Human Tumor–Derived Matrix Improves the Predictability of Head and Neck Cancer Drug Testing
Abstract
1. Introduction
2. Results
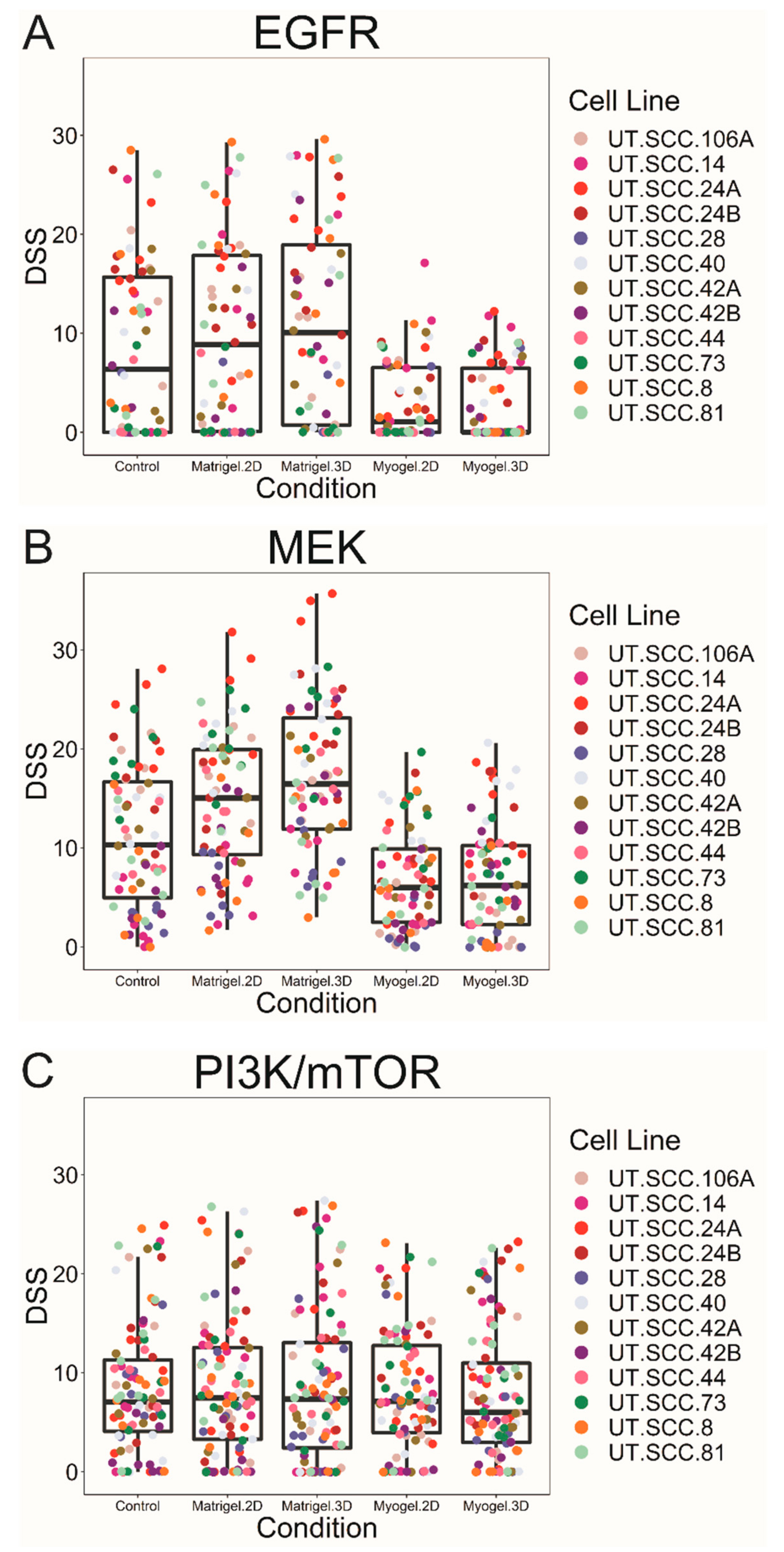
2.1. In Vitro Drug Screen
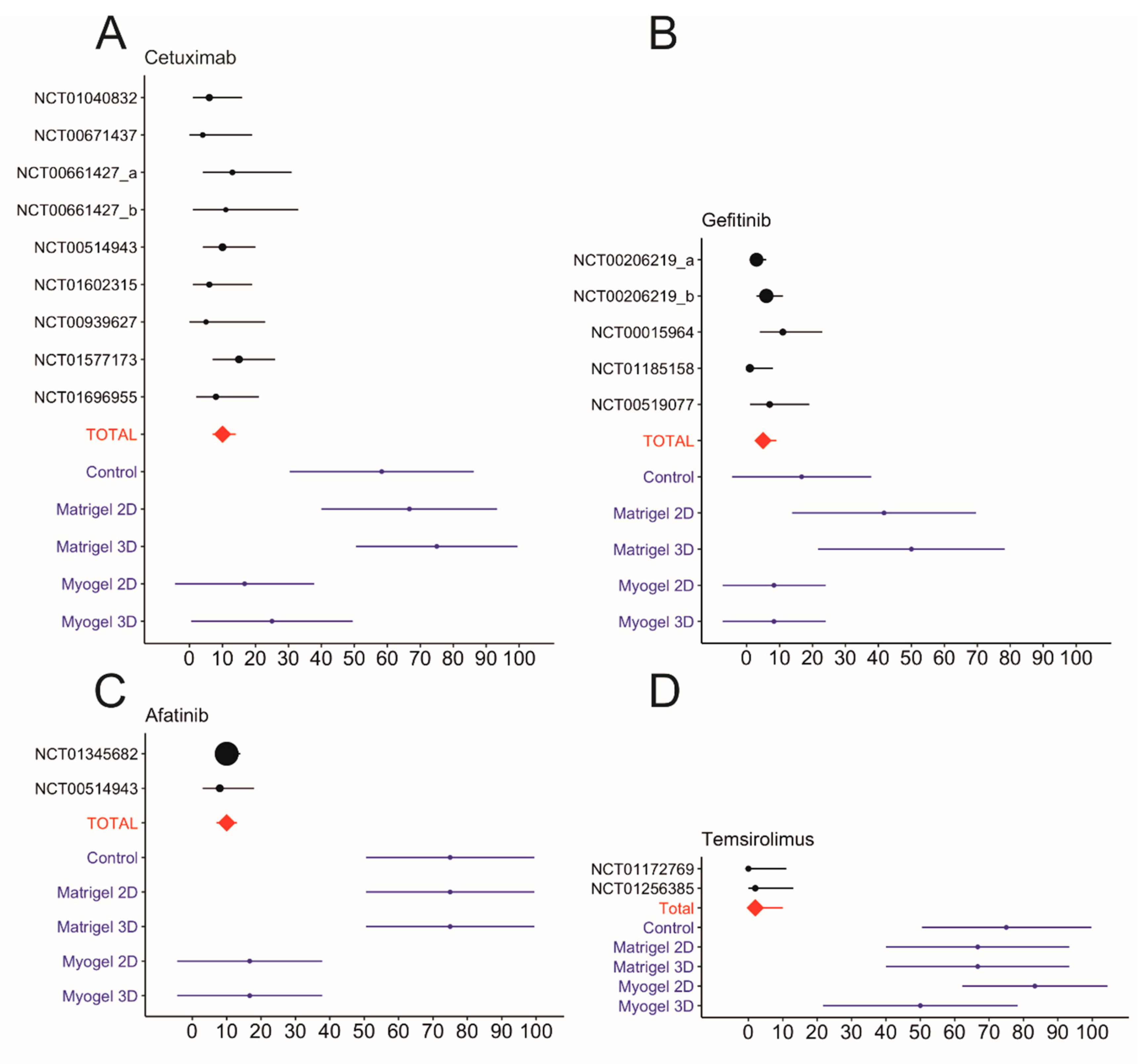
2.2. Clinical Trial Data Collection
2.3. Comparison of In Vitro Drug Testing and Clinical Trial Responses
| EGFR | Myogel 2D vs. Myogel 3D | Myogel 2D vs. control | Myogel 3D vs. control | Myogel 3D vs. Matrigel 2D | Myogel 3D vs. Matrigel 3D | Myogel 2D vs. Matrigel 3D | Myogel 2D vs. Matrigel 2D | Control vs. Matrigel 3D | Control vs. Matrigel 2D | Matrigel 2D vs. Matrigel 3D |
|---|---|---|---|---|---|---|---|---|---|---|
| Erbitux | 1.000 | 0.118 | 0.081 | 0.081 | 0.003 | 0.005 | 0.118 | 1.000 | 1.000 | 1.000 |
| Gefitinib | 0.098 | 1.000 | 0.814 | 0.055 | 0.012 | 0.098 | 0.332 | 1.000 | 1.000 | 1.000 |
| Erlotinib | 1.000 | 1.000 | 1.000 | 0.454 | 0.142 | 0.142 | 0.454 | 1.000 | 1.000 | 1.000 |
| Afatinib | 1.000 | 0.118 | 0.118 | 0.008 | 0.001 | 0.001 | 0.008 | 1.000 | 1.000 | 1.000 |
| Ganertinib | 1.000 | 0.118 | 0.019 | 0.002 | 0.003 | 0.024 | 0.019 | 1.000 | 1.000 | 1.000 |
| No. of sig. cases | 0 | 0 | 1 | 2 | 4 | 3 | 2 | 0 | 0 | 0 |
| % | 0.0 | 0.0 | 20.0 | 40.0 | 80.0 | 60.0 | 40.0 | 0.0 | 0.0 | 0.0 |
| MEK | ||||||||||
| Pimasertib | 1.000 | 1.000 | 1.000 | 0.012 | 0.000 | 0.000 | 0.005 | 0.008 | 0.282 | 1.000 |
| Trametinib | 1.000 | 0.707 | 1.000 | 0.030 | 0.000 | 0.000 | 0.003 | 0.019 | 0.707 | 1.000 |
| Refametinib | 1.000 | 1.000 | 1.000 | 0.012 | 0.000 | 0.000 | 0.008 | 0.019 | 0.707 | 1.000 |
| Binimetinib | 1.000 | 1.000 | 1.000 | 0.004 | 0.000 | 0.000 | 0.004 | 0.005 | 0.389 | 1.000 |
| TAK-733 | 1.000 | 0.707 | 1.000 | 0.012 | 0.000 | 0.000 | 0.003 | 0.019 | 0.528 | 1.000 |
| Selumetinib | 1.000 | 1.000 | 0.707 | 0.004 | 0.000 | 0.002 | 0.019 | 0.169 | 0.814 | 1.000 |
| No. of sig. cases | 0 | 0 | 0 | 6 | 6 | 6 | 6 | 5 | 0 | 0 |
| % | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | 100.0 | 100.0 | 83.3 | 0.0 | 0.0 |
| mTOR/PI3K | ||||||||||
| Everolimus | 1.000 | 1.000 | 1.000 | 0.142 | 0.010 | 0.008 | 0.118 | 0.612 | 1.000 | 1.000 |
| Temsirolimus | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sirolimus | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Ridaforolimus | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Dactolisib | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Apitolisib | 1.000 | 0.707 | 1.000 | 0.528 | 0.067 | 0.008 | 0.098 | 1.000 | 1.000 | 1.000 |
