PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis
Abstract
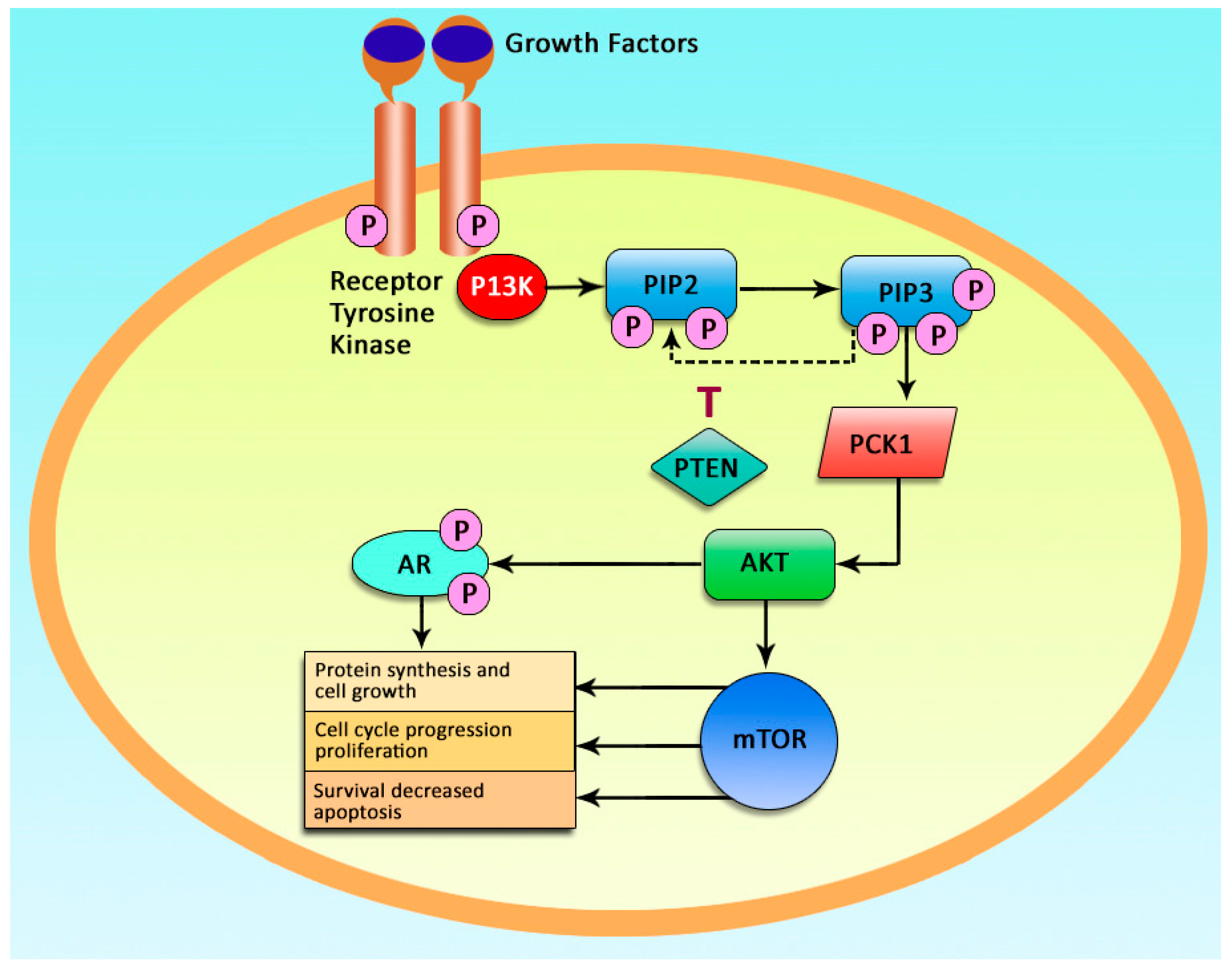
1. Introduction
1.1. PIK3CA Mutations in Breast Cancer
1.2. Prognostic Importance of PIK3CA Genetic Mutations in Colorectal Cancer
1.3. Prognostic Role of PIK3CA Gene Mutation in Lung Cancer
1.4. The Role of PIK3CA Gene Mutations in Thyroid Cancer
1.5. PIK3CA Gene Mutations Frequency in Head and Neck Squamous Cell Cancer (HNSCC)
1.6. Prognostic Role of PIK3CA Gene Mutations in Esophageal Cancer
1.7. The Importance of PIK3CA Gene Mutations in Pathogenesis of Renal Cell Cancer
1.8. Association of PIK3CA Genetic Mutations with Cervical, Ovarian, and Urothelial Cancer
2. PI3K Inhibitors
3. Conclusions
Funding
Conflicts of Interest
References
- Karakas, B.; Bachman, K.E.; Park, B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer 2006, 94, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Graziani, A.; Gramaglia, D.; Cantley, L.C.; Comoglio, P.M. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J. Biol. Chem. 1991, 266, 22087–22090. [Google Scholar] [PubMed]
- Bellacosa, A.; Testa, J.R.; Staal, S.P.; Tsichlis, P.N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 1991, 254, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Staal, S.P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA 1987, 84, 5034–5037. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef]
- Steck, P.A.; Pershouse, M.A.; Jasser, S.A.; Yung, W.K.; Lin, H.; Ligon, A.H.; Langford, L.A.; Baumgard, M.L.; Hattier, T.; Davis, T.; et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997, 15, 356–362. [Google Scholar] [CrossRef]
- Knobbe, C.B.; Reifenberger, G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3′-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003, 13, 507–518. [Google Scholar] [CrossRef]
- Shayesteh, L.; Lu, Y.; Kuo, W.L.; Baldocchi, R.; Godfrey, T.; Collins, C.; Pinkel, D.; Powell, B.; Mills, G.B.; Gray, J.W. PIK3CA is implicated as an oncogene in ovarian cancer. Nat. Genet. 1999, 21, 99–102. [Google Scholar] [CrossRef]
- Philp, A.J.; Campbell, I.G.; Leet, C.; Vincan, E.; Rockman, S.P.; Whitehead, R.H.; Thomas, R.J.; Phillips, W.A. The phosphatidylinositol 3’-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001, 61, 7426–7429. [Google Scholar]
- Millis, S.Z.; Jardim, D.L.; Albacker, L.; Ross, J.S.; Miller, V.A.; Ali, S.M.; Kurzrock, R. Phosphatidylinositol 3-kinase pathway genomic alterations in 60,991 diverse solid tumors informs targeted therapy opportunities. Cancer 2019, 125, 1185–1199. [Google Scholar] [CrossRef]
- Elwy, F.; Helwa, R.; El Leithy, A.A.; Shehab El din, Z.; Assem, M.M.; Hassan, N.H. PIK3CA mutations in HER2-positive Breast Cancer Patients; Frequency and Clinicopathological Perspective in Egyptian Patients. Asian Pac. J. Cancer Prev. 2017, 18, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef] [PubMed]
- Bachman, K.E.; Argani, P.; Samuels, Y.; Silliman, N.; Ptak, J.; Szabo, S.; Konishi, H.; Karakas, B.; Blair, B.G.; Lin, C.; et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol. Ther. 2004, 3, 772–775. [Google Scholar] [CrossRef]
- Saal, L.H.; Holm, K.; Maurer, M.; Memeo, L.; Su, T.; Wang, X.; Yu, J.S.; Malmstrom, P.O.; Mansukhani, M.; Enoksson, J.; et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005, 65, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Jhawer, M.; Goel, S.; Wilson, A.J.; Montagna, C.; Ling, Y.H.; Byun, D.S.; Nasser, S.; Arango, D.; Shin, J.; Klampfer, L.; et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008, 68, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Martini, M.; Molinari, F.; Veronese, S.; Nichelatti, M.; Artale, S.; Di Nicolantonio, F.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009, 69, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Liao, X.; Imamura, Y.; Yamauchi, M.; McCleary, N.J.; Ng, K.; Niedzwiecki, D.; Saltz, L.B.; Mayer, R.J.; Whittom, R.; et al. Predictive and prognostic analysis of PIK3CA mutation in stage III colon cancer intergroup trial. J. Natl. Cancer Inst. 2013, 105, 1789–1798. [Google Scholar] [CrossRef]
- Reggiani Bonetti, L.; Barresi, V.; Maiorana, A.; Manfredini, S.; Caprera, C.; Bettelli, S. Clinical Impact and Prognostic Role of KRAS/BRAF/PIK3CA Mutations in Stage I Colorectal Cancer. Dis. Markers 2018, 2018, 2959801. [Google Scholar] [CrossRef]
- Gong, J.; Cho, M.; Sy, M.; Salgia, R.; Fakih, M. Molecular profiling of metastatic colorectal tumors using next-generation sequencing: A single-institution experience. Oncotarget 2017, 8, 42198–42213. [Google Scholar] [CrossRef]
- Benvenuti, S.; Frattini, M.; Arena, S.; Zanon, C.; Cappelletti, V.; Coradini, D.; Daidone, M.G.; Pilotti, S.; Pierotti, M.A.; Bardelli, A. PIK3CA cancer mutations display gender and tissue specificity patterns. Hum. Mutat. 2008, 29, 284–288. [Google Scholar] [CrossRef]
