Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood–Brain Barrier
Abstract
1. Introduction
2. Results
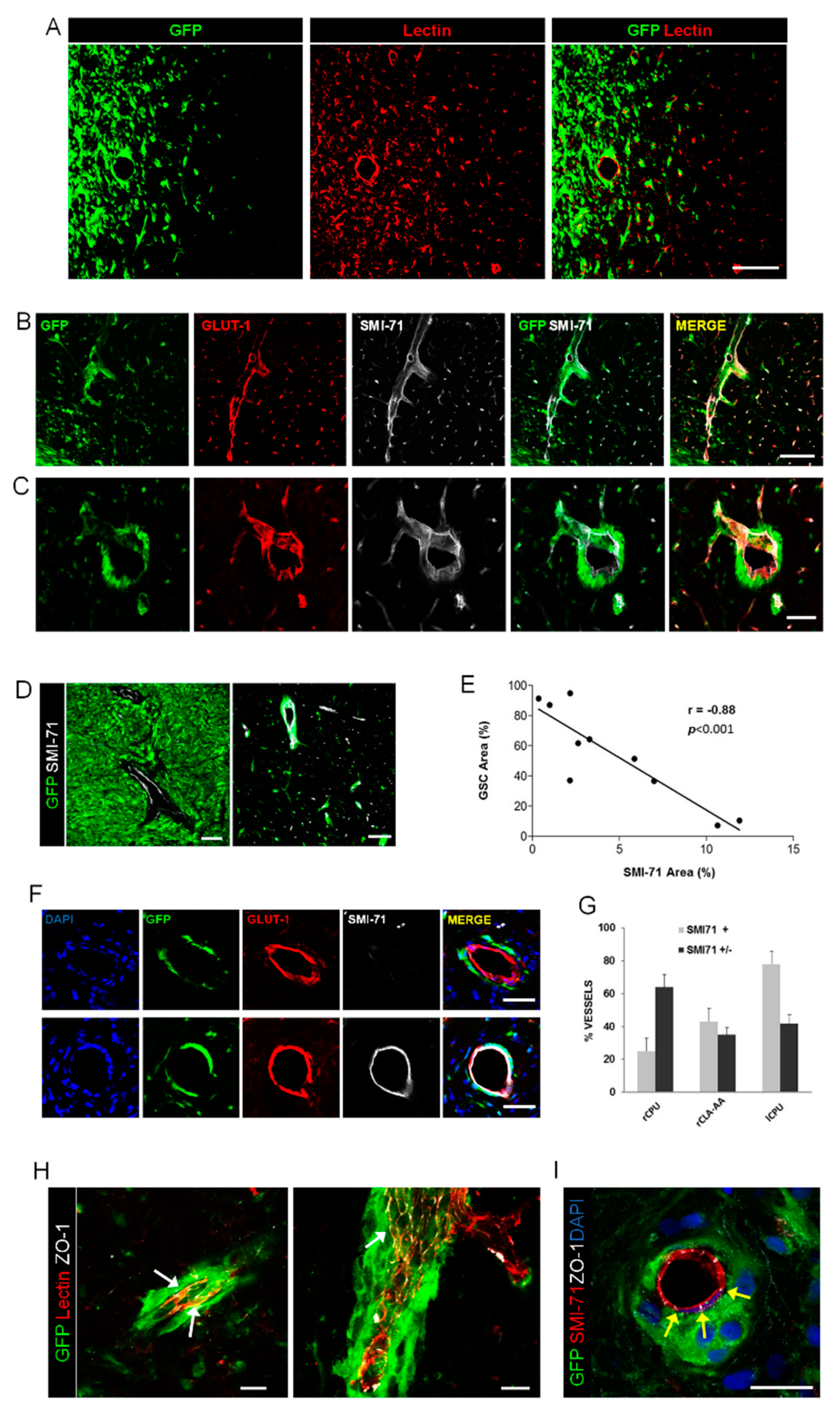
2.1. Association of Glioma Cells with Blood Vessels and Disruption of BBB in Models of Brain Xenograft
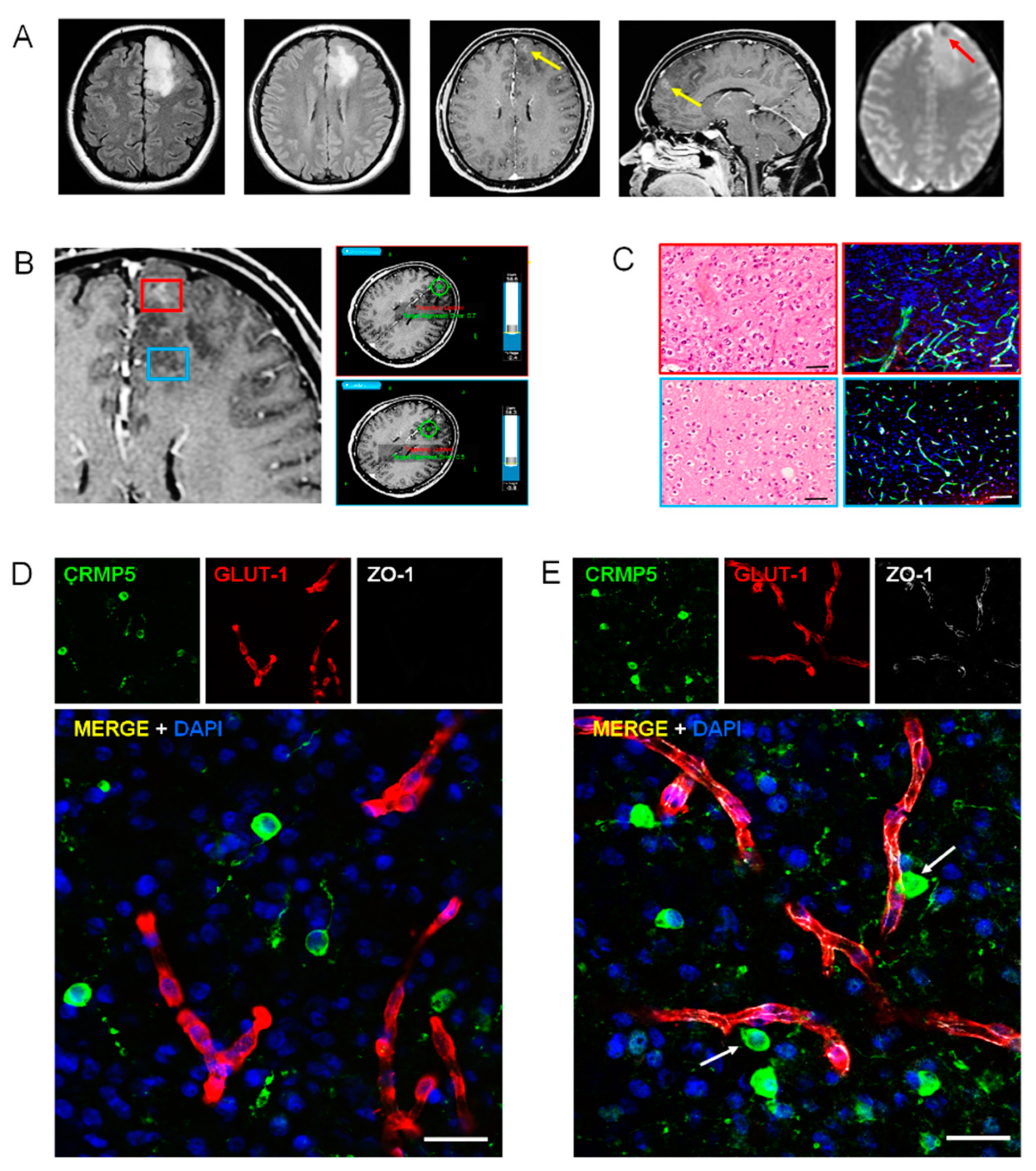
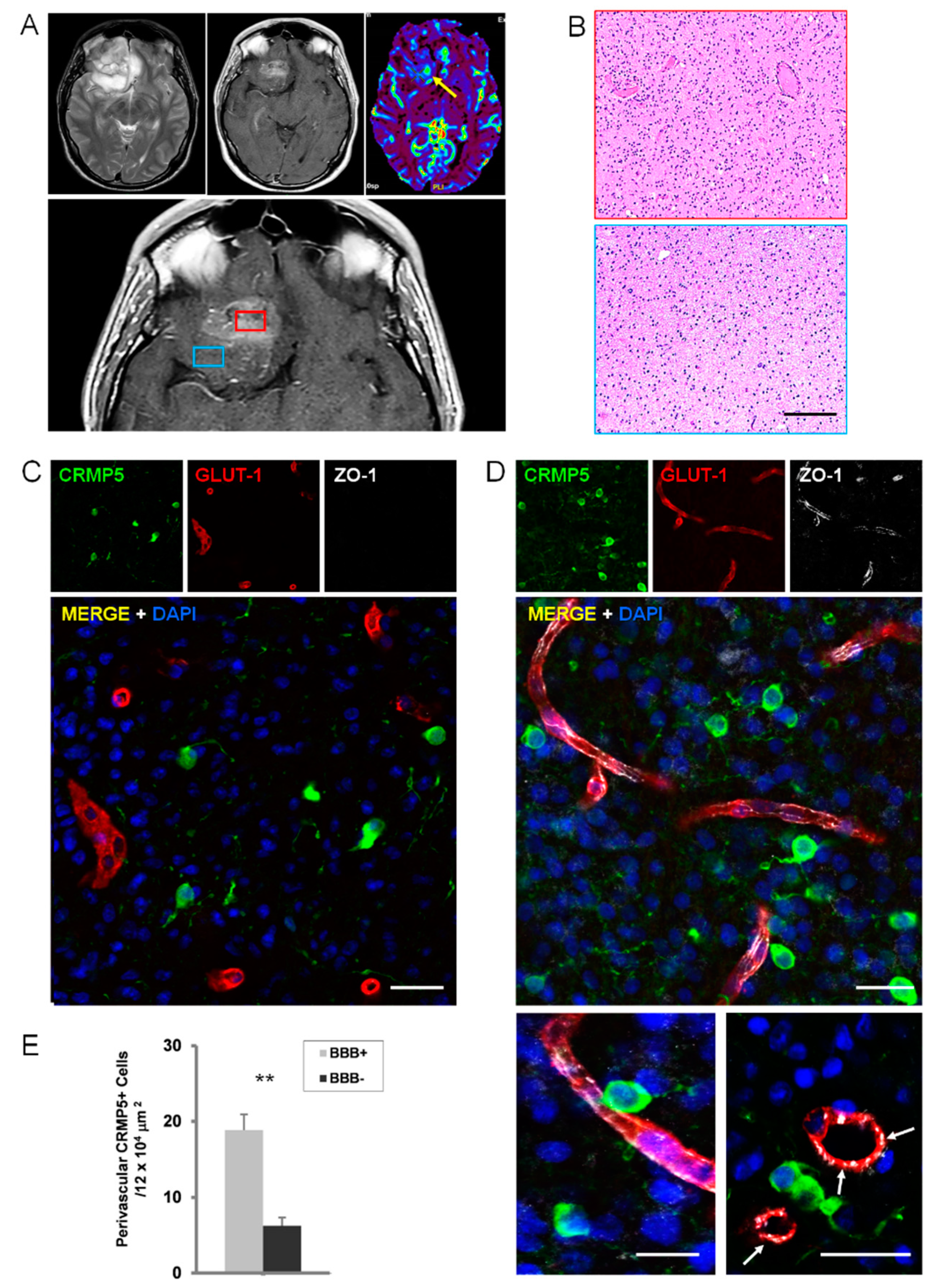
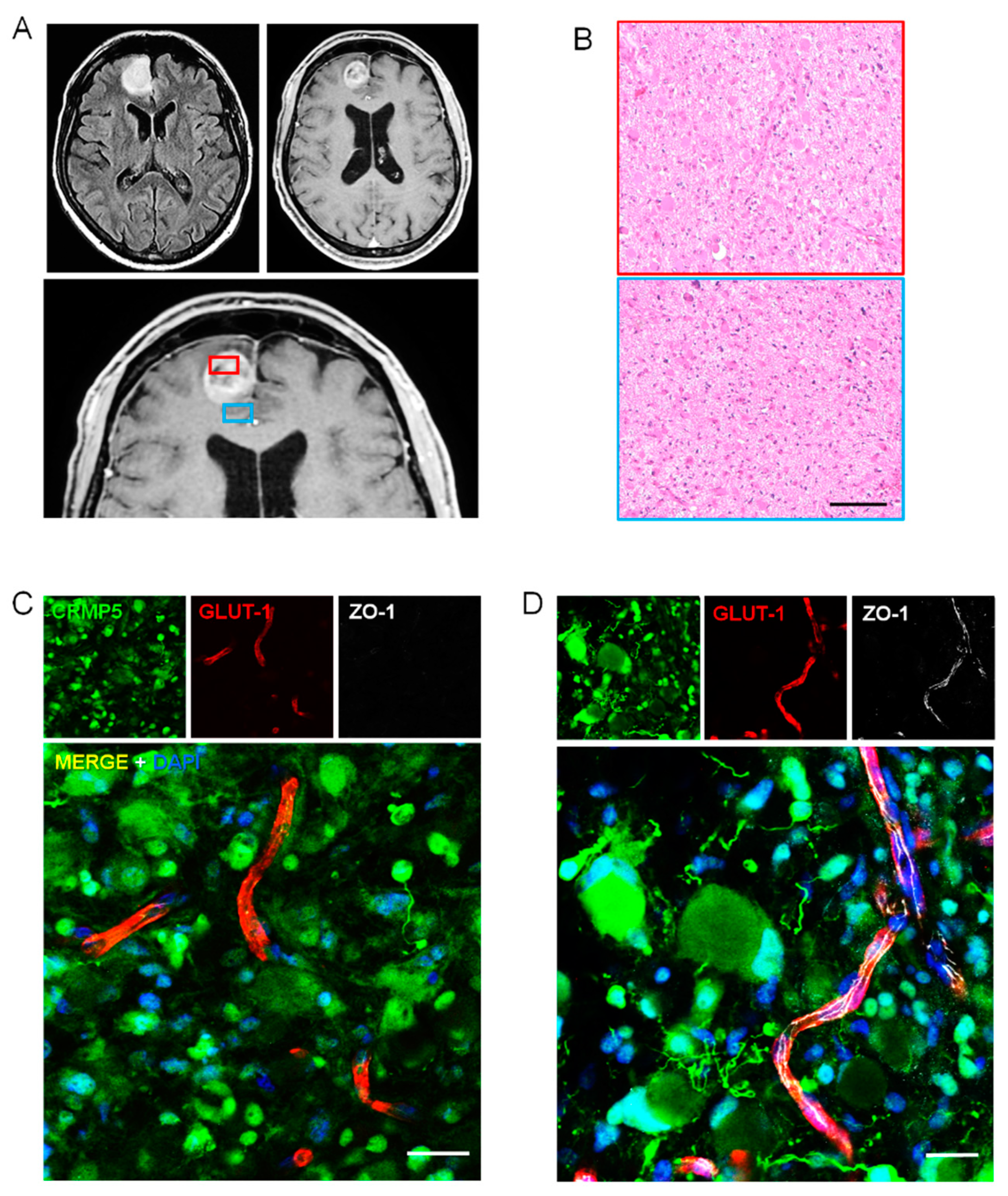
2.2. Perivascular Invasion and Disruption of BBB in Human Specimens of Gliomas
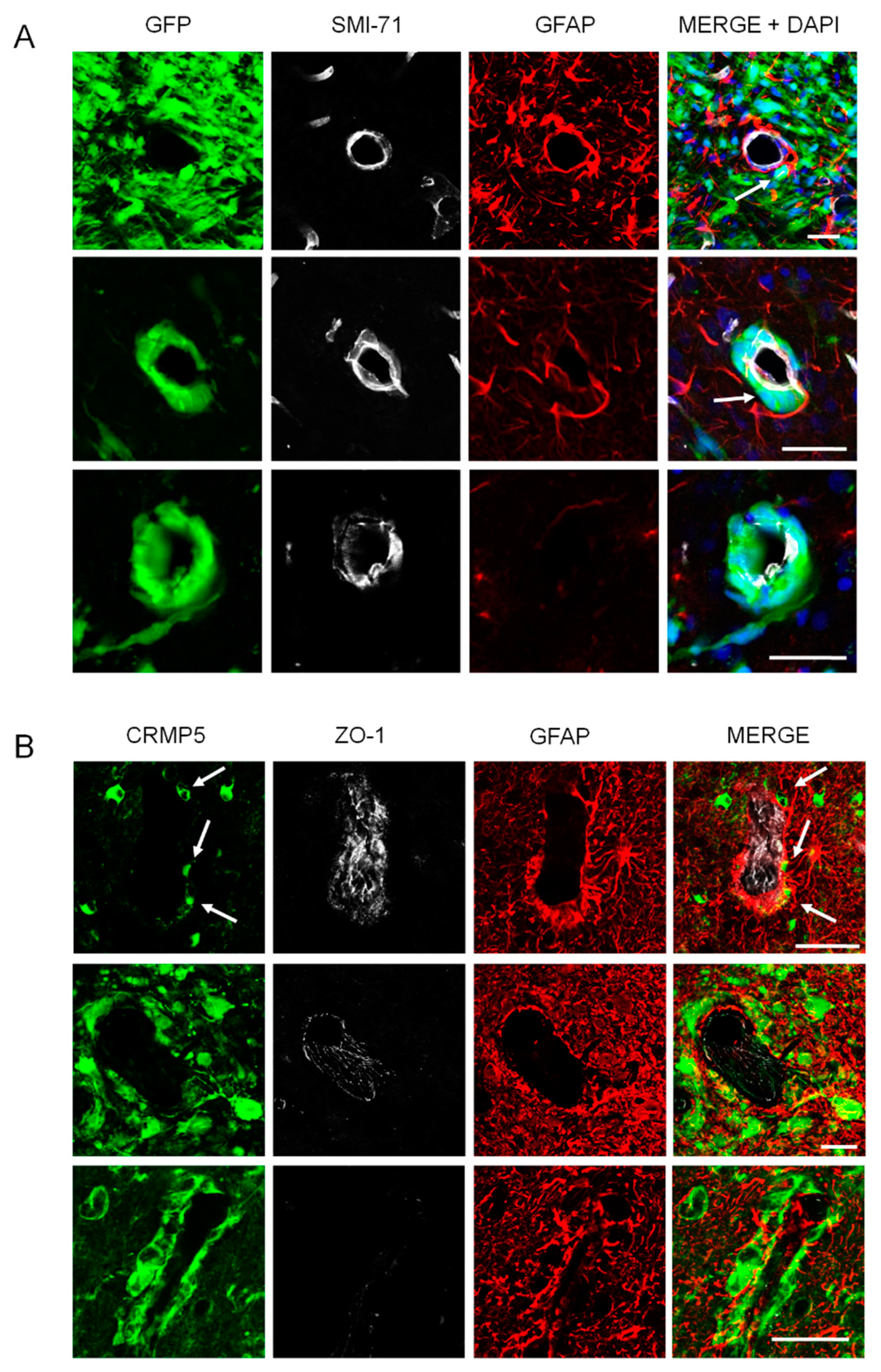
2.3. Relationships of Invading Glioma Cells and Perivascular Astrocytes in GSC Brain Xenografts and Patients’ Tumors
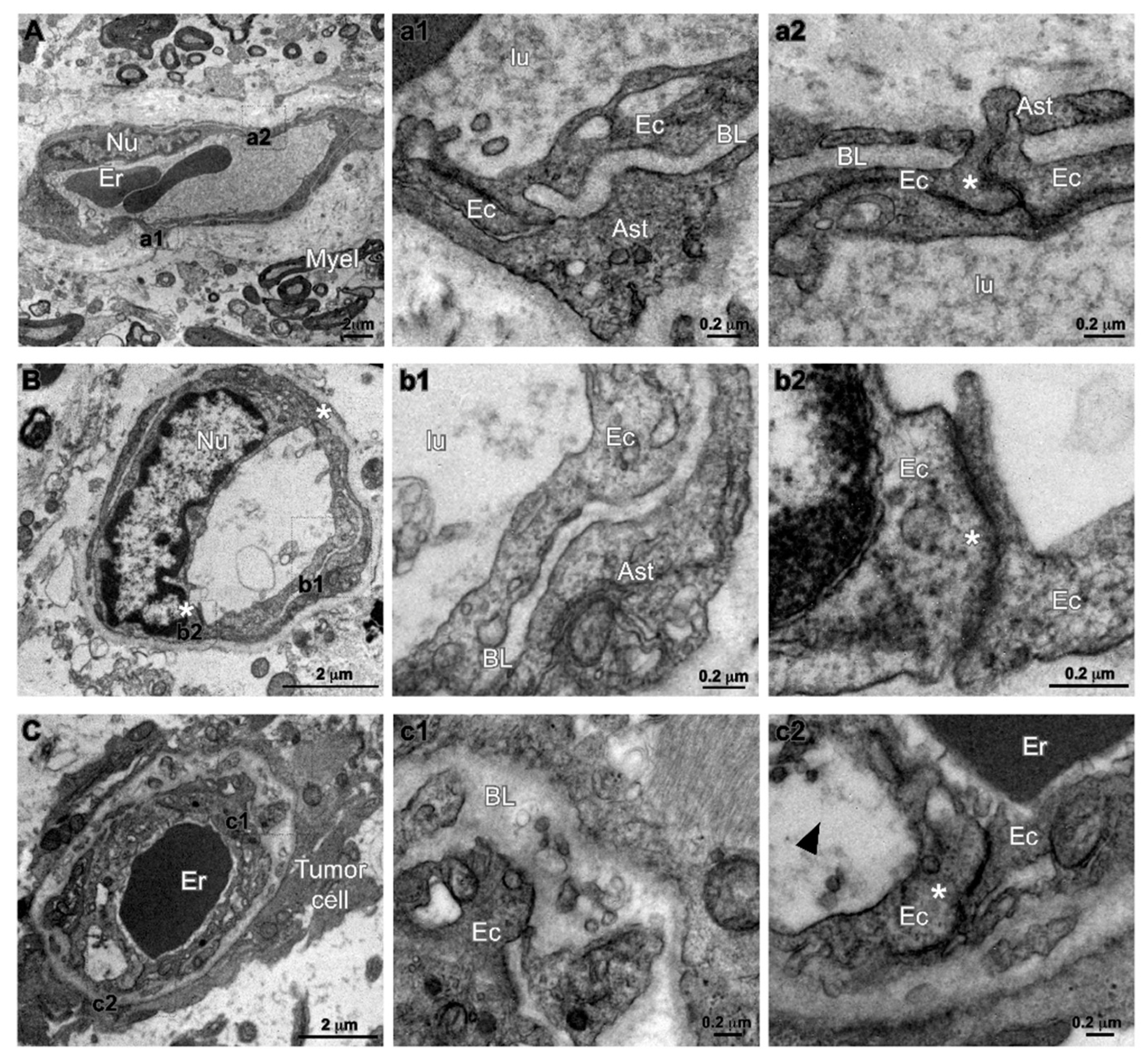
2.4. Characterization of Tumor Endothelium in Malignant Glioma by Transmission Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Compliance with Ethical Standards
4.2. Culture of Tumor Cells and Lentiviral Infection
4.3. Intracranial Xenografts of GFP Expressing U87MG, GSC1, and GSC275 Cells
4.4. Fluorescence Microscopy and Immunofluorescence of Brain Tumor Xenografts
4.5. Clinical Material
4.6. Antigen Retrieval and Auto-Fluorescence Removal in Brain Tumor Xenografts and Human Specimens
