In the Absence of a TCR Signal IL-2/IL-12/18-Stimulated γδ T Cells Demonstrate Potent Anti-Tumoral Function Through Direct Killing and Senescence Induction in Cancer Cells
Abstract
1. Introduction
2. Methods
2.1. Bioethics
2.2. PBMC Isolation and Culture
2.3. Isolation of γδ T Cells from PBMCs
2.4. Cell Culture of γδ T Cells
2.5. Flow Cytometric Analysis
2.6. Cell Trace Violet (CTV) Proliferation Assay
2.7. Cytotoxicity Assay via Electric Cell-Substrates Impedance Sensing
2.8. Cell Cycle Analysis
2.9. SA-β-Galactosidase Staining
2.10. RNA Extraction, cDNA Synthesis
2.11. Real Time PCR
2.12. Statistical Analysis
3. Results
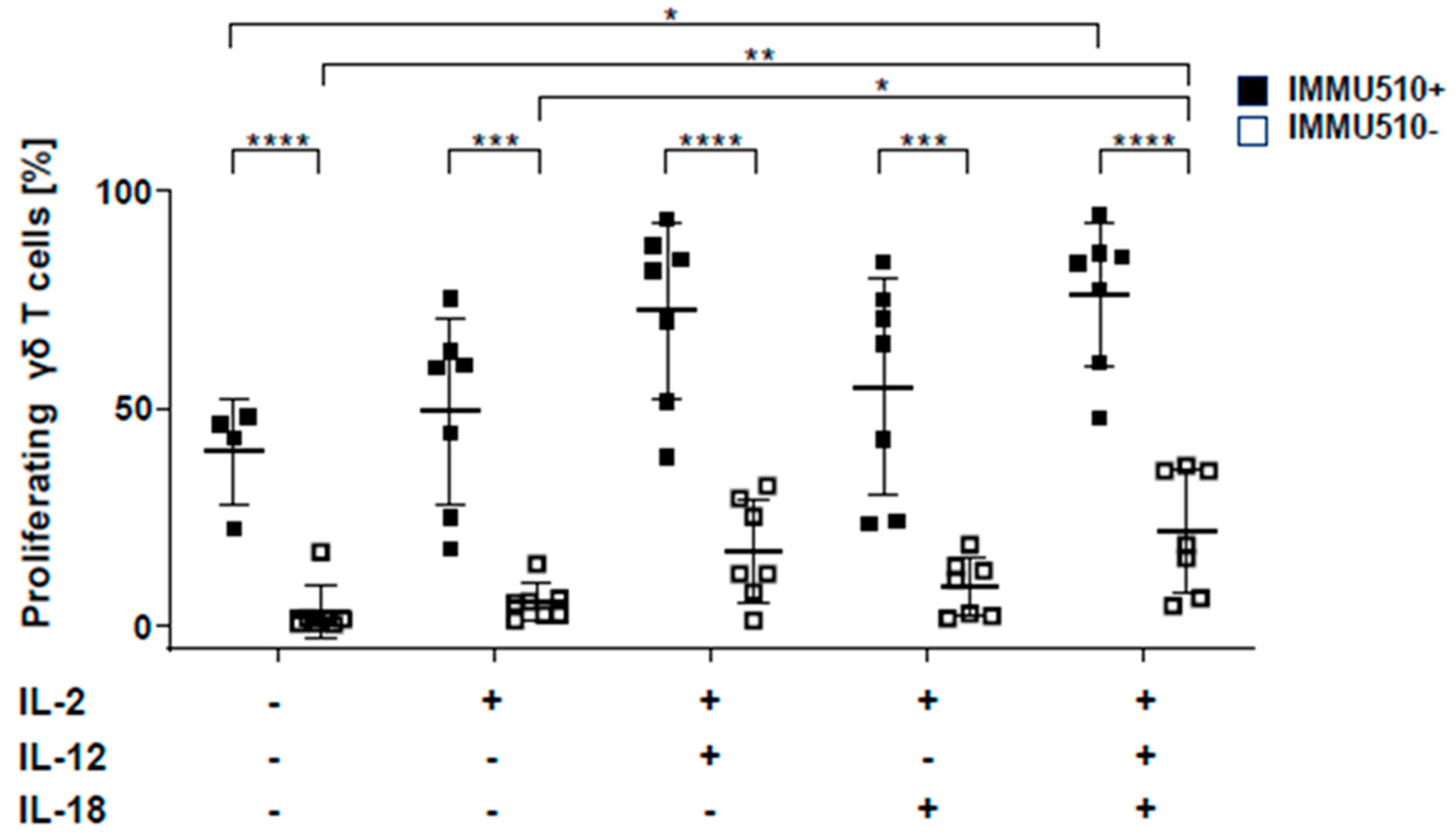
3.1. IL-12 Combined with IL-18 Induces the Proliferation of γδ T Cells both in the Presence and Absence of TCR Stimulation
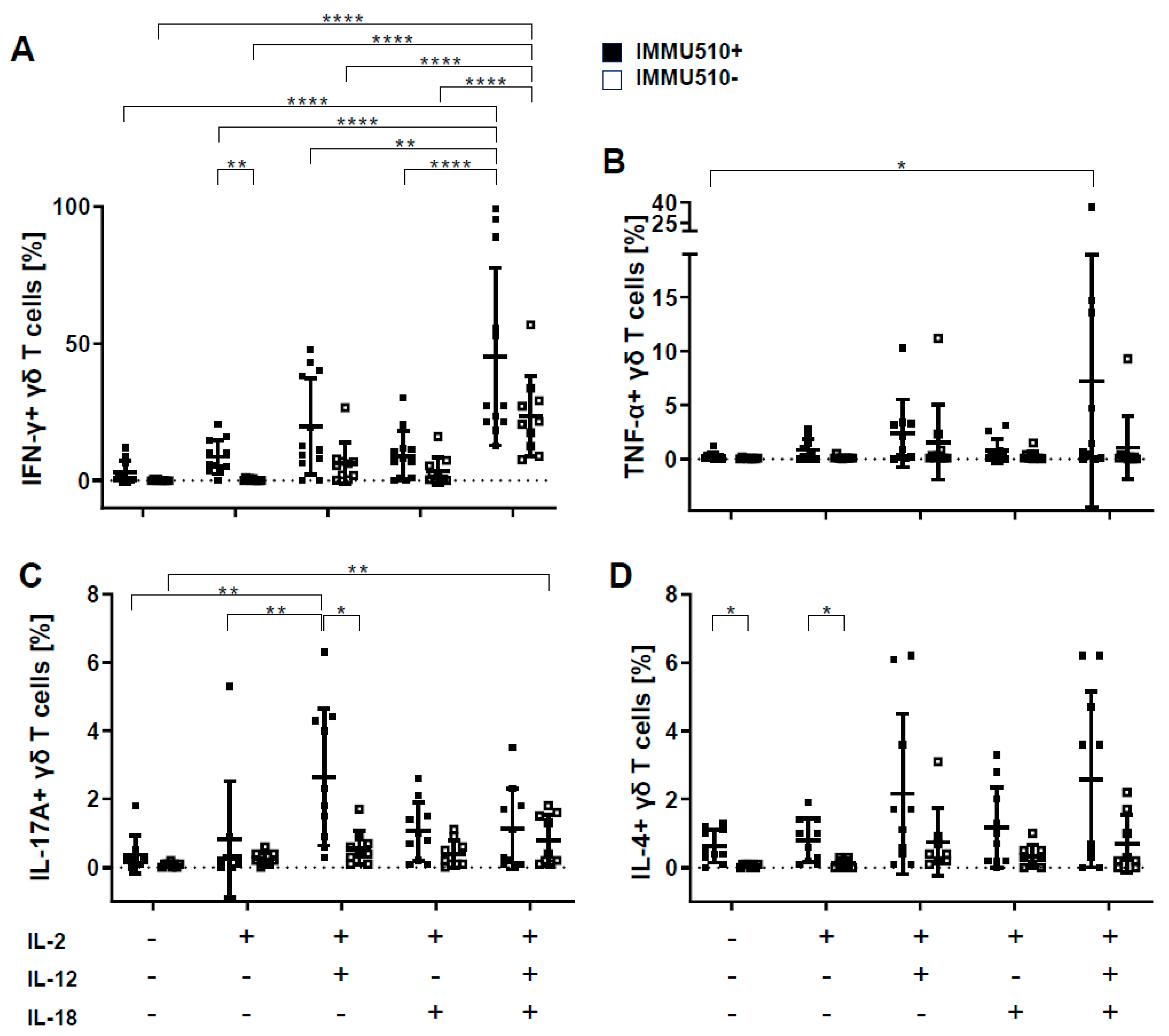
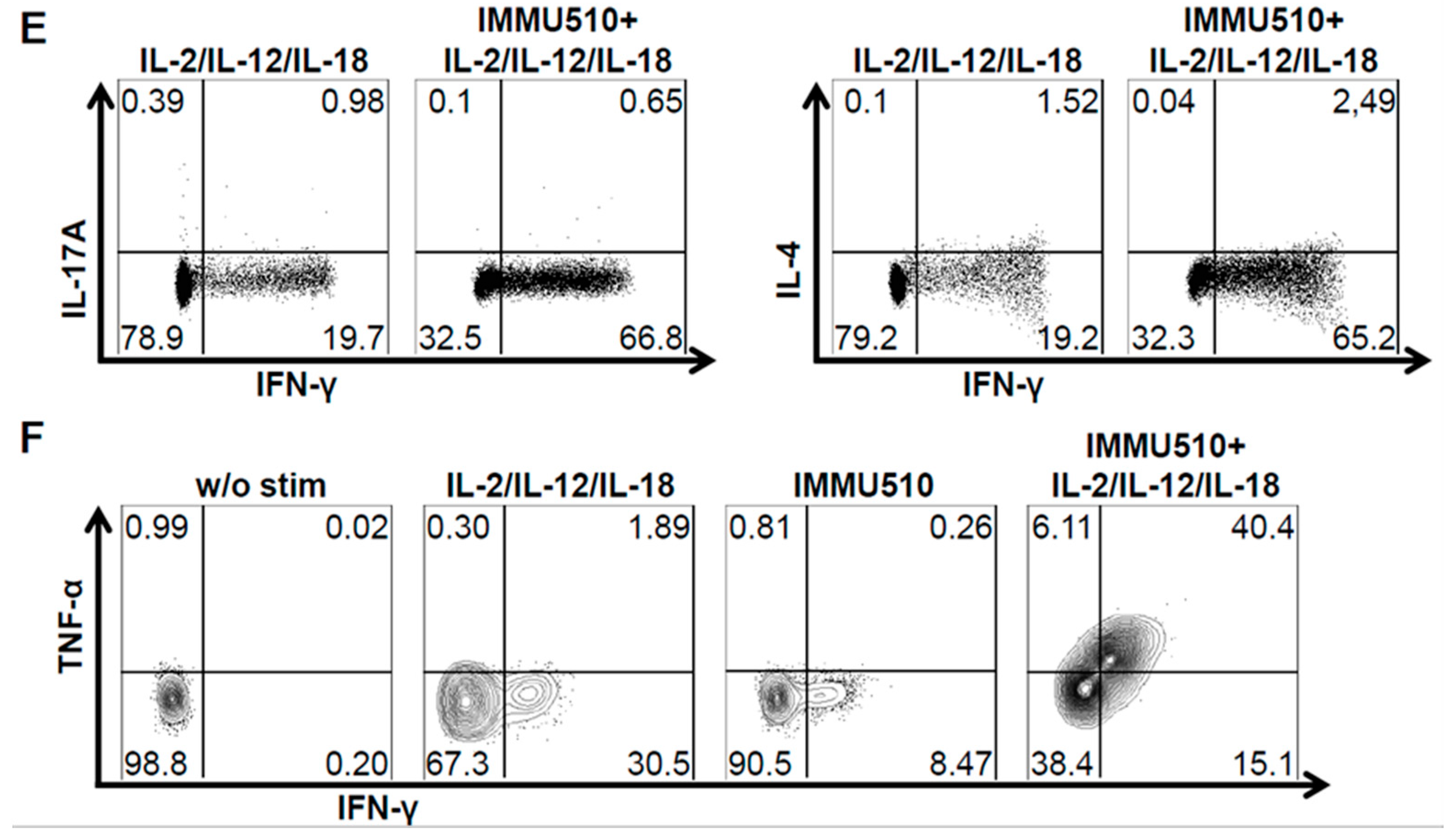
3.2. γδ T Cells Produce IFN-γ, TNF-α, and IL-17 in Response to the Combination of IL-2, IL-12 and IL-18
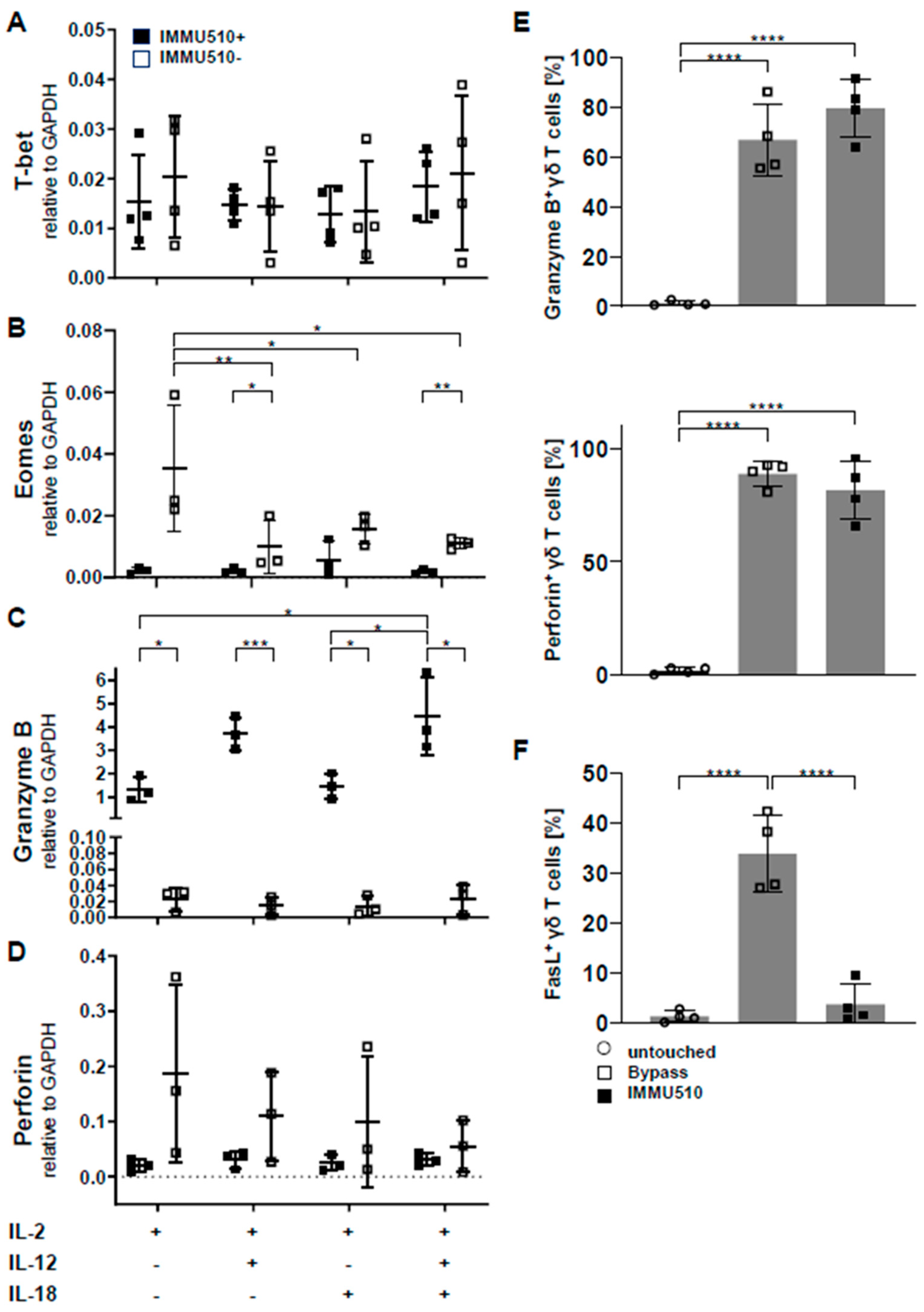
3.3. Neither T-Bet nor Eomes are Induced by Cytokine Stimulation
3.4. Granzyme B/Perforin Production by γδ T Cells is Increased by TCR Stimulation and the Combination of IL-2/IL-12/IL-18
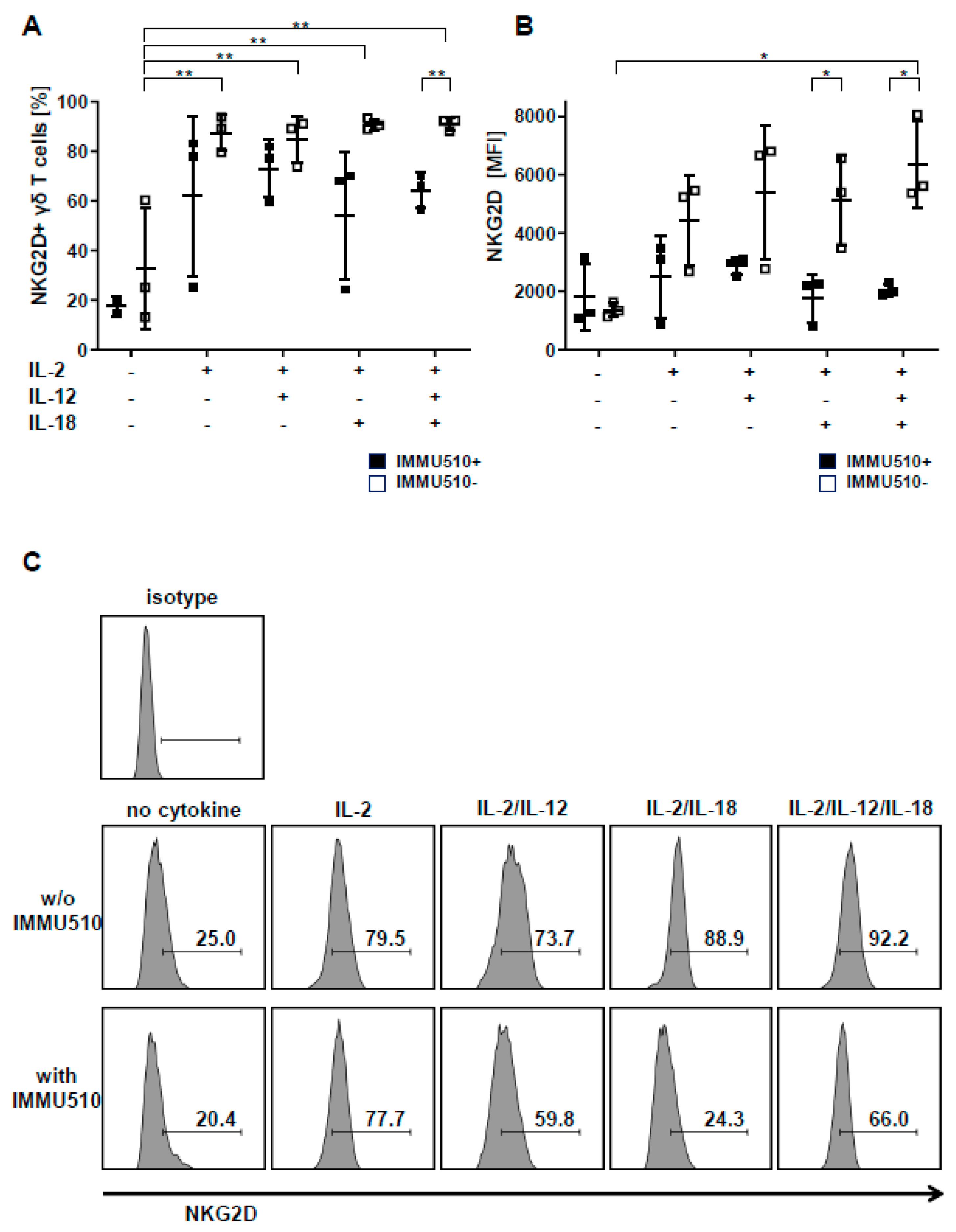