- Fang, W.L.; Huang, K.H.; Lan, Y.T.; Lin, C.H.; Chang, S.C.; Chen, M.H.; Chao, Y.; Lin, W.C.; Lo, S.S.; Li, A.F.; et al. Mutations in PI3K/AKT pathway genes and amplifications of PIK3CA are associated with patterns of recurrence in gastric cancers. Oncotarget 2016, 7, 6201–6220. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Ahnen, D.J.; Cheng, I.; Newcomb, P.A.; Win, A.K.; Burnett, T. PIK3CA Somatic Mutation Status in Relation to Patient and Tumor Factors in Racial/Ethnic Minorities with Colorectal Cancer. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.J.; Zeng, T.; Wang, L.J.; Lei, H.B.; Ge, W.; Wang, Z. Genes with mutation significance were highly associated with the clinical pattern of patients with breast cancer. Oncotarget 2017, 8, 98094–98102. [Google Scholar] [CrossRef] [PubMed]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Xie, N.; Tian, C.; Yang, X.; Liu, L.; Li, J.; Xiao, H.; Wu, H.; Lu, J.; Gao, J.; et al. Identifying Circulating Tumor DNA Mutation Profiles in Metastatic Breast Cancer Patients with Multiline Resistance. EBioMedicine 2018, 32, 111–118. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.; Du, J.; Li, H.; Wang, H.; Huang, L.; Xiang, H.; Xie, J.; Liu, X.; Li, H.; et al. The distinct clinicopathological and prognostic implications of PIK3CA mutations in breast cancer patients from Central China. Cancer Manag. Res. 2019, 11, 1473–1492. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; Xu, Y.; Xie, Y. Co-mutation of TP53 and PIK3CA in residual disease after neoadjuvant chemotherapy is associated with poor survival in breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1235–1242. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Huang, S.C.; Lin, H.H.; Lin, C.C.; Lan, Y.T.; Wang, H.S.; Yang, S.H.; Jiang, J.K.; Chen, W.S.; Lin, T.C.; et al. Differences in gene mutations according to gender among patients with colorectal cancer. World J. Surg. Oncol. 2018, 16, 128. [Google Scholar] [CrossRef]
- Christensen, T.D.; Palshof, J.A.; Larsen, F.O.; Poulsen, T.S.; Hogdall, E.; Pfeiffer, P.; Jensen, B.V.; Yilmaz, M.K.; Nielsen, D. Associations between primary tumor RAS, BRAF and PIK3CA mutation status and metastatic site in patients with chemo-resistant metastatic colorectal cancer. Acta Oncol. 2018, 57, 1057–1062. [Google Scholar] [CrossRef]
- Barbareschi, M.; Buttitta, F.; Felicioni, L.; Cotrupi, S.; Barassi, F.; Del Grammastro, M.; Ferro, A.; Dalla Palma, P.; Galligioni, E.; Marchetti, A. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin. Cancer Res. 2007, 13, 6064–6069. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, X.; Sun, Y.; Wang, J.; Zhong, X.; Li, J.; Hu, M.; Zheng, H. Prevalence and Prognostic Role of PIK3CA/AKT1 Mutations in Chinese Breast Cancer Patients. Cancer Res. Treat. 2019, 51, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Perez-Tenorio, G.; Alkhori, L.; Olsson, B.; Waltersson, M.A.; Nordenskjold, B.; Rutqvist, L.E.; Skoog, L.; Stal, O. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin. Cancer Res. 2007, 13, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, M.; Susini, A.; Vacher, S.; Cizeron-Clairac, G.; Andrieu, C.; Driouch, K.; Fourme, E.; Lidereau, R.; Bieche, I. PIK3CA mutation impact on survival in breast cancer patients and in ERalpha, PR and ERBB2-based subgroups. Breast Cancer Res. 2012, 14, R28. [Google Scholar] [CrossRef] [PubMed]
- Buttitta, F.; Felicioni, L.; Barassi, F.; Martella, C.; Paolizzi, D.; Fresu, G.; Salvatore, S.; Cuccurullo, F.; Mezzetti, A.; Campani, D.; et al. PIK3CA mutation and histological type in breast carcinoma: High frequency of mutations in lobular carcinoma. J. Pathol. 2006, 208, 350–355. [Google Scholar] [CrossRef]
- Mangone, F.R.; Bobrovnitchaia, I.G.; Salaorni, S.; Manuli, E.; Nagai, M.A. PIK3CA exon 20 mutations are associated with poor prognosis in breast cancer patients. Clinics 2012, 67, 1285–1290. [Google Scholar] [CrossRef]
- Deng, L.; Chen, J.; Zhong, X.R.; Luo, T.; Wang, Y.P.; Huang, H.F.; Yin, L.J.; Qiu, Y.; Bu, H.; Lv, Q.; et al. Correlation between activation of PI3K/AKT/mTOR pathway and prognosis of breast cancer in Chinese women. PLoS ONE 2015, 10, e0120511. [Google Scholar] [CrossRef][Green Version]
- Seo, Y.; Park, Y.H.; Ahn, J.S.; Im, Y.H.; Nam, S.J.; Cho, S.Y.; Cho, E.Y. PIK3CA Mutations and Neoadjuvant Therapy Outcome in Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: A Sequential Analysis. J. Breast Cancer 2018, 21, 382–390. [Google Scholar] [CrossRef]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Hauptmann, M.; et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef]
- Maruyama, N.; Miyoshi, Y.; Taguchi, T.; Tamaki, Y.; Monden, M.; Noguchi, S. Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clin. Cancer Res. 2007, 13, 408–414. [Google Scholar] [CrossRef]
- Eichhorn, P.J.; Gili, M.; Scaltriti, M.; Serra, V.; Guzman, M.; Nijkamp, W.; Beijersbergen, R.L.; Valero, V.; Seoane, J.; Bernards, R.; et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008, 68, 9221–9230. [Google Scholar] [CrossRef]
- Yang, L.; Ye, F.; Bao, L.; Zhou, X.; Wang, Z.; Hu, P.; Ouyang, N.; Li, X.; Shi, Y.; Chen, G.; et al. Somatic alterations of TP53, ERBB2, PIK3CA and CCND1 are associated with chemosensitivity for breast cancers. Cancer Sci. 2019, 110, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Hennessy, B.T.; Gonzalez-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; Garcia-Echeverria, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Cardone, L.; Tordai, A.; Yan, K.; Gomez, H.L.; Figureoa, L.J.; Hubbard, R.E.; Valero, V.; Souchon, E.A.; Symmans, W.F.; et al. PIK3CA-activating mutations and chemotherapy sensitivity in stage II-III breast cancer. Breast Cancer Res. 2008, 10, R27. [Google Scholar] [CrossRef]