4.7. Transmission Electron Microscopy (TEM)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegand, S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999, 284, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Farin, A.; Suzuki, S.O.; Weiker, M.; Goldman, J.E.; Bruce, J.N.; Canoll, P. Transplanted glioma cells migrate and proliferate on host brain vasculature: A dynamic analysis. Glia 2006, 53, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Sasaki, H.; Aoyagi, M.; Hirakawa, K. Invasion of experimental rat brain tumor: Early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol. 1993, 86, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zagzag, D.; Amirnovin, R.; Greco, M.A.; Yee, H.; Holash, J.; Wiegand, S.J.; Zabski, S.; Yancopoulos, G.D.; Grumet, M. Vascular apoptosis and involution in gliomas precede neovascularization: A novel concept for glioma growth and angiogenesis. Lab. Investig. 2000, 80, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef]
- D’Alessandris, Q.G.; Biffoni, M.; Martini, M.; Runci, D.; Buccarelli, M.; Cenci, T.; Signore, M.; Stancato, L.; Olivi, A.; De Maria, R.; et al. The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response. Neuro Oncol. 2017, 19, 1097–1108. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumor vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef]
- Haas, T.L.; Sciuto, M.R.; Brunetto, L.; Valvo, C.; Signore, M.; Fiori, M.E.; di Martino, S.; Giannetti, S.; Morgante, L.; Boe, A.; et al. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma. Cell Stem Cell 2017, 21, 35–50. [Google Scholar] [CrossRef]
- Marziali, G.; Buccarelli, M.; Giuliani, A.; Ilari, R.; Grande, S.; Palma, A.; D’Alessandris, Q.G.; Martini, M.; Biffoni, M.; Pallini, R.; et al. A three-microRNA signature identifies two subtypes of glioblastoma patients with different clinical outcomes. Mol. Oncol. 2017, 11, 1115–1129. [Google Scholar] [CrossRef][Green Version]
- Mazzetti, S.; Frigerio, S.; Gelati, M.; Salmaggi, A.; Vitellaro-Zuccarello, L. Lycopersicon esculentum lectin: An effective and versatile endothelial marker of normal and tumoral blood vessels in the central nervous system. Eur. J. Histochem. 2004, 48, 423–428. [Google Scholar] [CrossRef]
