mut | |
|---|---|---|---|---|---|---|---|---|---|
| n = 96 (33.5%) | n = 67 (23.3%) | n = 124 (43.2%) | n = 287 (100%) | ||||||
| Environmental characteristics | |||||||||
| Geographic areas | BZ | 33 of 96 (34.4%) | 30 of 67 (44.8%) | 43 of 124 (34.7%) | 106 of 287 (37%) | 0.2 ‡ | 0.2 ‡ | 0.3 ‡ | 0.4 ‡ |
| TS | 45 of 96 (46.9%) | 24 of 67 (35.8%) | 46 of 124 (37.1%) | 115 of 287 (40%) | |||||
| I/IL | 18 of 96 (18.8%) | 13 of 67 (19.4%) | 35 of 124 (28.2%) | 66 of 287 (23%) | |||||
| Altitude of residence | Mean (range) | 449.4 (13–1718) | 593 (46–2153) | 509 (10–2131) | 660 (10–2153) | 0.1 † | 0.4 †† | 0.3 †† | 0.1 †† |
| ≤300 m | 52 of 95 (54.7%) | 32 of 67 (47.8%) | 60 of 124 (48.4%) | 144 of 286 (50.3%) | 0.4 ‡ | 0.4 ‡ | 0.3 ‡ | 0.6 ‡ | |
| 300 < m <750 | 19 of 95 (20%) | 13 of 67 (19.4%) | 34 of 124 (27.4%) | 66 of 286 (23%) | |||||
| ≥750 m | 24 of 95 (25.3%) | 22 of 67 (32.8%) | 30 of 124 (24.2%) | 76 of 286 (26.6%) | |||||
| Demographic characteristics | |||||||||
| Gender | Male | 63 of 96 (65.6%) | 32 of 67 (47.8%) | 64 of 124 (51.6%) | 159 of 287 (55.4%) | 0.04‡ | 0.04‡ | 0.6 ‡ | 0.02‡ |
| Female | 33 of 96 (34.4%) | 35 of 67 (52.2%) | 60 of 124 (48.4%) | 128 of 287 (44.6%) | |||||
| Mean age | Years (range) | 62 (16–95) | 73 (32–101) | 71 (21–101) | 71 (16–101) | 0.0001† | 0.0005†† | 0.5 †† | 0.0001†† |
| Pathological characteristics | |||||||||
| Localization§ | Head&Neck&Face a | 20 of 96 (20.8%) | 11 of 67 (16.4%) | 38 of 124 (30.6%) | 69 of 287 (24%) | 0.02‡ | 0.02‡ | 0.1 ‡ | 0.08 ‡ |
| Trunk b | 43 of 96 (44.8%) | 21 of 67 (31.3%) | 33 of 124 (26.6%) | 97 of 287 (33.8%) | |||||
| Upper Limb c | 8 of 96 (8.3%) | 13 of 67 (19.4%) | 19 of 124 (15.3%) | 40 of 287 (14%) | |||||
| Lower Limb d | 25 of 96 (26%) | 22 of 67 (32.8%) | 29 of 124 (23.4%) | 76 of 287 (26.5%) | |||||
| Other sites e | 0 of 96 (0%) | 0 of 67 (0%) | 5 of 124 (4%) | 5 of 287 (1.7%) | |||||
| Stage | I B | 17 of 96 (17.7%) | 2 of 67 (3%) | 8 of 124 (6.5%) | 27 of 287 (9.4%) | 0.005‡ | 0.005‡ | 0.2 ‡ | 0.04‡ |
| II | 51 of 96 (53.1%) | 43 of 67 (64.2%) | 88 of 124 (71%) | 182 of 287 (63.4%) | |||||
| III | 23 of 96 (24%) | 15 of 67 (22.4%) | 15 of 124 (12.1%) | 53 of 287 (18.5%) | |||||
| IV | 5 of 96 (5.2%) | 3 of 67 (4.5%) | 7 of 124 (5.6%) | 15 of 287 (5.2%) | |||||
| unknown | 0 of 96 (0%) | 4 of 67 (6%) | 6 of 124 (4.8%) | 10 of 287 (3.5%) | |||||
| Lymph nodes | Positive | 25 of 96 (26%) | 14 of 67 (20.9%) | 16 of 124 (12.9%) | 55 of 287 (19.2%) | 0.07 ‡ | 0.02‡ | 0.1 ‡ | 0.5 ‡ |
| Negative | 71 of 96 (74%) | 50 of 67 (74.6%) | 101 of 124 (81.5%) | 222 of 287 (77.4%) | |||||
| unknown | 0 of 96 (0%) | 3 of 67 (4.5%) | 7 of 124 (5.6%) | 10 of 287 (3.5%) | |||||
| Breslow’s thickness | Median in mm (range) | 3.2 (1.8−15) | 3.6 (1.9−30) | 3.5 (1.8−22) | 4 (1.8−30) | 0.1 † | 0.3†† | 0.2†† | 0.07 †† |
| Ulceration status | yes | 45 of 96 (47%) | 31 of 67 (46.3%) | 57 of 124 (46%) | 133 of 287 (46.3%) | 0.9 ‡ | 0.7 ‡ | 0.9 ‡ | 0.8 ‡ |
| no | 49 of 96 (51%) | 32 of 67 (47.8%) | 57 of 124 (46%) | 138 of 287 (48.1%) | |||||
| unknown | 2 of 96 (2%) | 4 of 67 (6%) | 10 of 124 (8%) | 16 of 287 (5.6%) | |||||
| Histotype ¶ | NOS f | 28 of 96 (29.2%) | 16 of 67 (23.9%) | 31 of 124 (25%) | 75 of 287 (26.1%) | 0.1 ‡ | 0.08 ‡ | 0.2 ‡ | 0.7 ‡ |
| Nodular g | 47 of 96 (49%) | 33 of 67 (49.3%) | 56 of 124 (45.2%) | 136 of 287 (47.5%) | |||||
| Superficial spreading h | 14 of 96 (14.6%) | 12 of 67 (17.9%) | 13 of 124 (10.5%) | 39 of 287 (13.6%) | |||||
