Chronic Non-Malignant Pain in Patients with Cancer Seen at a Timely Outpatient Palliative Care Clinic
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
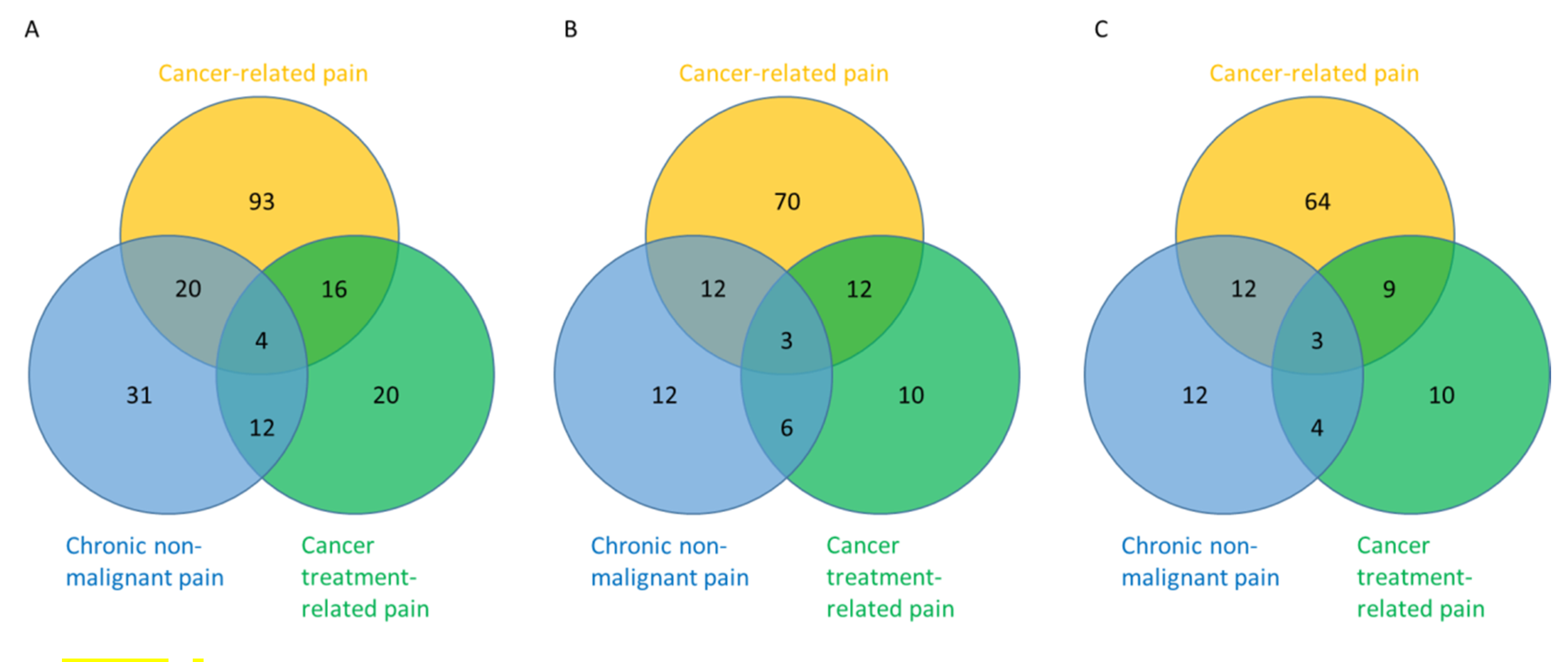
2.2. Prevalence of Chronic Non-Malignant Pain
2.3. Pain Diagnoses, Characteristics, and Treatments
2.4. Pain Management
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Data Collection
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Solano, J.P.; Gomes, B.; Higginson, I.J. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J. Pain Symptom Manag. 2006, 31, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, S.C.; Wesker, W.; Kruitwagen, C.; de Haes, H.C.; Voest, E.E.; de Graeff, A. Symptom prevalence in patients with incurable cancer: A systematic review. J. Pain Symptom Manag. 2007, 34, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W. Cancer pain and its impact on diagnosis, survival and quality of life. Nat. Rev. Neurosci. 2006, 7, 797–809. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. A personalized approach to assessing and managing pain in patients with cancer. J. Clin. Oncol. 2014, 32, 1640–1646. [Google Scholar] [CrossRef]
- Hui, D.; Hannon, B.L.; Zimmermann, C.; Bruera, E. Improving patient and caregiver outcomes in oncology: Team-based, timely, and targeted palliative care. CA Cancer J. Clin. 2018, 68, 356–376. [Google Scholar] [CrossRef]
- Hui, D.; Anderson, L.; Tang, M.; Park, M.; Liu, D.; Bruera, E. Examination of referral criteria for outpatient palliative care among patients with advanced cancer. Support. Care Cancer 2020, 28, 295–301. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA 2016. [Google Scholar] [CrossRef]
- Nuckols, T.K.; Anderson, L.; Popescu, I.; Diamant, A.L.; Doyle, B.; Di Capua, P.; Chou, R. Opioid prescribing: A systematic review and critical appraisal of guidelines for chronic pain. Ann. Intern. Med. 2014, 160, 38–47. [Google Scholar] [CrossRef]
- Manchikanti, L.; Abdi, S.; Atluri, S.; Balog, C.C.; Benyamin, R.M.; Boswell, M.V.; Brown, K.R.; Bruel, B.M.; Bryce, D.A.; Burks, P.A.; et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I—Evidence assessment. Pain Phys. 2012, 15, S1–S65. [Google Scholar]
- Manchikanti, L.; Abdi, S.; Atluri, S.; Balog, C.C.; Benyamin, R.M.; Boswell, M.V.; Brown, K.R.; Bruel, B.M.; Bryce, D.A.; Burks, P.A.; et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2—Guidance. Pain Phys. 2012, 15, S67–S116. [Google Scholar]