- Rosenstein, J.M.; Krum, J.M.; Sternberger, L.A.; Pulley, M.T.; Sternberger, N.H. Immunocytochemical expression of the endothelial barrier antigen (EBA) during brain angiogenesis. Brain Res. Dev. Brain Res. 1992, 66, 47–54. [Google Scholar] [CrossRef]
- Sternberger, N.H.; Sternberger, L.A. Blood-brain barrier protein recognized by monoclonal antibody. Proc. Natl. Acad. Sci. USA 1987, 84, 8169–8173. [Google Scholar] [CrossRef] [PubMed]
- Seidner, G.; Alvarez, M.G.; Yeh, J.I.; O’Driscoll, K.R.; Klepper, J.; Stump, T.S.; Wang, D.; Spinner, N.B.; Birnbaum, M.J.; De Vivo, D.C. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood- brain barrier hexose carrier. Nat. Genet. 1998, 18, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Uldry, M.; Thorens, B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004, 447, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (Zonula Occludens) in a variety of epithelia. J. Cell. Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell. Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- Romero, I.A.; Radewicz, K.; Jubin, E.; Michel, C.C.; Greenwood, J.; Couraud, P.O.; Adamson, P. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci. Lett. 2003, 344, 112–116. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron. 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Afonso, P.V.; Ozden, S.; Prevost, M.-C.; Schmitt, C.; Seilhean, D.; Weksler, B.; Couraud, P.-O.; Gessain, A.; Romero, I.A.; Ceccaldi, P.-E. Human blood-brain barrier disruption by retroviral-infected lymphocytes: Role of myosin light chain kinase in endothelial tight-junction disorganization. J. Immunol. 2007, 179, 2576–2583. [Google Scholar] [CrossRef]
- Liebner, S.; Fischmann, A.; Rascher, G.; Duffner, F.; Grote, E.H.; Kalbacher, H.; Wolburg, H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000, 100, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Küsters, B.; Leenders, W.P.J.; Wesseling, P.; Smits, D.; Verrijp, K.; Ruiter, D.J.; Peters, J.P.W.; Van der Kogel, A.J.; De Waal, R.M.W. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002, 62, 341–345. [Google Scholar] [PubMed]
- Gambarota, G.; Leenders, W. Characterization of tumor vasculature in mouse brain by USPIO contrast-enhanced MRI. Methods Mol. Biol. 2011, 771, 477–487. [Google Scholar] [PubMed]