| Omipalisib | 1.000 | 0.814 | 1.000 | 0.201 | 0.004 | 0.000 | 0.012 | 0.067 | 1.000 | 1.000 |
| PF-04691502 | 1.000 | 1.000 | 1.000 | 0.332 | 0.008 | 0.003 | 0.169 | 0.332 | 1.000 | 1.000 |
| No. of sig. cases | 0 | 0 | 0 | 0 | 3 | 4 | 1 | 0 | 0 | 0 |
| % | 0.0 | 0.0 | 0.0 | 0.0 | 37.5 | 50.0 | 12.5 | 0.0 | 0.0 | 0.0 |
| Clinical Trial Number | Total Enrollment | Phase | Completion Year | Monotherapy Treated Patients | Responded Patients | Evaluation Criteria * | ORR% * | Notes | |
|---|---|---|---|---|---|---|---|---|---|
| Afatinib | NCT01345682 | 483 | 3 | 2016 | 322 | 33 | RECIST 1.1 | 10.2 | |
| NCT00514943 | 124 | 2 | 2013 | 62 | 5 | RECIST 1.0 | 8.1 | ORR is based on independent central review (ICR) | |
| NCT01415674 | 61 | 2 | 2006 | 41 | 3 | RECIST1.1 | 7.3 | Neoadjuvant treatment | |
| Gefitinib | NCT00206219a [16] | 486 | 3 | 2007 | 158 | 4 | RECIST | 2.7 | Drug dose 250 mg/day |
| NCT00206219b [16] | 166 | 10 | RECIST | 7.6 | Drug dose 500 mg/day | ||||
| NCT00015964 [17] | 51 | 2 | 2005 | 47 | 5 | N/A | 10.6 | ||
| NCT01185158 [18] | 70 | 2 | 2004 | 70 | 1 | RECIST | 1.4 | ||
| NCT00519077 | 44 | 2 | 2013 | 44 | 3 | RECIST | 6.81 | ||
| Cetuximab | NCT01040832 | 107 | 2 | 2012 | 53 | 3 | RECIST 1.0 | 5.7 | |
| NCT00671437 | 42 | 2 | 2015 | 27 | 1 | RECIST 1.0 | 3.7 | ORR is based on CT scans | |
| NCT00661427a | 61 | 2 | 2012 | 30 | 4 | RECIST | 13.3 | Drug dose 500 mg/m2 | |
| NCT00661427b | 19 | 2 | RECIST | 10.5 | Drug dose 750 mg/m2 | ||||
| NCT00514943 | 124 | 2 | 2013 | 62 | 6 | RECIST 1.0 | 9.7 | ORR is based on independent central review (ICR) | |
| NCT01602315 | 27 | 2 | 2016 | 35 | 2 | RECIST 1.1 | 5.7 | ||
| NCT00939627 | 55 | 2 | 2014 | 22 | 1 | RECIST 1.1 | 4.5 | ||
| NCT01577173 | 122 | 2 | 2015 | 62 | 9 | RECIST 1.1 | 14.5 | ||
| NCT01696955 | 79 | 2 | 2017 | 38 | 3 | RECIST 1.0 | 7.9 | ||
| Temsirolimus | NCT01172769 [15] | 42 | 2 | 2012 | 33 | 0 | RECIST | 0 | |
| NCT01256385 | 86 | 2 | 2013 | 40 | 1 | RECIST 1.0 | 2.5 | ||
| Sirolimus | NCT01195922 [19] | 37 | 1 & 2 | 2015 | 16 | 4 | RECIST 1.1 | 25.0 | Neoadjuvant treatment |
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Anticancer Compounds
4.2. 3D Matrices and Culturing Conditions