- Swarm, R.A.; Abernethy, A.P.; Anghelescu, D.L.; Benedetti, C.; Buga, S.; Cleeland, C.; Deleon-Casasola, O.A.; Eilers, J.G.; Ferrell, B.; Green, M.; et al. Adult cancer pain. J. Natl. Compr. Canc. Netw. 2013, 11, 992–1022. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A.; Portenoy, R.; Lacchetti, C.; Campbell, T.; Cheville, A.; Citron, M.; Constine, L.S.; Cooper, A.; Glare, P.; Keefe, F.; et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3325–3345. [Google Scholar] [CrossRef] [PubMed]
- Stromgren, A.S.; Groenvold, M.; Petersen, M.A.; Goldschmidt, D.; Pedersen, L.; Spile, M.; Irming-Pedersen, G.; Sjogren, P. Pain characteristics and treatment outcome for advanced cancer patients during the first week of specialized palliative care. J. Pain Symptom Manag. 2004, 27, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Massaccesi, M.; Deodato, F.; Caravatta, L.; Macchia, G.; Padula, G.D.; Di Rito, S.; Woldemariam, A.A.; Rossi, M.; Di Falco, C.; Tambaro, R.; et al. Incidence and management of noncancer pain in cancer patients referred to a radiotherapy center. Clin. J. Pain 2013, 29, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.; Molloy, S.; Earle, C.C. Frequency of non-cancer-related pain in patients with cancer. J. Clin. Oncol. 2013, 31, 2837. [Google Scholar] [CrossRef]
- Childers, J.W.; King, L.A.; Arnold, R.M. Chronic Pain and Risk Factors for Opioid Misuse in a Palliative Care Clinic. Am. J. Hosp. Palliat. Care 2015, 32, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.R.; Rodin, G.; Zimmermann, C.; Warr, D.; Moore, M.; Shepherd, F.; Gagliese, L. Acceptance of pain: A study in patients with advanced cancer. Pain 2009, 143, 147–154. [Google Scholar] [CrossRef]
- Grond, S.; Zech, D.; Diefenbach, C.; Radbruch, L.; Lehmann, K.A. Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain 1996, 64, 107–114. [Google Scholar] [CrossRef]
- Bruera, E.; Hui, D. Integrating supportive and palliative care in the trajectory of cancer: Establishing goals and models of care. J. Clin. Oncol. 2010, 28, 4013–4017. [Google Scholar] [CrossRef]
- Dalal, S.; Bruera, S.; Hui, D.; Yennu, S.; Dev, R.; Williams, J.; Masoni, C.; Ihenacho, I.; Obasi, E.; Bruera, E. Use of Palliative Care Services in a Tertiary Cancer Center. Oncologist 2016, 21, 110–118. [Google Scholar] [CrossRef]
- Gaertner, J.; Boehlke, C.; Simone, C.B., 2nd; Hui, D. Early palliative care and the opioid crisis: Ten pragmatic steps towards a more rational use of opioids. Ann. Palliat. Med. 2019. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- Bennett, M.I.; Eisenberg, E.; Ahmedzai, S.H.; Bhaskar, A.; O’Brien, T.; Mercadante, S.; Krcevski Skvarc, N.; Vissers, K.; Wirz, S.; Wells, C.; et al. Standards for the management of cancer-related pain across Europe-A position paper from the EFIC Task Force on Cancer Pain. Eur. J. Pain 2019, 23, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Craigie, S.; Juurlink, D.N.; Buckley, D.N.; Wang, L.; Couban, R.J.; Agoritsas, T.; Akl, E.A.; Carrasco-Labra, A.; Cooper, L.; et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017, 189, E659–E666. [Google Scholar] [CrossRef]