- Moutal, A.; Honnorat, J.; Massoma, P.; Désormeaux, P.; Bertrand, C.; Malleval, C.; Watrin, C.; Chounlamountri, N.; Mayeur, M.E.; Besançon, R.; et al. CRMP5 controls glioblastoma cell proliferation and survival through Notch-dependent signaling. Cancer Res. 2015, 75, 3519–3528. [Google Scholar] [CrossRef]
- Brot, S.; Malleval, C.; Benetollo, C.; Auger, C.; Meyronet, D.; Rogemond, V.; Honnorat, J.; Moradi-Améli, M. Identification of a new CRMP5 isoform present in the nucleus of cancer cells and enhancing their proliferation. Exp. Cell Res. 2013, 319, 588–599, PMID:23298946. [Google Scholar] [CrossRef]
- Falchetti, M.L.; D’Alessandris, Q.G.; Pacioni, S.; Buccarelli, M.; Morgante, L.; Giannetti, S.; Lulli, V.; Martini, M.; Larocca, L.M.; Vakana, E.; et al. Glioblastoma endothelium drives bevacizumab-induced infiltrative growth via modulation of PLXDC1. Int. J. Cancer 2019, 144, 1331–1344. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell. Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Kumar, S.; Sharife, H.; Kreisel, T.; Mogilevsky, M.; Bar-Lev, L.; Grunewald, M.; Aizenshtein, E.; Karni, R.; Paldor, I.; Shlomi, T.; et al. Intra-tumoral metabolic zonation and resultant phenotypic diversification are dictated by blood vessel proximity. Cell. Metabolism. 2019, 30, 201–211. [Google Scholar] [CrossRef]