4.3. Drug Sensitivity and Resistance Testing
4.4. Cell Viability Assay
4.5. Data Analysis
4.6. Clinical Trial Data Collection
4.7. Meta-Analysis of Clinical Data
4.8. Immunoblot Analysis of EGFR, ERK1/2 and pERK1/2 Expressions in Growing Cells on Plastic, Matrigel and Myogel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hutchinson, L.; Kirk, R. High drug attrition rates—Where are we going wrong? Nat. Rev. Clin. Oncol. 2011, 8, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Salo, T.; Dourado, M.R.; Sundquist, E.; Apu, E.H.; Alahuhta, I.; Tuomainen, K.; Vasara, J.; Al-Samadi, A. Organotypic three-dimensional assays based on human leiomyoma-derived matrices. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Salo, T.; Sutinen, M.; Hoque Apu, E.; Sundquist, E.; Cervigne, N.K.; de Oliveira, C.E.; Akram, S.U.; Ohlmeier, S.; Suomi, F.; Eklund, L.; et al. A novel human leiomyoma tissue derived matrix for cell culture studies. BMC Cancer 2015, 15, 981. [Google Scholar] [CrossRef] [PubMed]
- Naakka, E.; Tuomainen, K.; Wistrand, H.; Palkama, M.; Suleymanova, I.; Al-Samadi, A.; Salo, T. Fully Human Tumor-based Matrix in Three-dimensional Spheroid Invasion Assay. J. Vis. Exp. 2019, 147, e59567. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W. Role of Epidermal Growth Factor Receptor Pathway–Targeted Therapy in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2006, 24, 2659–2665. [Google Scholar] [CrossRef]
- Young, N.; Soneru, C.; Liu, J.; Grushko, T.; Hardeman, A.; Olopade, O.; Baum, A.; Solca, F.; Cohen, E. Afatinib efficacy against squamous cell carcinoma of the head and neck cell lines in vitro and in vivo. Target. Oncol. 2015, 10, 501–508. [Google Scholar] [CrossRef]
- Huang, S.; Bock, J.M.; Harari, P.M. Epidermal Growth Factor Receptor Blockade with C225 Modulates Proliferation, Apoptosis, and Radiosensitivity in Squamous Cell Carcinomas of the Head and Neck. Cancer Res. 1999, 59, 1935–1940. [Google Scholar]
- Schütze, C.; Dörfler, A.; Eicheler, W.; Zips, D.; Hering, S.; Solca, F.; Baumann, M.; Krause, M. Combination of EGFR/HER2 tyrosine kinase inhibition by BIBW 2992 and BIBW 2669 with irradiation in FaDu human squamous cell carcinoma. Strahlenther. Onkol. 2007, 183, 256–264. [Google Scholar] [CrossRef]