- Inoue, S.; Kobayashi, F.; Nishihara, M.; Arai, Y.C.; Ikemoto, T.; Kawai, T.; Inoue, M.; Hasegawa, T.; Ushida, T. Chronic Pain in the Japanese Community—Prevalence, Characteristics and Impact on Quality of Life. PLoS ONE 2015, 10, e0129262. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Rounds, L.A. Conjoint screening questionnaires for alcohol and other drug abuse: Criterion validity in a primary care practice. Wis. Med. J. 1995, 94, 135–140. [Google Scholar] [PubMed]
- Akbik, H.; Butler, S.F.; Budman, S.H.; Fernandez, K.; Katz, N.P.; Jamison, R.N. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP). J. Pain Symptom Manag. 2006, 32, 287–293. [Google Scholar] [CrossRef]
- Butler, S.F.; Budman, S.H.; Fernandez, K.; Jamison, R.N. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain 2004, 112, 65–75. [Google Scholar] [CrossRef]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present and Future Developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Yurkovich, M.; Avina-Zubieta, J.A.; Thomas, J.; Gorenchtein, M.; Lacaille, D. A systematic review identifies valid comorbidity indices derived from administrative health data. J. Clin. Epidemiol. 2015, 68, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singap. 1994, 23, 129–138. [Google Scholar] [PubMed]
- Caraceni, A.; Portenoy, R.K. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain 1999, 82, 263–274. [Google Scholar] [CrossRef]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Degenhardt, L.; Charlson, F.; Mathers, B.; Hall, W.D.; Flaxman, A.D.; Johns, N.; Vos, T. The global epidemiology and burden of opioid dependence: Results from the global burden of disease 2010 study. Addiction 2014, 109, 1320–1333. [Google Scholar] [CrossRef]
- Morley, K.I.; Ferris, J.A.; Winstock, A.R.; Lynskey, M.T. Polysubstance use and misuse or abuse of prescription opioid analgesics: A multi-level analysis of international data. Pain 2017, 158, 1138–1144. [Google Scholar] [CrossRef]
| Variables | Without Chronic Non-Malignant Pain (n = 129) | With Chronic Non-Malignant Pain (n = 67) | All Patients (n = 200) * | p-Value |
|---|---|---|---|---|
| Age, mean (range) | 57 (21.0, 84.0) | 66 (25, 92) | 60 (21, 92) | <0.001 |
| Sex | ||||
| Female | 68 (52.7%) | 36 (53.7%) | 106 (53.0%) | 0.89 |
| Male | 61 (47.3%) | 31 (46.3%) | 94 (47.0%) | |