- Brown, K.K.; Toker, A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000Prime Rep. 2015, 7, 13. [Google Scholar] [CrossRef]
- Baselga, J.; Cortes, J.; Kim, S.B.; Im, S.A.; Hegg, R.; Im, Y.H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Zhou, D.; Ouyang, Q.; Liu, L.; Liu, J.; Tang, Y.; Xiao, M.; Wang, Y.; He, Q.; Hu, Z.Y. Chemotherapy Modulates Endocrine Therapy-Related Resistance Mutations in Metastatic Breast Cancer. Transl. Oncol. 2019, 12, 764–774. [Google Scholar] [CrossRef]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Li, C.S.; Song, Y.; Lou, H.; Guan, D.; Jin, L. Surface Enhanced Raman Spectroscopy (SERS) for the Multiplex Detection of Braf, Kras, and Pik3ca Mutations in Plasma of Colorectal Cancer Patients. Theranostics 2018, 8, 1678–1689. [Google Scholar] [CrossRef]
- Mikami, M.; Nosho, K.; Yamamoto, H.; Takahashi, T.; Maehata, T.; Taniguchi, H.; Adachi, Y.; Imamura, A.; Fujita, M.; Hosokawa, M.; et al. Mutational analysis of beta-catenin and the RAS-RAF signalling pathway in early flat-type colorectal tumours. Eur. J. Cancer 2006, 42, 3065–3072. [Google Scholar] [CrossRef]
- Miyaki, M.; Iijima, T.; Yamaguchi, T.; Takahashi, K.; Matsumoto, H.; Yasutome, M.; Funata, N.; Mori, T. Mutations of the PIK3CA gene in hereditary colorectal cancers. Int. J. Cancer 2007, 121, 1627–1630. [Google Scholar] [CrossRef]
- Reggiani Bonetti, L.; Barresi, V.; Bettelli, S.; Caprera, C.; Manfredini, S.; Maiorana, A. Analysis of KRAS, NRAS, PIK3CA, and BRAF mutational profile in poorly differentiated clusters of KRAS-mutated colon cancer. Hum. Pathol. 2017, 62, 91–98. [Google Scholar] [CrossRef]
- Barault, L.; Veyrie, N.; Jooste, V.; Lecorre, D.; Chapusot, C.; Ferraz, J.M.; Lievre, A.; Cortet, M.; Bouvier, A.M.; Rat, P.; et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int. J. Cancer 2008, 122, 2255–2259. [Google Scholar] [CrossRef]
- Ogino, S.; Nosho, K.; Kirkner, G.J.; Shima, K.; Irahara, N.; Kure, S.; Chan, A.T.; Engelman, J.A.; Kraft, P.; Cantley, L.C.; et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J. Clin. Oncol. 2009, 27, 1477–1484. [Google Scholar] [CrossRef]
- Foltran, L.; De Maglio, G.; Pella, N.; Ermacora, P.; Aprile, G.; Masiero, E.; Giovannoni, M.; Iaiza, E.; Cardellino, G.G.; Lutrino, S.E.; et al. Prognostic role of KRAS, NRAS, BRAF and PIK3CA mutations in advanced colorectal cancer. Future Oncol. 2015, 11, 629–640. [Google Scholar] [CrossRef]
- Manceau, G.; Marisa, L.; Boige, V.; Duval, A.; Gaub, M.P.; Milano, G.; Selves, J.; Olschwang, S.; Jooste, V.; le Legrain, M.; et al. PIK3CA mutations predict recurrence in localized microsatellite stable colon cancer. Cancer Med. 2015, 4, 371–382. [Google Scholar] [CrossRef]
- Michel, P.; Boige, V.; Andre, T.; Aparicio, T.; Bachet, J.B.; Dahan, L.; Guimbaud, R.; Lepage, C.; Manfredi, S.; Tougeron, D.; et al. Aspirin versus placebo in stage III or high-risk stage II colon cancer with PIK3CA mutation: A French randomised double-blind phase III trial (PRODIGE 50-ASPIK). Dig. Liver Dis. 2018, 50, 305–307. [Google Scholar] [CrossRef]
- Li, P.; Wu, H.; Zhang, H.; Shi, Y.; Xu, J.; Ye, Y.; Xia, D.; Yang, J.; Cai, J.; Wu, Y. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: A meta-analysis. Gut 2015, 64, 1419–1425. [Google Scholar] [CrossRef]
- Massion, P.P.; Taflan, P.M.; Shyr, Y.; Rahman, S.M.; Yildiz, P.; Shakthour, B.; Edgerton, M.E.; Ninan, M.; Andersen, J.J.; Gonzalez, A.L. Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am. J. Respir. Crit. Care Med. 2004, 170, 1088–1094. [Google Scholar] [CrossRef]
- Youssef, O.; Knuuttila, A.; Piirila, P.; Bohling, T.; Sarhadi, V.; Knuutila, S. Hotspot Mutations Detectable by Next-generation Sequencing in Exhaled Breath Condensates from Patients with Lung Cancer. Anticancer Res. 2018, 38, 5627–5634. [Google Scholar] [CrossRef]
- Kawano, O.; Sasaki, H.; Endo, K.; Suzuki, E.; Haneda, H.; Yukiue, H.; Kobayashi, Y.; Yano, M.; Fujii, Y. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer 2006, 54, 209–215. [Google Scholar] [CrossRef]
- Okudela, K.; Suzuki, M.; Kageyama, S.; Bunai, T.; Nagura, K.; Igarashi, H.; Takamochi, K.; Suzuki, K.; Yamada, T.; Niwa, H.; et al. PIK3CA mutation and amplification in human lung cancer. Pathol. Int. 2007, 57, 664–671. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shigematsu, H.; Nomura, M.; Lockwood, W.W.; Sato, M.; Okumura, N.; Soh, J.; Suzuki, M.; Wistuba, I.; Fong, K.M.; et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008, 68, 6913–6921. [Google Scholar] [CrossRef]
- Shi, R.; Li, M.; Raghavan, V.; Tam, S.; Cabanero, M.; Pham, N.A.; Shepherd, F.A.; Moghal, N.; Tsao, M.S. Targeting the CDK4/6-Rb Pathway Enhances Response to PI3K Inhibition in PIK3CA-Mutant Lung Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 5990–6000. [Google Scholar] [CrossRef]
- Kawano, O.; Sasaki, H.; Okuda, K.; Yukiue, H.; Yokoyama, T.; Yano, M.; Fujii, Y. PIK3CA gene amplification in Japanese non-small cell lung cancer. Lung Cancer 2007, 58, 159–160. [Google Scholar] [CrossRef]
- Wu, S.G.; Liu, Y.N.; Yu, C.J.; Yang, J.C.; Shih, J.Y. Driver mutations of young lung adenocarcinoma patients with malignant pleural effusion. Genes Chromosomes Cancer 2018, 57, 513–521. [Google Scholar] [CrossRef]