| Acral i | 0 of 96 (0%) | 1 of 67 (1.5%) | 6 of 124 (4.8%) | 7 of 287 (2.4%) | |||||
| Others l, m, n, o, p, q, r | 7 of 96 (7.3%) | 4 of 67 (6%) | 18 of 124 (14.5%) | 29 of 287 (10.1%) | |||||
| Unknown | 0 of 96 (0%) | 1 of 67 (1.5%) | 0 of 124 (0%) | 1 of 287 (0.3%) | |||||
| Variable | Haz. Ratio | p > z | 95% Conf. Interval | |
|---|---|---|---|---|
| Age at diagnosis | 1.02 | 0.02 1 | 1.00−1.04 | |
| 1.012 | 0.2 | 0.99−1.04 | ||
| Gender | 0.62 | 0.1 | 0.33−1.14 | |
| 0.65 | 0.2 | 0.32−1.33 | ||
| Altitude of Residence | 1.00 | 0.004 | 1.00−1.00 | |
| 1.00 | 0.03 | 1.00−1.00 | ||
| Anatomical Site | 1.13 | 0.3 | 0.90−1.40 | |
| 1.01 | 0.9 | 0.77−1.32 | ||
| Geographic area | 1.13 | 0.5 | 0.69−1.87 | |
| 1.13 | 0.8 | 0.42−3.01 | ||
| TRPM1 | 0.76 | 0.5 | 0.36−1.61 | |
| 0.72 | 0.5 | 0.29−1.80 | ||
| TYRP1 | 2.43 | 0.01 | 1.24−4.77 | |
| 2.51 | 0.02 | 1.15−5.48 | ||
| PDL1 (CD274) | 0.71 | 0.3 | 0.37−1.35 | |
| 0.49 | 0.06 | 0.23−1.01 | ||
| CD2 | 0.75 | 0.4 | 0.39−1.42 | |
| 0.94 | 0.9 | 0.46−1.93 | ||
| MITF | 0.51 | 0.04 | 0.27−0.97 | |
| 0.98 | 0.9 | 0.44−2.19 | ||
| HIF1A | 1.15 | 0.6 | 0.66−2.00 | |
| 1.39 | 0.4 | 0.69−2.75 | ||
| miR-150-5p | 0.96 | 0.9 | 0.48−1.93 | |
| 0.62 | 0.3 | 0.27−1.42 | ||
| miR-155-5p | 0.95 | 0.9 | 0.50−1.79 | |
| 0.86 | 0.7 | 0.41−1.80 | ||
| miR-204-5p | 0.87 | 0.7 | 0.48−1.59 | |
| 1.08 | 0.8 | 0.53−2.19 | ||
| miR-211-5p | 0.99 | 1 | 0.44−2.25 | |
| 0.58 | 0.3 | 0.21−1.60 | ||
| Mutational Status | 1.98 | <0.001 | 1.40−2.25 | |
| 1.55 | 0.04 | 1.01−2.39 | ||
| Stage at diagnosis | II | 0.67 | 0.4 | 0.25−1.81 |
| 0.95 | 0.9 | 0.27−3.31 | ||
| III | 1.38 | 0.5 | 0.49−3.94 | |
| 1.87 | 0.4 | 0.48−7.32 | ||
| IV | 9.89 | <0.001 | 2.76−35.5 | |
| 11.1 | 0.008 | 1.88−65.8 | ||
| Ulceration | 1.3 | 0.4 | 0.72−2.34 | |
| 1.5 | 0.3 | 0.73−2.99 | ||
| phtest p = 0.3 | ||||
| phtest p = 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Martino, E.; Brunetti, D.; Canzonieri, V.; Conforti, C.; Eisendle, K.; Mazzoleni, G.; Nobile, C.; Rao, F.; Zschocke, J.; Jukic, E.; et al. The Association of Residential Altitude on the Molecular Profile and Survival of Melanoma: Results of an Interreg Study. Cancers 2020, 12, 2796. https://doi.org/10.3390/cancers12102796
De Martino E, Brunetti D, Canzonieri V, Conforti C, Eisendle K, Mazzoleni G, Nobile C, Rao F, Zschocke J, Jukic E, et al. The Association of Residential Altitude on the Molecular Profile and Survival of Melanoma: Results of an Interreg Study. Cancers. 2020; 12(10):2796. https://doi.org/10.3390/cancers12102796
Chicago/Turabian StyleDe Martino, Eleonora, Davide Brunetti, Vincenzo Canzonieri, Claudio Conforti, Klaus Eisendle, Guido Mazzoleni, Carla Nobile, Federica Rao, Johannes Zschocke, Emina Jukic, and et al. 2020. "The Association of Residential Altitude on the Molecular Profile and Survival of Melanoma: Results of an Interreg Study" Cancers 12, no. 10: 2796. https://doi.org/10.3390/cancers12102796
APA StyleDe Martino, E., Brunetti, D., Canzonieri, V., Conforti, C., Eisendle, K., Mazzoleni, G., Nobile, C., Rao, F., Zschocke, J., Jukic, E., Jaschke, W., Weinlich, G., Zelger, B., Schmuth, M., Stanta, G., Zanconati, F., Zalaudek, I., & Bonin, S. (2020). The Association of Residential Altitude on the Molecular Profile and Survival of Melanoma: Results of an Interreg Study. Cancers, 12(10), 2796. https://doi.org/10.3390/cancers12102796