| Race/Ethnicity | ||||
| White/Caucasian | 96 (74.4%) | 54 (80.6%) | 153 (76.5%) | 0.59 |
| Black/African American | 15 (11.6%) | 4 (6.0%) | 20 (10.0%) | |
| Asian | 4 (3.1%) | 1 (1.5%) | 5 (2.5%) | |
| American Indian/Alaskan Native | 1 (0.8%) | 1 (0.5%) | ||
| Hispanic/Latino | 13 (10.1%) | 8 (11.9%) | 21 (10.5%) | |
| Education | ||||
| Some high school or less | 4 (3.1%) | 4 (6.3%) | 8 (4.1%) | 0.48 |
| Completed high school | 23 (18.1%) | 13 (20.3%) | 36 (18.6%) | |
| Some college | 33 (26.0%) | 19 (29.7%) | 52 (26.8%) | |
| University college | 34 (26.8%) | 18 (28.1%) | 54 (27.8%) | |
| Advanced degree | 33 (26.0%) | 10 (15.6%) | 44 (22.7%) | |
| Marital Status | ||||
| Married | 94 (72.9%) | 46 (68.7%) | 141 (70.5%) | 0.048 |
| Single | 14 (10.9%) | 4 (6.0%) | 19 (9.5%) | |
| Divorced | 17 (13.2%) | 8 (11.9%) | 27 (13.5%) | |
| Widowed | 4 (3.1%) | 9 (13.4%) | 13 (6.5%) | |
| Cancer Diagnosis | ||||
| Head and neck | 18 (14.0%) | 5 (7.5%) | 23 (11.5%) | 0.42 |
| Breast | 14 (10.9%) | 10 (14.9%) | 26 (13.0%) | |
| Respiratory | 23 (17.8%) | 11 (16.4%) | 35 (17.5%) | |
| Gastrointestinal | 28 (21.7%) | 15 (22.4%) | 43 (21.5%) | |
| Genitourinary | 9 (7.0%) | 10 (14.9%) | 20 (10.0%) | |
| Gynecological | 11 (8.5%) | 3 (4.5%) | 14 (7.0%) | |
| Hematologic | 8 (6.2%) | 4 (6.0%) | 12 (6.0%) | |
| Other | 18 (14.0%) | 9 (13.4%) | 27 (13.5%) | |
| Cancer Stage | ||||
| Localized | 12 (9.3%) | 10 (14.9%) | 22 (11.0%) | 0.06 |
| Locally advanced | 10 (7.8%) | 7 (10.4%) | 17 (8.5%) | |
| Metastatic | 96 (74.4%) | 39 (58.2%) | 138 (69.0%) | |
| Recurrent | 6 (4.7%) | 2 (3.0%) | 9 (4.5%) | |
| Other | 5 (3.9%) | 9 (13.4%) | 14 (7.0%) | |
| CAGE-AID Score | ||||
| <2 | 114 (89.1%) | 61 (91.0%) | 178 (89.4%) | 0.67 |
| ≥2 | 14 (10.9%) | 6 (9.0%) | 21 (10.6%) | |
| SOAPP Score, mean (SD) | 4.0 (3.6) | 3.5 (3.0) | 3.8 (3.4) | 0.42 |
| Charlson Comorbidity Index, mean (SD) | 6.6 (2.4) | 7.2 (3.1) | 6.8 (2.6) | 0.054 |
| Karnofsky Performance Status, mean (SD) | 73.1 (15.7) | 76.4 (14.1) | 74.2 (15.2) | 0.19 |
| Number of pain types | ||||
| 1 | 73 (56.6%) | 10 (14.9%) | 84 (42.0%) | <0.001 |
| 2 | 41 (31.8%) | 35 (52.2%) | 79 (39.5%) | |
| ≥3 | 15 (11.6%) | 22 (32.8%) | 37 (18.5%) | |
| Morphine equivalent daily dose, mg/day | ||||
| Mean (SD) | 18 (40) | 10 (13) | 16 (35) | 0.001 |
| Median (IQR) | 5.0 (3.0, 13.0) | 5.6 (1.0,17.0) | 5.0 (2.8, 15) | |
| One-year survival % (95% CI) | 71 (61, 79) | 87 (77, 94) | 77 (70, 83) | 0.03 |
| Edmonton Symptom Assessment System, mean (SD) | ||||
| Pain | 5.1 (2.7) | 5 (2.7) | 5.1 (2.7) | 0.74 |
| Fatigue | 4.8 (3) | 5 (2.8) | 4.9 (2.9) | 0.63 |