- Jing, C.; Mao, X.; Wang, Z.; Sun, K.; Ma, R.; Wu, J.; Cao, H. Nextgeneration sequencingbased detection of EGFR, KRAS, BRAF, NRAS, PIK3CA, Her2 and TP53 mutations in patients with nonsmall cell lung cancer. Mol. Med. Rep. 2018, 18, 2191–2197. [Google Scholar] [CrossRef]
- Li, W.; Qiu, T.; Ling, Y.; Gao, S.; Ying, J. Subjecting appropriate lung adenocarcinoma samples to next-generation sequencing-based molecular testing: challenges and possible solutions. Mol. Oncol. 2018, 12, 677–689. [Google Scholar] [CrossRef]
- Lockney, N.A.; Yang, T.J.; Barron, D.; Gelb, E.; Gelblum, D.Y.; Yorke, E.; Shi, W.; Zhang, Z.; Rimner, A.; Wu, A.J. PIK3CA mutation is associated with increased local failure in lung stereotactic body radiation therapy (SBRT). Clin. Transl. Radiat. Oncol. 2017, 7, 91–93. [Google Scholar] [CrossRef]
- Zhao, J.; Han, Y.; Li, J.; Chai, R.; Bai, C. Prognostic value of KRAS/TP53/PIK3CA in non-small cell lung cancer. Oncol. Lett. 2019, 17, 3233–3240. [Google Scholar] [CrossRef]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef]
- Xing, M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid 2010, 20, 697–706. [Google Scholar] [CrossRef]
- Jeon, M.J.; Song, D.E.; Jung, C.K.; Kim, W.G.; Kwon, H.; Lee, Y.M.; Sung, T.Y.; Yoon, J.H.; Chung, K.W.; Hong, S.J.; et al. Impact of Reclassification on Thyroid Nodules with Architectural Atypia: From Non-Invasive Encapsulated Follicular Variant Papillary Thyroid Carcinomas to Non-Invasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. PLoS ONE 2016, 11, e0167756. [Google Scholar] [CrossRef]
- Garcia-Rostan, G.; Costa, A.M.; Pereira-Castro, I.; Salvatore, G.; Hernandez, R.; Hermsem, M.J.; Herrero, A.; Fusco, A.; Cameselle-Teijeiro, J.; Santoro, M. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005, 65, 10199–10207. [Google Scholar] [CrossRef]
- Wu, M.; Szporn, A.H.; Zhang, D.; Wasserman, P.; Gan, L.; Miller, L.; Burstein, D.E. Cytology applications of p63 and TTF-1 immunostaining in differential diagnosis of lung cancers. Diagn. Cytopathol. 2005, 33, 223–227. [Google Scholar] [CrossRef]
- Qiu, W.; Tong, G.X.; Manolidis, S.; Close, L.G.; Assaad, A.M.; Su, G.H. Novel mutant-enriched sequencing identified high frequency of PIK3CA mutations in pharyngeal cancer. Int. J. Cancer 2008, 122, 1189–1194. [Google Scholar] [CrossRef]
- Abubaker, J.; Jehan, Z.; Bavi, P.; Sultana, M.; Al-Harbi, S.; Ibrahim, M.; Al-Nuaim, A.; Ahmed, M.; Amin, T.; Al-Fehaily, M.; et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J. Clin. Endocrinol. Metab. 2008, 93, 611–618. [Google Scholar] [CrossRef]
- Santarpia, L.; El-Naggar, A.K.; Cote, G.J.; Myers, J.N.; Sherman, S.I. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2008, 93, 278–284. [Google Scholar] [CrossRef]
- Schechter, R.B.; Nagilla, M.; Joseph, L.; Reddy, P.; Khattri, A.; Watson, S.; Locati, L.D.; Licitra, L.; Greco, A.; Pelosi, G.; et al. Genetic profiling of advanced radioactive iodine-resistant differentiated thyroid cancer and correlation with axitinib efficacy. Cancer Lett. 2015, 359, 269–274. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Murugan, A.K.; Qasem, E.; Alswailem, M.; Al-Hindi, H.; Shi, Y. Single Point Mutations in Pediatric Differentiated Thyroid Cancer. Thyroid 2017, 27, 189–196. [Google Scholar] [CrossRef]
- Charles, R.P.; Silva, J.; Iezza, G.; Phillips, W.A.; McMahon, M. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol. Cancer Res. 2014, 12, 979–986. [Google Scholar] [CrossRef]
- Xing, J.C.; Tufano, R.P.; Murugan, A.K.; Liu, D.; Wand, G.; Ladenson, P.W.; Xing, M.; Trink, B. Single nucleotide polymorphism rs17849071 G/T in the PIK3CA gene is inversely associated with follicular thyroid cancer and PIK3CA amplification. PLoS ONE 2012, 7, e49192. [Google Scholar] [CrossRef]
- Lee, M.Y.; Ku, B.M.; Kim, H.S.; Lee, J.Y.; Lim, S.H.; Sun, J.M.; Lee, S.H.; Park, K.; Oh, Y.L.; Hong, M.; et al. Genetic Alterations and Their Clinical Implications in High-Recurrence Risk Papillary Thyroid Cancer. Cancer Res. Treat. 2017, 49, 906–914. [Google Scholar] [CrossRef]
- Morandi, L.; Righi, A.; Maletta, F.; Rucci, P.; Pagni, F.; Gallo, M.; Rossi, S.; Caporali, L.; Sapino, A.; Lloyd, R.V.; et al. Somatic mutation profiling of hobnail variant of papillary thyroid carcinoma. Endocr. Relat. Cancer 2017, 24, 107–117. [Google Scholar] [CrossRef]
- Bandoh, N.; Akahane, T.; Goto, T.; Kono, M.; Ichikawa, H.; Sawada, T.; Yamaguchi, T.; Nakano, H.; Kawase, Y.; Kato, Y.; et al. Targeted next-generation sequencing of cancer-related genes in thyroid carcinoma: A single institution’s experience. Oncol. Lett. 2018, 16, 7278–7286. [Google Scholar] [CrossRef]
- Kozaki, K.; Imoto, I.; Pimkhaokham, A.; Hasegawa, S.; Tsuda, H.; Omura, K.; Inazawa, J. PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci. 2006, 97, 1351–1358. [Google Scholar] [CrossRef]
- Murugan, A.K.; Hong, N.T.; Fukui, Y.; Munirajan, A.K.; Tsuchida, N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int. J. Oncol. 2008, 32, 101–111. [Google Scholar] [CrossRef]
- Ludwig, M.L.; Kulkarni, A.; Birkeland, A.C.; Michmerhuizen, N.L.; Foltin, S.K.; Mann, J.E.; Hoesli, R.C.; Devenport, S.N.; Jewell, B.M.; Shuman, A.G.; et al. The genomic landscape of UM-SCC oral cavity squamous cell carcinoma cell lines. Oral Oncol. 2018, 87, 144–151. [Google Scholar] [CrossRef]