- Wakeling, A.E.; Guy, S.P.; Woodburn, J.R.; Ashton, S.E.; Curry, B.J.; Barker, A.J.; Gibson, K.H. ZD1839 (Iressa). Cancer Res. 2002, 62, 5749–5754. [Google Scholar]
- Magné, N.; Fischel, J.L.; Dubreuil, A.; Formento, P.; Poupon, M.F.; Laurent-Puig, P.; Milano, G. Influence of epidermal growth factor receptor (EGFR), p53 and intrinsic MAP kinase pathway status of tumour cells on the antiproliferative effect of ZD1839 (‘Iressa’). Br. J. Cancer 2002, 86, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Resteghini, C.; Paielli, N.; Licitra, L.; Pilotti, S.; Perrone, F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 74362–74379. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Pemovska, T.; Szwajda, A.; Kulesskiy, E.; Kontro, M.; Karjalainen, R.; Majumder, M.M.; Malani, D.; Murumagi, A.; Knowles, J.; et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Sci. Rep. 2014, 4, 5193. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Grünwald, V.; Keilholz, U.; Boehm, A.; Guntinas-Lichius, O.; Hennemann, B.; Schmoll, H.J.; Ivanyi, P.; Abbas, M.; Lehmann, U.; Koch, A.; et al. TEMHEAD: A single-arm multicentre phase II study of temsirolimus in platin- and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO). Ann. Oncol. 2015, 26, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.S.; Cohen, E.E.; Licitra, L.; Van Herpen, C.M.; Khorprasert, C.; Soulieres, D.; Vodvarka, P.; Rischin, D.; Garin, A.M.; Hirsch, F.R.; et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J. Clin. Oncol. 2009, 27, 1864–1871. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Rosen, F.; Stadler, W.M.; Recant, W.; Stenson, K.; Huo, D.; Vokes, E.E. Phase II Trial of ZD1839 in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2003, 21, 1980–1987. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Kane, M.A.; List, M.A.; Brockstein, B.E.; Mehrotra, B.; Huo, D.; Mauer, A.M.; Pierce, C.; Dekker, A.; Vokes, E.E. Phase II Trial of Gefitinib 250 mg Daily in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2005, 11, 8418–8424. [Google Scholar] [CrossRef]