3.5. Expression of NKG2D on γδ T Cells is Reduced by TCR Stimulation
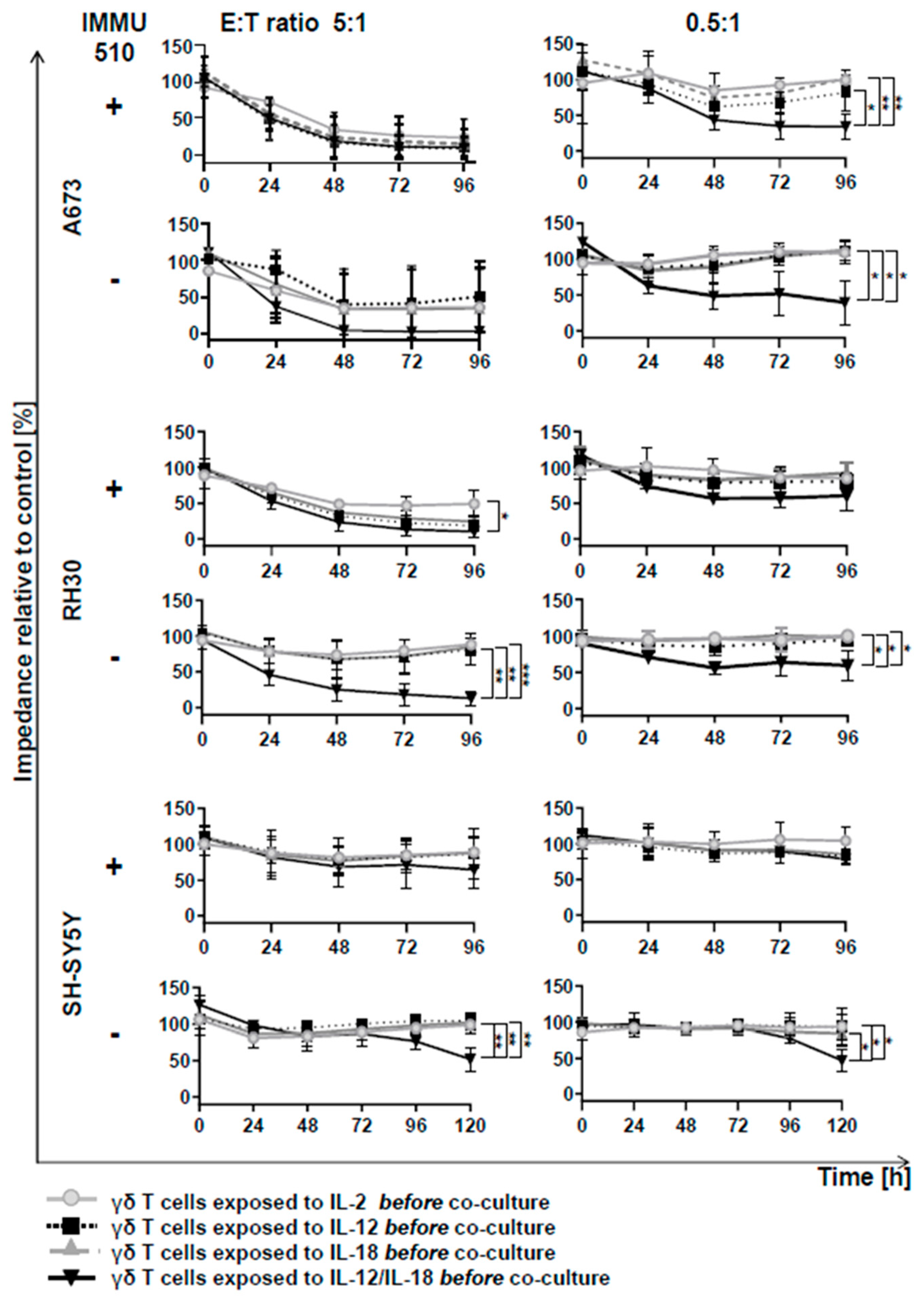
3.6. Anti-Tumor Activity of γδ T Cells is Remarkably Enhanced by the Combination of IL-2/IL-12/IL-18 even in the Absence of a TCR Stimulus
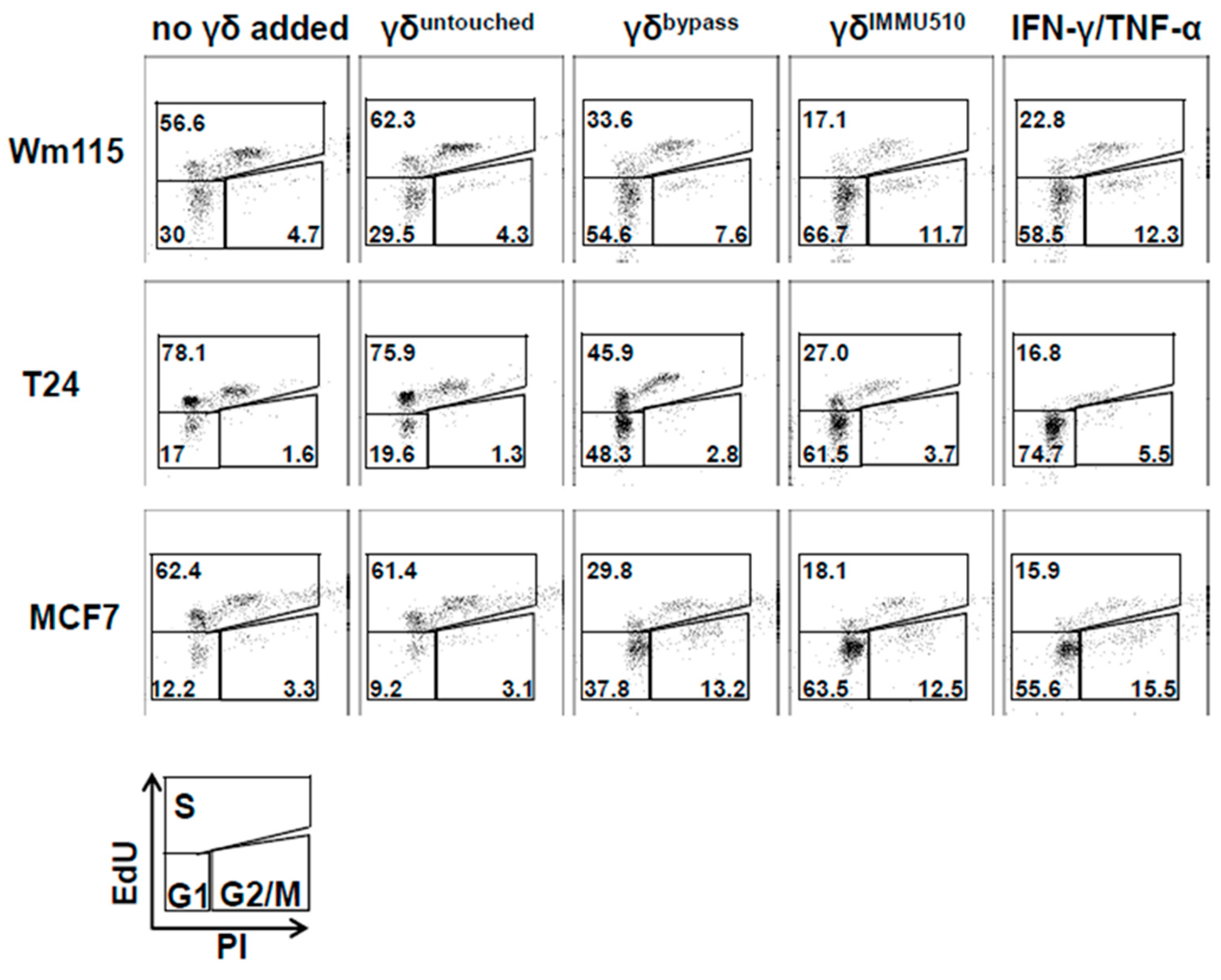
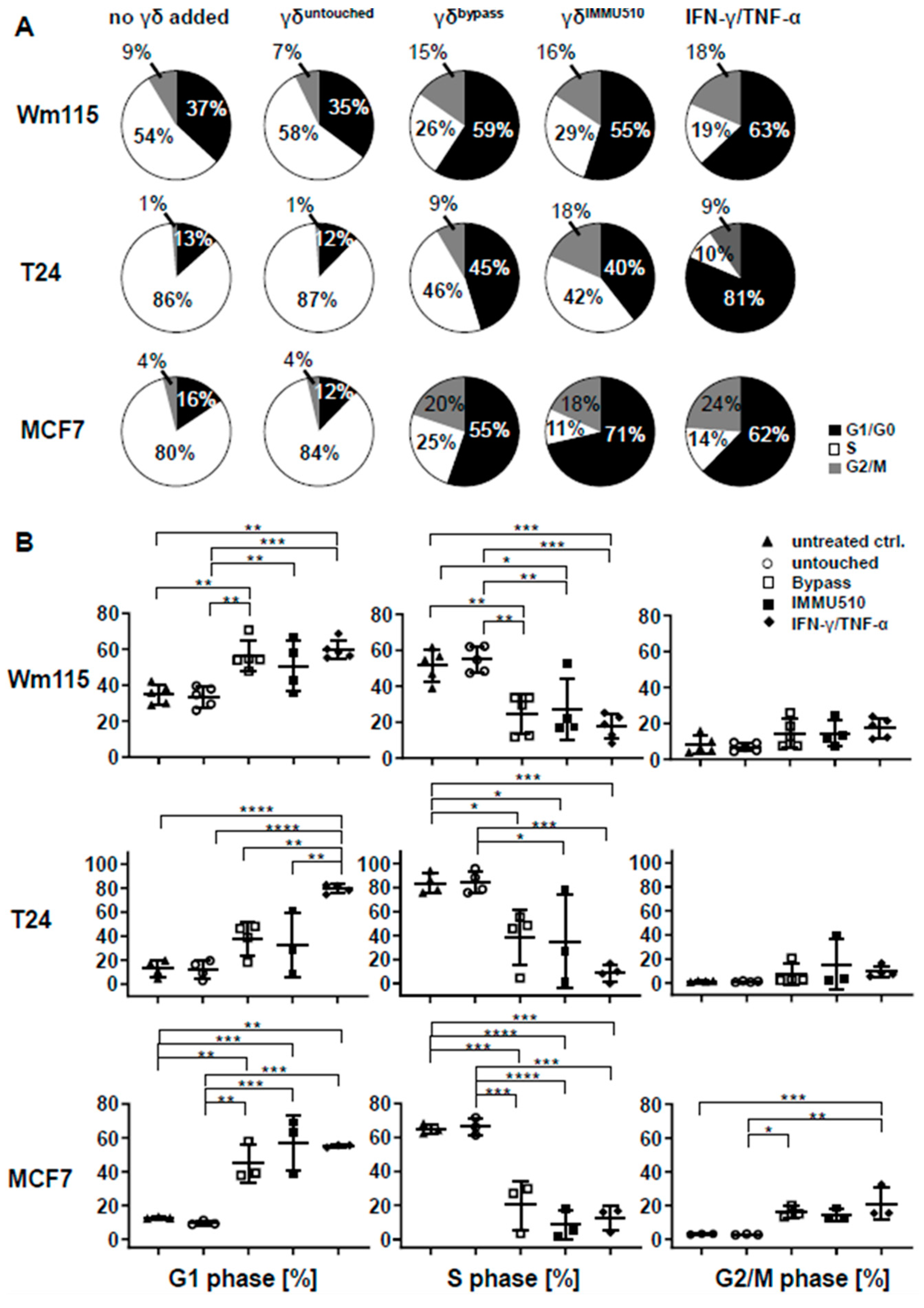
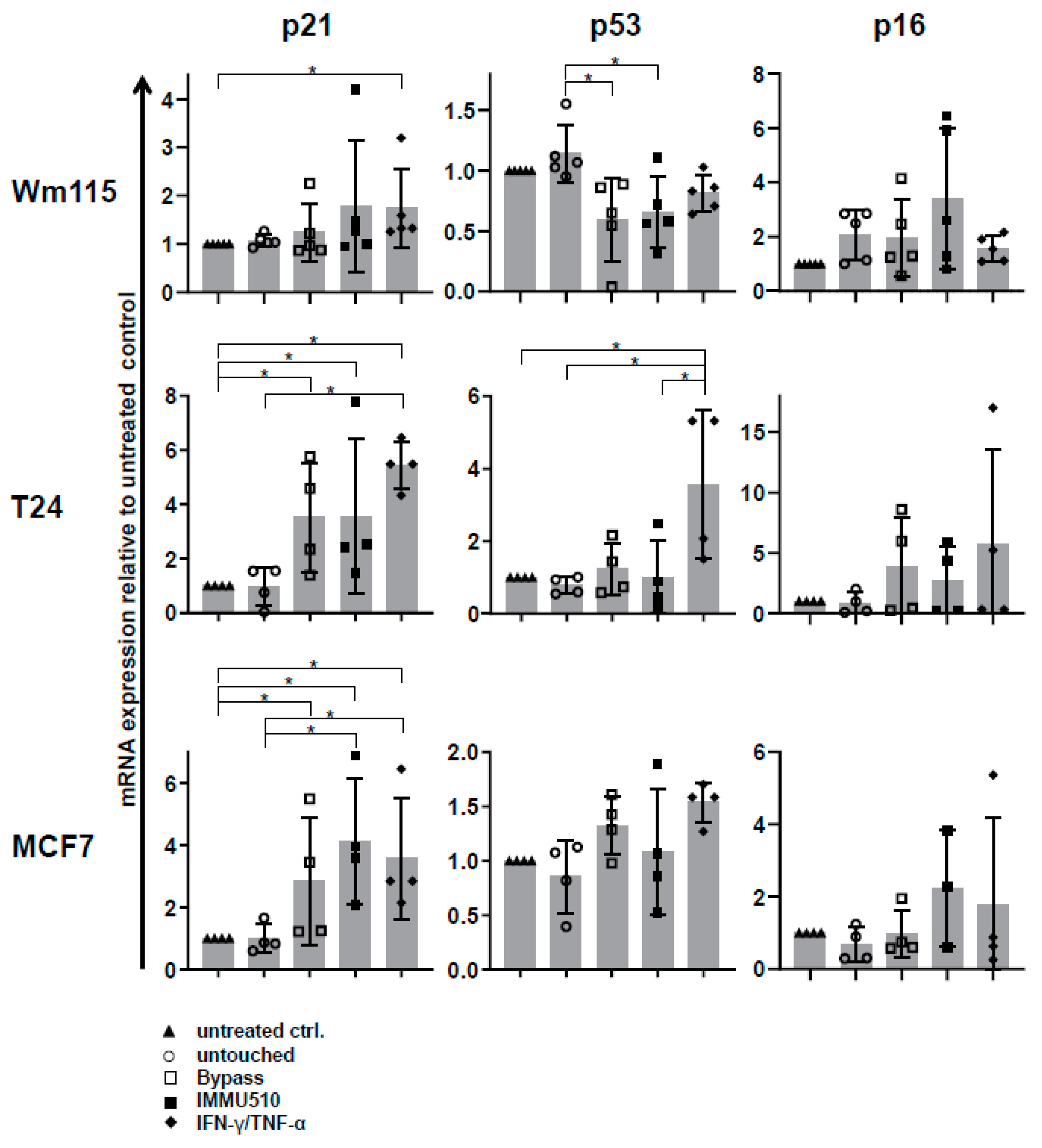
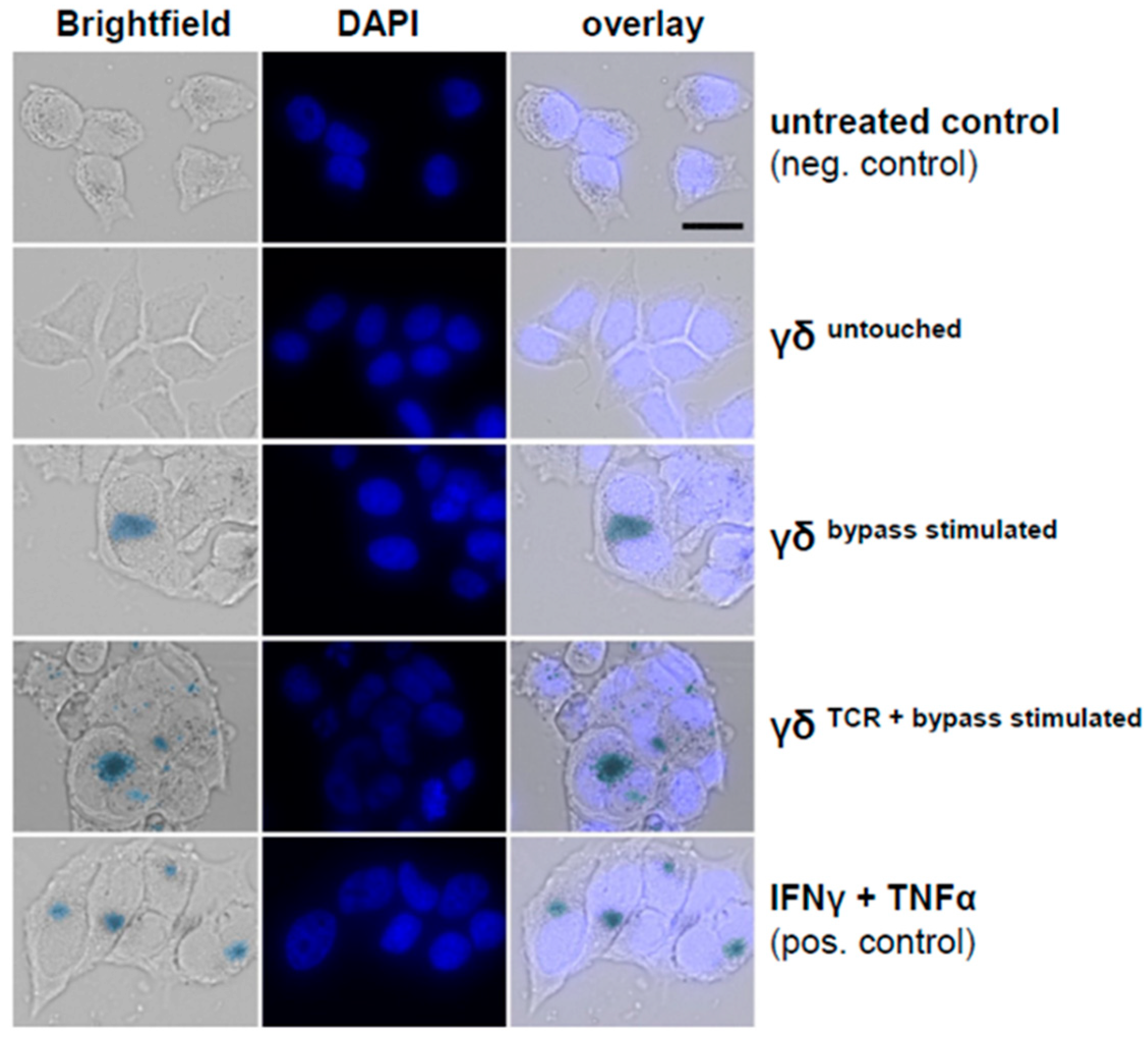
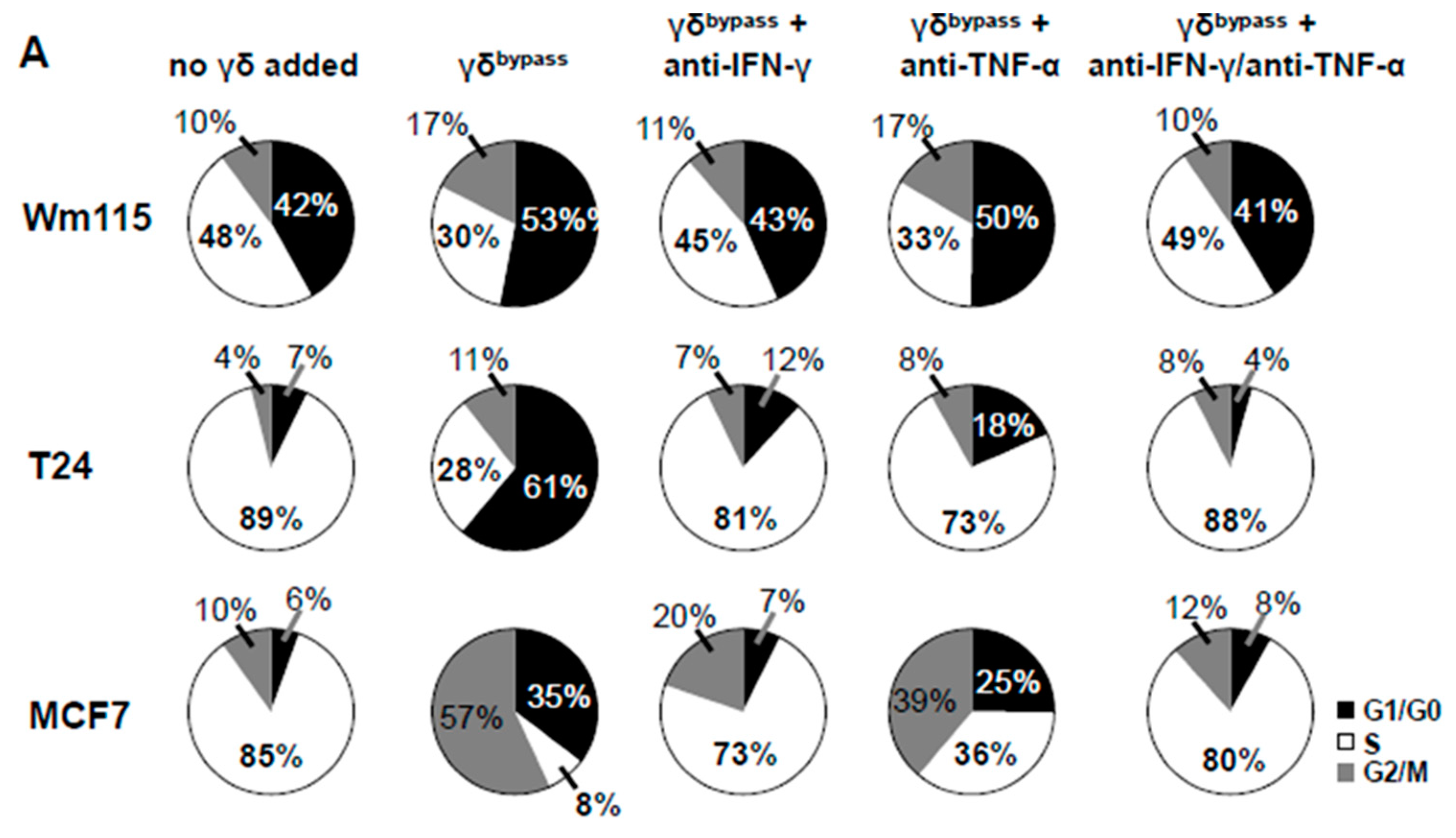
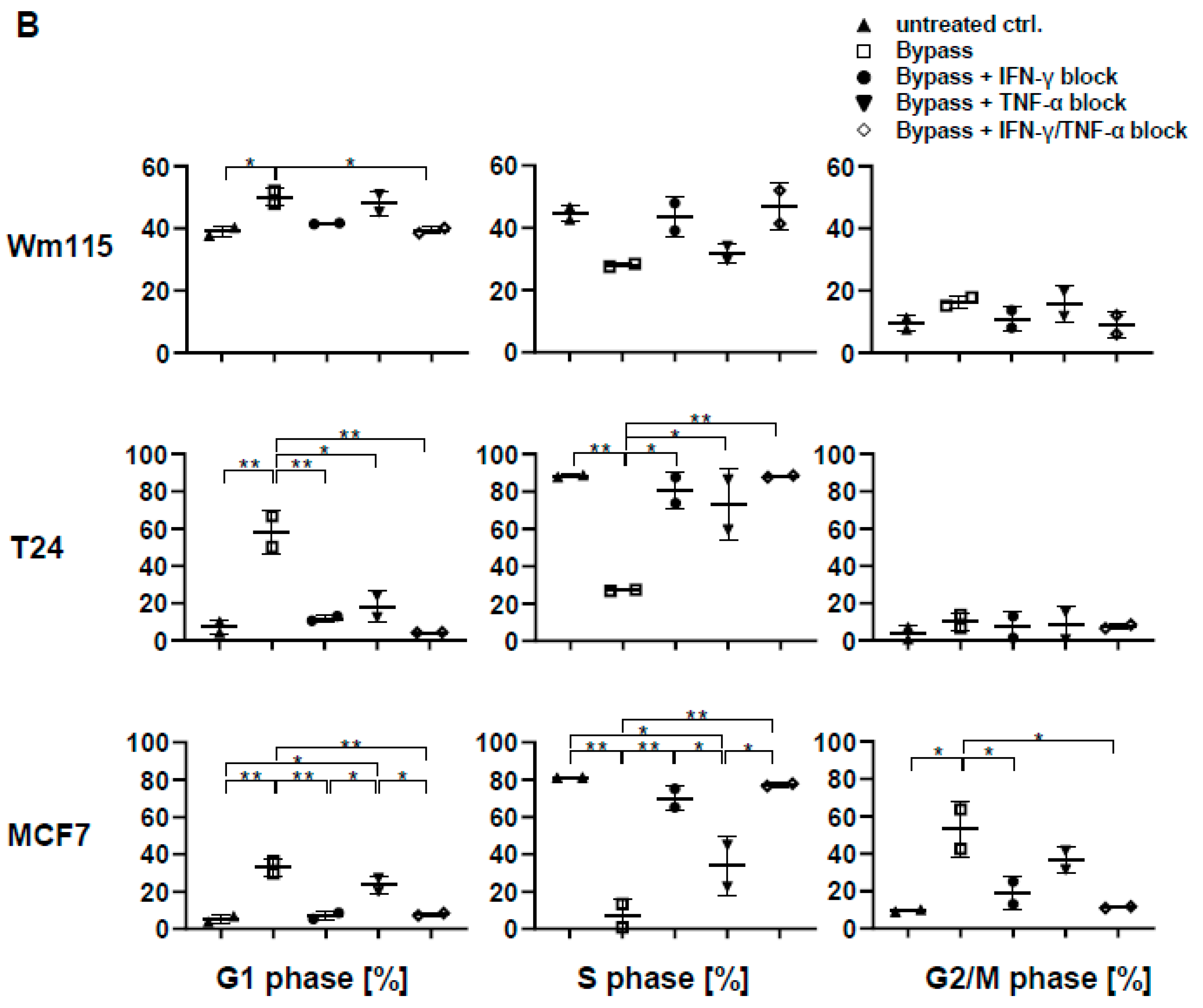
3.7. Combined IL-2/IL-12/IL-18 Induces Senescence in Tumor Cells Independent of a TCR Stimulus