- Campos, B.; Wan, F.; Farhadi, M.; Ernst, A.; Zeppernick, F.; Tagscherer, K.E.; Ahmadi, R.; Lohr, J.; Dictus, C.; Gdynia, G.; et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin. Cancer Res. 2010, 16, 2715–2728. [Google Scholar] [CrossRef]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; De Pascalis, I.; Coccè, V.; Bonomi, A.; Pascucci, L.; Alessandri, G.; Pessina, A.; et al. Mesenchymal stromal cells loaded with paclitaxel induce cytotoxic damage in glioblastoma brain xenografts. Stem Cell Res. Ther. 2015, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Bokman, S.H.; Ward, W.W. Renaturation of Aequorea green-fluorescent protein. Biochem. Biophys. Res. Commun. 1981, 101, 1372–1380. [Google Scholar] [CrossRef]
- Ward, W.W.; Bokman, S.H. Reversible Denaturation of Aequorea Green-Fluorescent Protein: Physical Separation and Characterization of the Renatured Protein. Biochemistry 1982, 21, 4535–4540. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacioni, S.; D’Alessandris, Q.G.; Buccarelli, M.; Boe, A.; Martini, M.; Larocca, L.M.; Bolasco, G.; Ricci-Vitiani, L.; Falchetti, M.L.; Pallini, R. Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood–Brain Barrier. Cancers 2020, 12, 18. https://doi.org/10.3390/cancers12010018
Pacioni S, D’Alessandris QG, Buccarelli M, Boe A, Martini M, Larocca LM, Bolasco G, Ricci-Vitiani L, Falchetti ML, Pallini R. Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood–Brain Barrier. Cancers. 2020; 12(1):18. https://doi.org/10.3390/cancers12010018
Chicago/Turabian StylePacioni, Simone, Quintino Giorgio D’Alessandris, Mariachiara Buccarelli, Alessandra Boe, Maurizio Martini, Luigi Maria Larocca, Giulia Bolasco, Lucia Ricci-Vitiani, Maria Laura Falchetti, and Roberto Pallini. 2020. "Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood–Brain Barrier" Cancers 12, no. 1: 18. https://doi.org/10.3390/cancers12010018
APA StylePacioni, S., D’Alessandris, Q. G., Buccarelli, M., Boe, A., Martini, M., Larocca, L. M., Bolasco, G., Ricci-Vitiani, L., Falchetti, M. L., & Pallini, R. (2020). Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood–Brain Barrier. Cancers, 12(1), 18. https://doi.org/10.3390/cancers12010018