- Schmidt, H.; Kulasinghe, A.; Allcock, R.J.N.; Tan, L.Y.; Mokany, E.; Kenny, L.; Punyadeera, C. A Pilot Study to Non-Invasively Track PIK3CA Mutation in Head and Neck Cancer. Diagnostics 2018, 8, 79. [Google Scholar] [CrossRef]
- Saintigny, P.; Mitani, Y.; Pytynia, K.B.; Ferrarotto, R.; Roberts, D.B.; Weber, R.S.; Kies, M.S.; Maity, S.N.; Lin, S.H.; El-Naggar, A.K. Frequent PTEN loss and differential HER2/PI3K signaling pathway alterations in salivary duct carcinoma: Implications for targeted therapy. Cancer 2018, 124, 3693–3705. [Google Scholar] [CrossRef]
- Nakagaki, T.; Tamura, M.; Kobashi, K.; Omori, A.; Koyama, R.; Idogawa, M.; Ogi, K.; Hiratsuka, H.; Tokino, T.; Sasaki, Y. Targeted next-generation sequencing of 50 cancer-related genes in Japanese patients with oral squamous cell carcinoma. Tumour Biol. 2018, 40, 1010428318800180. [Google Scholar] [CrossRef]
- Gillison, M.L.; Akagi, K.; Xiao, W.; Jiang, B.; Pickard, R.K.L.; Li, J.; Swanson, B.J.; Agrawal, A.D.; Zucker, M.; Stache-Crain, B.; et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 2019, 29, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ruicci, K.M.; Meens, J.; Sun, R.X.; Rizzo, G.; Pinto, N.; Yoo, J.; Fung, K.; MacNeil, D.; Mymryk, J.S.; Barrett, J.W.; et al. A controlled trial of HNSCC patient-derived xenografts reveals broad efficacy of PI3Kalpha inhibition in controlling tumor growth. Int. J. Cancer 2019, 145, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Pettinga, D.; Schubert, A.D.; Ladenson, P.W.; Ball, D.W.; Chung, J.H.; Schrock, A.B.; Madison, R.; Frampton, G.M.; Stephens, P.J.; et al. Genomic Profiling of Parathyroid Carcinoma Reveals Genomic Alterations Suggesting Benefit from Therapy. Oncologist 2019, 24, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Chou, M.J.; Tzen, C.Y. PIK3CA mutation occurs in nasopharyngeal carcinoma but does not significantly influence the disease-specific survival. Med. Oncol. 2009, 26, 322–326. [Google Scholar] [CrossRef]
- Hedberg, M.L.; Peyser, N.D.; Bauman, J.E.; Gooding, W.E.; Li, H.; Bhola, N.E.; Zhu, T.R.; Zeng, Y.; Brand, T.M.; Kim, M.O.; et al. Use of nonsteroidal anti-inflammatory drugs predicts improved patient survival for PIK3CA-altered head and neck cancer. J. Exp. Med. 2019, 216, 419–427. [Google Scholar] [CrossRef]
- Hanna, G.J.; Kacew, A.; Chau, N.G.; Shivdasani, P.; Lorch, J.H.; Uppaluri, R.; Haddad, R.I.; MacConaill, L.E. Improved outcomes in PI3K-pathway-altered metastatic HPV oropharyngeal cancer. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Resteghini, C.; Perrone, F.; Miceli, R.; Bergamini, C.; Alfieri, S.; Orlandi, E.; Guzzo, M.; Granata, R.; Galbiati, D.; Cavalieri, S.; et al. Prognostic role of PIK3CA and TP53 in human papillomavirus-negative oropharyngeal cancers. Tumori 2018, 104, 213–220. [Google Scholar] [CrossRef]
- Dunn, L.A.; Fury, M.G.; Xiao, H.; Baxi, S.S.; Sherman, E.J.; Korte, S.; Pfister, C.; Haque, S.; Katabi, N.; Ho, A.L.; et al. A phase II study of temsirolimus added to low-dose weekly carboplatin and paclitaxel for patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). Ann. Oncol. 2017, 28, 2533–2538. [Google Scholar] [CrossRef]
- Munari, F.F.; Cruvinel-Carloni, A.; Lacerda, C.F.; de Oliveira, A.T.T.; Scapulatempo-Neto, C.; da Silva, S.R.M.; Crema, E.; Adad, S.J.; Rodrigues, M.A.M.; Henry, M.; et al. PIK3CA mutations are frequent in esophageal squamous cell carcinoma associated with chagasic megaesophagus and are associated with a worse patient outcome. Infect. Agent Cancer 2018, 13, 43. [Google Scholar] [CrossRef]
- Akagi, I.; Miyashita, M.; Makino, H.; Nomura, T.; Hagiwara, N.; Takahashi, K.; Cho, K.; Mishima, T.; Ishibashi, O.; Ushijima, T.; et al. Overexpression of PIK3CA is associated with lymph node metastasis in esophageal squamous cell carcinoma. Int. J. Oncol. 2009, 34, 767–775. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yamaguchi, T.; Maekawa, S.; Takano, S.; Kuno, T.; Tanaka, K.; Tsukui, Y.; Iwamoto, F.; Yoshida, T.; Asakawa, Y.; et al. Target sequencing of cancer-related genes in early esophageal squamous neoplasia resected by endoscopic resection in Japanese patients. Oncotarget 2018, 9, 36793–36803. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, T.; Ren, K.; Li, Z.L.; Ren, J.; Wu, G.; Han, X. RUNX2 Plays An Oncogenic Role in Esophageal Carcinoma by Activating the PI3K/AKT and ERK Signaling Pathways. Cell Physiol. Biochem. 2018, 49, 217–225. [Google Scholar] [CrossRef]
- Ge, X.Q.; Yang, Y.Z.; Li, S.S.; Hou, L.; Ren, J.L.; Yang, K.P.; Fa, X.E. No significant association between PIK3CA mutation and survival of esophageal squamous cell carcinoma: A meta-analysis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 462–468. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, W.; Chughtai, E.A.; Qiao, Z.; Jiang, J.T.; Li, S.M.; Zhang, W.; Zhang, J. PIK3CA gene mutations in Northwest Chinese esophageal squamous cell carcinoma. World J. Gastroenterol. 2017, 23, 2585–2591. [Google Scholar] [CrossRef]
- Brugarolas, J. Molecular genetics of clear-cell renal cell carcinoma. J. Clin. Oncol. 2014, 32, 1968–1976. [Google Scholar] [CrossRef]