- Day, T.A.; Shirai, K.; O’Brien, P.E.; Matheus, M.G.; Godwin, K.; Sood, A.J.; Kompelli, A.; Vick, J.A.; Martin, D.; Vitale-Cross, L.; et al. Inhibition of mTOR Signaling and Clinical Activity of Rapamycin in Head and Neck Cancer in a Window of Opportunity Trial. Clin. Cancer Res. 2019, 25, 1156–1164. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Grandis, J.R. Emerging drugs for head and neck cancer. Expert Opin. Emerg. Drugs 2015, 20, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Lepikhova, T.; Karhemo, P.; Louhimo, R.; Yadav, B.; Murumägi, A.; Kulesskiy, E.; Kivento, M.; Sihto, H.; Grénman, R.; Syrjänen, S.M.; et al. Drug-Sensitivity Screening and Genomic Characterization of 45 HPV-Negative Head and Neck Carcinoma Cell Lines for Novel Biomarkers of Drug Efficacy. Mol. Cancer Ther. 2018, 17, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef]
- Khaznadar, S.S.; Khan, M.; Schmid, E.; Gebhart, S.; Becker, E.; Krahn, T.; von Ahsen, O. EGFR overexpression is not common in patients with head and neck cancer. Cell lines are not representative for the clinical situation in this indication. Oncotarget 2018, 9, 28965–28975. [Google Scholar] [CrossRef]
- Lui, V.W.Y.; Hedberg, M.L.; Li, H.; Vangara, B.S.; Pendleton, K.; Zeng, Y.; Lu, Y.; Zhang, Q.; Du, Y.; Gilbert, B.R.; et al. Frequent Mutation of the PI3K Pathway in Head and Neck Cancer Defines Predictive Biomarkers. Cancer Discov. 2013, 3, 761–769. [Google Scholar] [CrossRef]
- Wang, Z.; Valera, J.C.; Zhao, X.; Chen, Q.; Silvio Gutkind, J. mTOR co-targeting strategies for head and neck cancer therapy. Cancer Metastasis Rev. 2017, 36, 491–502. [Google Scholar] [CrossRef]
- Pemovska, T.; Kontro, M.; Yadav, B.; Edgren, H.; Eldfors, S.; Szwajda, A.; Almusa, H.; Bespalov, M.M.; Ellonen, P.; Elonen, E.; et al. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discov. 2013, 3, 1416–1429. [Google Scholar] [CrossRef]
- Grenman, R.; Pekkola-Heino, K.; Joensuu, H.; Aitasalo, K.; Klemi, P.; Lakkala, T. UT-MUC-1, a new mucoepidermoid carcinoma cell line, and its radiosensitivity. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 542–547. [Google Scholar] [CrossRef]
- Troiani, T.; Martinelli, E.; Napolitano, S.; Vitagliano, D.; Ciuffreda, L.P.; Costantino, S.; Morgillo, F.; Capasso, A.; Sforza, V.; Nappi, A.; et al. Increased TGF-α as a Mechanism of Acquired Resistance to the Anti-EGFR Inhibitor Cetuximab through EGFR–MET Interaction and Activation of MET Signaling in Colon Cancer Cells. Clin. Cancer Res. 2013, 19, 6751–6765. [Google Scholar] [CrossRef]