3.8. Direct Comparison of Two Different Anti-γδTCR Monoclonal Antibodies Regarding Cytokine Production and Efficacy in Senescence Induction
3.9. Senescence Induction in Tumor Cells by γδ T Cells Treated with IL-2/IL-12/IL-18 is Mediated by IFN-γ/TNF-α
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Champagne, E. Gammadelta T cell receptor ligands and modes of antigen recognition. Archivum Immunologiae et Therapiae Experimentalis 2011, 59, 117–137. [Google Scholar] [CrossRef]
- Hayday, A.; Vantourout, P. A long-playing CD about the gammadelta TCR repertoire. Immunity 2013, 39, 994–996. [Google Scholar] [CrossRef]
- Dieli, F.; Vermijlen, D.; Fulfaro, F.; Caccamo, N.; Meraviglia, S.; Cicero, G.; Roberts, A.; Buccheri, S.; D’Asaro, M.; Gebbia, N.; et al. Targeting human gammadeltaTcells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7450–7457. [Google Scholar] [CrossRef]
- Sakamoto, M.; Nakajima, J.; Murakawa, T.; Fukami, T.; Yoshida, Y.; Murayama, T.; Takamoto, S.; Matsushita, H.; Kakimi, K. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded gammadeltaTcells: A phase I clinical study. J. Immunother. 2011, 34, 202–211. [Google Scholar] [CrossRef]