- Yokota, T.; Serizawa, M.; Hosokawa, A.; Kusafuka, K.; Mori, K.; Sugiyama, T.; Tsubosa, Y.; Koh, Y. PIK3CA mutation is a favorable prognostic factor in esophageal cancer: molecular profile by next-generation sequencing using surgically resected formalin-fixed, paraffin-embedded tissue. BMC Cancer 2018, 18, 826. [Google Scholar] [CrossRef]
- Liu, Q.; Cornejo, K.M.; Cheng, L.; Hutchinson, L.; Wang, M.; Zhang, S.; Tomaszewicz, K.; Cosar, E.F.; Woda, B.A.; Jiang, Z. Next-Generation Sequencing to Detect Deletion of RB1 and ERBB4 Genes in Chromophobe Renal Cell Carcinoma: A Potential Role in Distinguishing Chromophobe Renal Cell Carcinoma from Renal Oncocytoma. Am. J. Pathol. 2018, 188, 846–852. [Google Scholar] [CrossRef]
- Gasinska, A.; Jaszczynski, J.; Adamczyk, A.; Janecka-Widla, A.; Wilk, W.; Cichocka, A.; Stelmach, A. Biomarkers of epithelial-mesenchymal transition in localized, surgically treated clear-cell renal cell carcinoma. Folia Histochem. Cytobiol. 2018, 56, 195–206. [Google Scholar] [CrossRef]
- Gripp, K.W.; Baker, L.; Kandula, V.; Conard, K.; Scavina, M.; Napoli, J.A.; Griffin, G.C.; Thacker, M.; Knox, R.G.; Clark, G.R.; et al. Nephroblastomatosis or Wilms tumor in a fourth patient with a somatic PIK3CA mutation. Am. J. Med. Genet. A 2016, 170, 2559–2569. [Google Scholar] [CrossRef]
- Isharwal, S.; Hu, W.; Sarungbam, J.; Chen, Y.B.; Gopalan, A.; Fine, S.W.; Tickoo, S.K.; Sirintrapun, S.J.; Jadallah, S.; Loo, F.L.; et al. Genomic landscape of inverted urothelial papilloma and urothelial papilloma of the bladder. J. Pathol. 2019, 248, 260–265. [Google Scholar] [CrossRef]
- Li, C.; Bonazzoli, E.; Bellone, S.; Choi, J.; Dong, W.; Menderes, G.; Altwerger, G.; Han, C.; Manzano, A.; Bianchi, A.; et al. Mutational landscape of primary, metastatic, and recurrent ovarian cancer reveals c-MYC gains as potential target for BET inhibitors. Proc. Natl. Acad. Sci. USA 2019, 116, 619–624. [Google Scholar] [CrossRef]
- Van Nieuwenhuysen, E.; Busschaert, P.; Neven, P.; Han, S.N.; Moerman, P.; Liontos, M.; Papaspirou, M.; Kupryjanczyk, J.; Hogdall, C.; Hogdall, E.; et al. The genetic landscape of 87 ovarian germ cell tumors. Gynecol. Oncol. 2018, 151, 61–68. [Google Scholar] [CrossRef]
- Malentacchi, F.; Turrini, I.; Sorbi, F.; Projetto, E.; Castiglione, F.; Fambrini, M.; Petraglia, F.; Pillozzi, S.; Noci, I. Pilot investigation of the mutation profile of PIK3CA/PTEN genes (PI3K pathway) in grade 3 endometrial cancer. Oncol. Rep. 2019, 41, 1560–1574. [Google Scholar] [CrossRef]
- Patibandla, J.R.; Fehniger, J.E.; Levine, D.A.; Jelinic, P. Small cell cancers of the female genital tract: Molecular and clinical aspects. Gynecol. Oncol. 2018, 149, 420–427. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.; Zhang, X.; Stendahl, U.; Andersson, S.; Wallin, K.L. Mutation of PIK3CA: Possible risk factor for cervical carcinogenesis in older women. Int. J. Oncol. 2009, 34, 409–416. [Google Scholar]
- Lachkar, B.; Minaguchi, T.; Akiyama, A.; Liu, S.; Zhang, S.; Xu, C.; Shikama, A.; Tasaka, N.; Sakurai, M.; Nakao, S.; et al. Prognostic significance of PIK3CA mutation in stage IIB to IVA cervical cancers treated by concurrent chemoradiotherapy with weekly cisplatin. Medicine 2018, 97, e11392. [Google Scholar] [CrossRef]
- Zieba, S.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Goryca, K.; Piascik, A.; Misiek, M.; Bakula-Zalewska, E.; Kopczynski, J.; Kowalski, K.; et al. Somatic mutation profiling of vulvar cancer: Exploring therapeutic targets. Gynecol. Oncol. 2018, 150, 552–561. [Google Scholar] [CrossRef]
- Ousati Ashtiani, Z.; Mehrsai, A.R.; Pourmand, M.R.; Pourmand, G.R. High Resolution Melting Analysis for Rapid Detection of PIK3CA Gene Mutations in Bladder Cancer: A Mutated Target for Cancer Therapy. Urol. J. 2018, 15, 26–31. [Google Scholar] [CrossRef]
- Arjumand, W.; Merry, C.D.; Wang, C.; Saba, E.; McIntyre, J.B.; Fang, S.; Kornaga, E.; Ghatage, P.; Doll, C.M.; Lees-Miller, S.P. Phosphatidyl inositol-3 kinase (PIK3CA) E545K mutation confers cisplatin resistance and a migratory phenotype in cervical cancer cells. Oncotarget 2016, 7, 82424–82439. [Google Scholar] [CrossRef]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. Efficacy of PI3K/AKT/mTOR pathway inhibitors for the treatment of advanced solid cancers: A literature-based meta-analysis of 46 randomised control trials. PLoS ONE 2018, 13, e0192464. [Google Scholar] [CrossRef]
- Jehan, Z.; Bavi, P.; Sultana, M.; Abubaker, J.; Bu, R.; Hussain, A.; Alsbeih, G.; Al-Sanea, N.; Abduljabbar, A.; Ashari, L.H.; et al. Frequent PIK3CA gene amplification and its clinical significance in colorectal cancer. J. Pathol. 2009, 219, 337–346. [Google Scholar] [CrossRef]
- La Monica, S.; Galetti, M.; Alfieri, R.R.; Cavazzoni, A.; Ardizzoni, A.; Tiseo, M.; Capelletti, M.; Goldoni, M.; Tagliaferri, S.; Mutti, A.; et al. Everolimus restores gefitinib sensitivity in resistant non-small cell lung cancer cell lines. Biochem. Pharmacol. 2009, 78, 460–468. [Google Scholar] [CrossRef]