- Iida, M.; Brand, T.M.; Starr, M.M.; Huppert, E.J.; Luthar, N.; Bahrar, H.; Coan, J.P.; Pearson, H.E.; Salgia, R.; Wheeler, D.L. Overcoming acquired resistance to cetuximab by dual targeting HER family receptors with antibody-based therapy. Mol. Cancer 2014, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Narvi, E.; Vaparanta, K.; Karrila, A.; Chakroborty, D.; Knuutila, S.; Pulliainen, A.; Sundvall, M.; Elenius, K. Different responses of colorectal cancer cells to alternative sequences of cetuximab and oxaliplatin. Sci. Rep. 2018, 8, 16579. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T.; Okuyama, H.; Endo, H.; Kawada, K.; Ashida, Y.; Ohue, M.; Sakai, Y.; Inoue, M. In vivo and ex vivo cetuximab sensitivity assay using three-dimensional primary culture system to stratify KRAS mutant colorectal cancer. PLoS ONE 2017, 12, e0174151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chung, T.D.Y.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Carey, T.E.; Kimmel, K.A.; Schwartz, D.R.; Richter, D.E.; Baker, S.R.; Krause, C.J. Antibodies to human squamous cell carcinoma. Otolaryngol. Head Neck Surg. 1983, 91, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuomainen, K.; Al-Samadi, A.; Potdar, S.; Turunen, L.; Turunen, M.; Karhemo, P.-R.; Bergman, P.; Risteli, M.; Åström, P.; Tiikkaja, R.; et al. Human Tumor–Derived Matrix Improves the Predictability of Head and Neck Cancer Drug Testing. Cancers 2020, 12, 92. https://doi.org/10.3390/cancers12010092
Tuomainen K, Al-Samadi A, Potdar S, Turunen L, Turunen M, Karhemo P-R, Bergman P, Risteli M, Åström P, Tiikkaja R, et al. Human Tumor–Derived Matrix Improves the Predictability of Head and Neck Cancer Drug Testing. Cancers. 2020; 12(1):92. https://doi.org/10.3390/cancers12010092
Chicago/Turabian StyleTuomainen, Katja, Ahmed Al-Samadi, Swapnil Potdar, Laura Turunen, Minna Turunen, Piia-Riitta Karhemo, Paula Bergman, Maija Risteli, Pirjo Åström, Riia Tiikkaja, and et al. 2020. "Human Tumor–Derived Matrix Improves the Predictability of Head and Neck Cancer Drug Testing" Cancers 12, no. 1: 92. https://doi.org/10.3390/cancers12010092
APA StyleTuomainen, K., Al-Samadi, A., Potdar, S., Turunen, L., Turunen, M., Karhemo, P.-R., Bergman, P., Risteli, M., Åström, P., Tiikkaja, R., Grenman, R., Wennerberg, K., Monni, O., & Salo, T. (2020). Human Tumor–Derived Matrix Improves the Predictability of Head and Neck Cancer Drug Testing. Cancers, 12(1), 92. https://doi.org/10.3390/cancers12010092