| Nausea | 2 (2.9) | 1.1 (2.1) | 1.7 (2.7) | 0.01 |
| Depression | 1.7 (2.4) | 1.4 (2.4) | 1.6 (2.4) | 0.06 |
| Anxiety | 2.4 (2.7) | 2.1 (2.9) | 2.3 (2.7) | 0.17 |
| Drowsiness | 3.2 (3) | 2.4 (2.8) | 2.9 (2.9) | 0.06 |
| Dyspnea | 2.2 (2.8) | 1.7 (2.3) | 2 (2.7) | 0.31 |
| Appetite | 4.2 (3) | 3.4 (2.6) | 4 (2.9) | 0.07 |
| Well-being | 4.6 (2.6) | 4.1 (2.2) | 4.4 (2.5) | 0.17 |
| Sleep | 4.4 (2.8) | 4.4 (2.6) | 4.4 (2.7) | 0.96 |
| Financial distress | 2.2 (2.7) | 2 (2.7) | 2.1 (2.7) | 0.51 |
| Spiritual pain | 1 (2) | 0.6 (1.3) | 0.9 (1.8) | 0.37 |
| Variables | Cancer-Related Pain (n = 182) | Cancer Treatment-Related Pain (n = 60) | Non-Malignant Pain (n = 94) | Total (n = 361) * | p-Value ǂ |
|---|---|---|---|---|---|
| Pain rank | |||||
| 1 (most important) | 115 (63.2) | 32 (53.3) | 41 (43.6) | 200 (55.4) | 0.003 |
| 2 | 51 (28.0) | 17 (28.3) | 34 (36.2) | 115 (31.9) | |
| ≥3 | 16 (8.8) | 11 (18.3) | 19 (20.2) | 46 (12.7) | |
| Duration in months median (IQR) | 3.0 (1.5, 8.0) | 3.0 (1.0, 12.0) | 17.5 (3.0, 96.0) | 4.0 (1.5, 12.0) | <0.0001 |
| Brief Pain Inventory | |||||
| Worst pain intensity | 6.1 (2.9) | 5.4 (2.6) | 5.4 (2.7) | 5.8 (2.8) | 0.01 |
| Least pain intensity | 2 (1.9) | 2 (1.9) | 1.8 (1.7) | 1.9 (1.9) | 0.64 |
| Average pain intensity | 4.2 (2.5) | 3.5 (2.3) | 3.7 (2.2) | 3.9 (2.4) | 0.08 |
| Pain intensity now | 3.6 (2.8) | 3 (2.3) | 2.7 (2.4) | 3.2 (2.6) | 0.03 |
| Pain relief % | 5.6 (3.1) | 5.6 (3.2) | 4.9 (3.2) | 5.4 (3.2) | 0.41 |
| Activity interference | 4.7 (3.5) | 3.2 (3.3) | 3.5 (3.1) | 4.1 (3.4) | 0.006 |
| Mood interference | 3.2 (3.2) | 2.5 (3.3) | 1.9 (2.8) | 2.7 (3.1) | 0.006 |
| Walking interference | 2.8 (3.4) | 2.4 (3.4) | 3.1 (3.4) | 2.8 (3.4) | 0.52 |
| Work interference | 4.3 (3.6) | 2.3 (3.2) | 3.5 (3.4) | 3.7 (3.5) | 0.002 |
| Relations interference | 1.8 (2.7) | 1.3 (2.5) | 0.9 (2.3) | 1.4 (2.6) | 0.03 |
| Sleep interference | 4.2 (3.7) | 3.5 (3.4) | 2.8 (3.4) | 3.7 (3.6) | 0.02 |
| Enjoyment interference | 3.5 (3.3) | 2.4 (2.9) | 2 (3) | 2.9 (3.2) | 0.003 |
| BPI pain intensity score | 4.0 (2.2) | 3.5 (2.0) | 3.4 (1.9) | 3.7 (2.1) | 0.08 |
| BPI pain interference score | 3.5 (2.5) | 2.5 (2.4) | 2.5 (2.3) | 3.0 (2.5) | 0.004 |
| Personalized pain goal mean (SD) | 2.2 (1.1) | 2.1 (1) | 2.1 (1.3) | 2.2 (1.1) | 0.64 |
| Level of supporting evidence | |||||
| Definite | 180 (100.0) | 57 (95.0) | 77 (81.9) | 314 (94.0) | NA |
| Possible | 0 (0) | 1 (1.7) | 7 (7.4) | 8 (2.4) | |
| Probable | 0 (0) | 2 (3.3) | 10 (10.6) | 12 (3.6) |
| Variables | Cancer-Related Pain (n = 182) | Cancer Treatment-Related Pain (n = 60) | Non-Malignant Pain (n = 94) | Total (n = 361) * | p-Value ǂ |