- Madsen, R.R.; Vanhaesebroeck, B.; Semple, R.K. Cancer-Associated PIK3CA Mutations in Overgrowth Disorders. Trends Mol. Med. 2018, 24, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Sapp, J.C.; Lindhurst, M.J.; Parker, V.E.; Blumhorst, C.; Darling, T.; Tosi, L.L.; Huson, S.M.; Whitehouse, R.W.; Jakkula, E.; et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am. J. Med. Genet. A 2014, 164a, 1713–1733. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Mermel, C.H.; Robinson, J.T.; Garraway, L.A.; Golub, T.R.; Meyerson, M.; Gabriel, S.B.; Lander, E.S.; Getz, G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014, 505, 495–501. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- Millis, S.Z.; Ikeda, S.; Reddy, S.; Gatalica, Z.; Kurzrock, R. Landscape of Phosphatidylinositol-3-Kinase Pathway Alterations Across 19784 Diverse Solid Tumors. JAMA Oncol. 2016, 2, 1565–1573. [Google Scholar] [CrossRef]
- Semple, R.K.; Vanhaesebroeck, B. Lessons for cancer drug treatment from tackling a non-cancerous overgrowth syndrome. Nature 2018, 558, 523–525. [Google Scholar] [CrossRef]
| Cancer Type | Reference | Clinicopathological and Prognostic Parameters |
|---|---|---|
| Colon Cancer | [15] | Nodal metastases, high pathological TNM stage, and lymphatic invasion |
| [16] | Decreased risk of peritoneal metastases | |
| [17,18] | Diffuse-type and poorly differentiated gastric cancers and peritoneal recurrence | |
| Not associated with patient outcomes such as survival | ||
| [19,20] | Not associated with the overall survival | |
| [21] | Increased five-year relapse-free interval | |
| [22,23] | Against anti-EGFR antibodies | |
| [24] | Poor prognosis | |
| Breast Cancer | [14] | Nodal involvement |
| [25] | Hormone receptor positive and HER2-positive status | |
| [26,27] | ER-positive, PR-positive, low Ki67 labeling index and negatively correlated with triple-negative breast cancer subtype | |
| [28,29] | Poor survival rates | |
| [30] | Mutations in exon 9 are associated with poor prognosis but mutations in exon 20 are associated with better prognosis | |
| [31] | Reduced disease-free survival | |
| [32] | Risk factors for progression-free survival | |
| [33] | Poor survival | |
| [34,35,36,37] | Better survival | |
| [30] | Exon 9 mutations are independently associated with early recurrence and death, whereas exon 20 PIK3CA mutations are associated with optimal prognosis | |
| [38,39,40,41] | Resistant to antibody-based therapeutic therapy and chemotherapy |
| Clinical Trial/Author | Year | Tumor | Phase | Target | Protocol | Primary End-Point |
|---|---|---|---|---|---|---|
| Andre (BOLERO-3) | 2014 | Breast | III | mTORC1 | Everolimus + Vinorelbine + trastuzumab vs. placebo + Vinorelbine + trastuzumab | PFS |
| Bachelot (GINECO) | 2012 | Breast | II | mTORC1 | Everolimus + tamoxifen vs. tamoxifen | CBR |
| Baselga (BOLERO-2) ** | 2012 | Breast | III | mTORC1 | Everolimus + exemestane vs. placebo+ exemestane | PFS |
| Baselga (BELLE-2) | 2017 | Breast | III | Pan-PI3K | Buparlisib + fulvestrant vs. placebo+ fulvestrant | PFS |
| Baselga | 2017 | Breast | II | mTORC1 | Ridaforolimus + dalotuzumab + exemestane vs. exemestane | PFS |
| Hurvitz (BOLERO-1) | 2015 | Breast | III | mTORC1 | Everolimus+ Trastuzumab+ Paclitaxel vs. placebo+ Trastuzumab + Paclitaxel | PFS |
| Kim (LOTUS) | 2017 | Breast | II | AKT | Ipatasertib+ paclitaxel vs. placebo+ paclitaxel | PFS |
| Krop (FERGI) | 2016 | Breast | II | Pan-PI3K | Pictilisib+ fulvestrant vs. placebo+ fulvestrant | PFS |
| Martin (BELLE-4) | 2017 | Breast | III | Pan-PI3K | Buparlisib + paclitaxel vs. placebo + paclitaxel | PFS |
| Vuylsteke (PEGGY) | 2016 | Breast | II | Pan-PI3K | Pictilisib+ paclitaxel vs. placebo+ paclitaxel | PFS |
| Wolff (HORIZON) | 2013 | Breast | III | mTORC1 | Temsirolimus + letrozole vs. placebo + letrozole | PFS |
| Yardley | 2015 | Breast | II | mTORC1 | Everolimus +Paclitaxel+ Bevacizumab vs. Placebo + Paclitaxel+ Bevacizumab | PFS |
| Armstrong (ASPEN) | 2016 | RCC | II | mTORC1 | Everolimus vs. sunitinib | PFS |
| Choueiri (METEOR) | 2016 | RCC | III | mTORC1 | Everolimus vs. cabozantinib | PFS |
| Cirkel (ROPETAR) | 2016 | RCC | II | mTORC1 | Everolimus + pazopanib vs. pazopanib | PFS |
| Dutcher#a; b | 2009 | RCC | III | mTORC1 | Temsirolimus vs. interferon (a: cc-RCC; b: non-cc-RCC) | OS |
| Flaherty#a; b; c (ECOG2804) | 2015 | RCC | II | mTORC1 | (a) Bevacizumab + temsirolimus vs. bevacizumab alone (b) Bevacizumab + temsirolimus vs. bevacizumab + sorafenib (c) Sorafenib + temsirolimus vs. bevacizumab + sorafenib | PFS |
| Hudes#a; b ** | 2007 | RCC | III | mTORC1 | (a) Temsirolimus vs. interferon (b) Temsirolimus + interferon vs. interferon | OS |
| Hutson | 2013 | RCC | III | mTORC1 | Temsirolimus vs. sorafenib | PFS |
| Motzer (RECORD-1) ** | 2008 | RCC | III | mTORC1 | Everolimus vs. placebo | PFS |
| Motzer (RECORD-3) | 2014 | RCC | II | mTORC1 | Everolimus vs. sunitinib | PFS |
| Motzer | 2015 | RCC | III | mTORC1 | Everolimus vs. Nivolumab | OS |
| Negrier (TORAVA) | 2011 | RCC | II | mTORC1 | Temsirolimus + bevacizumab vs. INF-α + bevacizumab | PFS |
| Rini (INTORACT) | 2013 | RCC | III | mTORC1 | Temsirolimus+ bevacizumab vs. IFN + bevacizumab | PFS |
| Tannir | 2015 | RCC | II | mTORC1 | Temsirolimus vs. sunitinib | PFS |
| Besse | 2014 | Lung | II | mTORC1 | Everolimus + erlotinib vs. erlotinib | DCR |
| Levy | 2014 | Lung | II | Pan-PI3K | PX-866+ docetaxel vs. docetaxel | PFS |
| Papadimitrakopoulou (BATTLE-2) | 2016 | Lung | II | AKT | MK-2206+erlotinib vs. erlotinib | DCR |
| Socinski (TAX 326) | 2010 | Lung | II | AKT | Enzastaurin+ carboplatin vs. carboplatin | TTP |
| Zhu (EVOLVE-1) | 2014 | Lung | III | mTORC1 | Everolimus vs. placebo | OS |
| Bendell | 2011 | CRC | II | PI3K/Akt/mTOR pathway | Perifosine + capecitabine vs. placebo + capecitabine | TTP |
| Bowles | 2016 | CRC | II | Pan-PI3K | PX-866 + cetuximab vs. placebo + cetuximab | PFS |
| Ohtsu (GRANITE-1) | 2013 | Gastric cancer | III | mTORC1 | Everolimus vs. placebo | OS |
| Jimeno | 2015 | HNSCC | II | Pan-PI3K | PX-866 + cetuximab vs. cetuximab | PFS |
| Jimeno | 2016 | HNSCC | II | Pan-PI3K | PX-866 + docetaxel vs. docetaxel | PFS |
| Soulieres (BERIL-1) | 2017 | HNSCC | II | Pan-PI3K | Buparlisib + paclitaxel vs. placebo + paclitaxel | PFS |
| Rachards | 2011 | Pancreatic | II | AKT | Enzastaurin + gemcitabine vs gemcitabine | OS |
| Pavel (RADIANT-2) | 2011 | NET | III | mTORC1 | Everolimus + octreotide LAR vs placebo+ octreotide LAR | PFS |
| Yao (RADIANT-3) ** | 2011 | NET | III | mTORC1 | Everolimus vs. placebo | PFS |
| Yao (RADIANT-4) ** | 2016 | NET | III | mTORC1 | Everolimus vs. placebo | PFS |
| Eroglu | 2015 | Sarcoma | II | mTORC1 | Temsirolimus + selumetinib vs. selumetinib | PFS |
| Demetri | 2013 | Sarcoma | III | mTORC1 | Redaforolimus vs. placebo | PFS |
| Oza | 2015 | Endometrial cancer | II | mTORC1 | Ridaforolimusvs progestin or chemotherapy | PFS |
| Wick (EORTC 26082) | 2016 | Glioblastoma | II | mTORC1 | Temsirolimus vs. temozolomide | OS |
| Margolin (S0438) | 2012 | Melanoma | II | mTORC1 | Temsirolimus+ sorafenib vs. tipifarnib+ sorafenib | PFS |
| Biomarker | Drug | Target | Population | Study Phase | Clinicaltrials.gov Registration |
|---|---|---|---|---|---|
| PIK3CA mutation or amplification | Sirolimus | mTROC1 | Advanced-stage solid cancers | II | NCT02449564 |
| PIK3CA mutation or amplification or PTEN loss | Copanlisib | Pan-PI3K | Advanced HNSCC | I/II | NCT02822482 |
| PIK3CA mutation | Alpelisib + fulvestrant | PI3K-α | Advanced-stage HR+/HER2− breast cancer | III | NCT02437318 |
| Alpelisib + fulvestrant ± letrozole | PI3K-α | Advanced-stage HR+/HER2− breast cancer | II | NCT03056755 | |
| Taselisib | PI3K-α | Advanced-stage SCC of the lung | II | NCT02154490 | |
| PIK3CA and/or BRAF mutations | ASN003 | PI3K-α AND BRAF | Advanced-stage solid cancers | I | NCT02961283 |
| PIK3CA, AKT, or PTEN mutations | MK-2206 | AKT | Advanced-stage lung and thymus cancers | II | NCT01306045 |
| Ipatasertib + paclitazel | AKT | Advanced-stage breast cancer | III | NCT03337724 | |
| PIK3CA mutation or amplification | AZD5363 + paclitaxel | AKT | Advanced-stage gastric cancer | II | NCT0251956 |
| PIK3CA or AKT mutations | Miransertib + carboplatin | AKT | Selected advanced-stage solid cancers | I | NCT02476955 |
| Drug | Target(s) | Trial | Population (n) | Results | Toxicities (Most Common) | Ref. |
|---|---|---|---|---|---|---|
| Temsirolimus | mTORC1 | Global ARCC (vs. INF-α vs. combination) | Untreated, mRCC (n = 626) | ↑ OS (10.9 vs. 7.3 months; p = 0.008) ↑ PFS in the temsirolimus monotherapy (p < 0.001) | Rash, HG, HL; mild | [5] |
| Everolimus | mTORC1 | RECORD-I (vs. placebo) | Previously treated, mRCC (n = 272) | ↑ PFS (4.0 vs. 1.9 months; p < 0.0001) No significant improvement in OS or in ORR | Stomatitis, rash, fatigue, pneumonitis, diarrhea | [6] |
| RADIANT-3 (vs. placebo) | Advanced pancreatic NET (n = 410) | ↑ PFS (mPFS 11.0 vs. 4.6 months; p < 0.001) No clear ORR benefit | Stomatitis, rash, fatigue, pneumonitis, diarrhea | [7] | ||
| RADIANT-4 (vs. placebo) | Other NET (n = 302) | ↑ PFS (11.0 vs. 3.9 months; p < 0.00001) No clear ORR benefit | Stomatitis, rash, fatigue, pneumonitis, diarrhea | [8] | ||
| +AI | BOLERO-2 (vs. placebo + AI) | HR+/HER2− breast cancer (n = 724) | ↑↑ ORR (9.5% vs. 0.5% p < 0.001) ↑ mPFS (6.9 vs. 2.8 months; p < 0.001) | Stomatitis, rash, fatigue, pneumonitis, diarrhea | [9] | |
| Copanlisib | Pan-PI3K | CHRONOS-1 (vs. placebo) | r/r B-NHL, Macroglobulinemia (n = 142) | ORR of 59% (12% CR and 47% PR), with a mPFS of 11.2 months | HG, nausea | [12] |
| Idelalisib + rituximab | PI3K-δ | NCT01539512 (vs. placebo+ rituximab) | Relapse CLL (n = 220) | ORR (81% vs. 13%), ↑ PFS at 24 weeks (93% vs. 46%; p < 0.001), ↑ 1-year OS (92% vs. 80%; p = 0.002). | Diarrhea, rash, immune-mediated hepatitis/ pneumonis | [14] |
| NCT01282424 (vs. placebo) | r/r B-NHL (FL) and SLL (n = 125) | ORR: 54% in FL patients and 58% in SLL (p < 0.001) | Diarrhea, rash, immune-mediated hepatitis, and pneumonitis | [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, A.; Ayesh, H.S.K.; Halawani, H. PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers 2020, 12, 93. https://doi.org/10.3390/cancers12010093
Alqahtani A, Ayesh HSK, Halawani H. PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers. 2020; 12(1):93. https://doi.org/10.3390/cancers12010093
Chicago/Turabian StyleAlqahtani, Ali, Hazem S. K. Ayesh, and Hafez Halawani. 2020. "PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis" Cancers 12, no. 1: 93. https://doi.org/10.3390/cancers12010093
APA StyleAlqahtani, A., Ayesh, H. S. K., & Halawani, H. (2020). PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers, 12(1), 93. https://doi.org/10.3390/cancers12010093