|---|---|---|---|---|---|
| Treatment at baseline prior to palliative care consultation | |||||
| Acetaminophen | 56 (30.8) | 10 (16.7) | 34 (36.2) | 108 (29.9) | 0.045 |
| NSAIDs | 31 (17.0) | 10 (16.7) | 27 (28.7) | 72 (19.9) | 0.26 |
| Opioids | 120 (65.9) | 28 (46.7) | 30 (31.9) | 188 (52.1) | <0.0001 |
| Gabapentinoids | 18 (9.9) | 17 (28.3) | 3 (3.2) | 39 (10.8) | 0.0001 |
| Tricyclic antidepressants | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Serotonin and norepinephrine reuptake inhibitors | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Corticosteroids | 3 (1.6) | 0 (0) | 0 (0) | 4 (1.1) | NA |
| Psychotherapy | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Physical therapy | 2 (1.1) | 0 (0) | 2 (2.1) | 4 (1.1) | NA |
| Nothing | 28 (15.4) | 14 (23.3) | 20 (21.3) | 69 (19.1) | 0.36 |
| Other | 8 (4.4) | 6 (10.0) | 8 (8.5) | 24 (6.6) | 0.33 |
| Proportion of rescue opioids for pain diagnosis | |||||
| 0% | 73 (40.1) | 37 (61.7) | 65 (69.1) | 190 (52.6) | NA |
| 1–10% | 4 (2.2) | 1 (1.7) | 0 | 5 (1.4) | |
| 11–30% | 8 (4.4) | 1 (1.7) | 6 (6.4) | 17 (4.7) | |
| 31–50% | 54 (29.7) | 7 (11.7) | 16 (17.0) | 84 (23.3) | |
| 51–99% | 1 (0.5) | 0 | 0 | 1 (0.3) | |
| 100% | 42 (23.1) | 14 (23.3) | 7 (7.4) | 64 (17.7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, D.; Abdelghani, E.; Chen, J.; Dibaj, S.; Zhukovsky, D.; Dev, R.; Tanco, K.; Haider, A.; Azhar, A.; Reddy, A.; et al. Chronic Non-Malignant Pain in Patients with Cancer Seen at a Timely Outpatient Palliative Care Clinic. Cancers 2020, 12, 214. https://doi.org/10.3390/cancers12010214
Hui D, Abdelghani E, Chen J, Dibaj S, Zhukovsky D, Dev R, Tanco K, Haider A, Azhar A, Reddy A, et al. Chronic Non-Malignant Pain in Patients with Cancer Seen at a Timely Outpatient Palliative Care Clinic. Cancers. 2020; 12(1):214. https://doi.org/10.3390/cancers12010214
Chicago/Turabian StyleHui, David, Eman Abdelghani, Joseph Chen, Shiva Dibaj, Donna Zhukovsky, Rony Dev, Kimberson Tanco, Ali Haider, Ahsan Azhar, Akhila Reddy, and et al. 2020. "Chronic Non-Malignant Pain in Patients with Cancer Seen at a Timely Outpatient Palliative Care Clinic" Cancers 12, no. 1: 214. https://doi.org/10.3390/cancers12010214
APA StyleHui, D., Abdelghani, E., Chen, J., Dibaj, S., Zhukovsky, D., Dev, R., Tanco, K., Haider, A., Azhar, A., Reddy, A., Epner, D., Arthur, J., Dalal, S., Heung, Y., Reddy, S., De La Cruz, M., Liu, D., & Bruera, E. (2020). Chronic Non-Malignant Pain in Patients with Cancer Seen at a Timely Outpatient Palliative Care Clinic. Cancers, 12(1), 214. https://doi.org/10.3390/cancers12010214