- Noguchi, A.; Kaneko, T.; Kamigaki, T.; Fujimoto, K.; Ozawa, M.; Saito, M.; Ariyoshi, N.; Goto, S. Zoledronate-activated Vgamma9gammadelta T cell-based immunotherapy is feasible and restores the impairment of gammadelta T cells in patients with solid tumors. Cytotherapy 2011, 13, 92–97. [Google Scholar] [CrossRef]
- Lang, J.M.; Kaikobad, M.R.; Wallace, M.; Staab, M.J.; Horvath, D.L.; Wilding, G.; Liu, G.; Eickhoff, J.C.; McNeel, D.G.; Malkovsky, M. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1447–1460. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Minato, N.; Tanabe, K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1075–1084. [Google Scholar] [CrossRef]
- Bennouna, J.; Bompas, E.; Neidhardt, E.M.; Rolland, F.; Philip, I.; Galéa, C.; Salot, S.; Saiagh, S.; Audrain, M.; Rimbert, M.; et al. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2008, 57, 1599–1609. [Google Scholar]
- Lo Presti, E.; Pizzolato, G.; Gulotta, E.; Cocorullo, G.; Gulotta, G.; Dieli, F.; Meraviglia, S. Current Advances in gammadelta T Cell-Based Tumor Immunotherapy. Front. Immunol. 2017, 8, 1401. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, P.; Xiao, Z.; Han, X.; Fu, F.; Fu, L. Gammadelta T cells in cancer immunotherapy. Oncotarget 2017, 8, 8900–8909. [Google Scholar]
- Scheper, W.; Sebestyen, Z.; Kuball, J. Cancer Immunotherapy Using gammadeltaT Cells: Dealing with Diversity. Front. Immunol. 2014, 5, 601. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D.; Peters, C.; Wesch, D.; Oberg, H.H. Regulatory functions of gammadelta T cells. Int. Immunopharmacol. 2013, 16, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Oberg, H.H.; Kabelitz, D.; Wesch, D. Phenotype and regulation of immunosuppressive Vdelta2-expressing gammadelta T cells. Cell Mol. Life Sci. 2014, 71, 1943–1960. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Kabelitz, D.; Wesch, D. Regulatory functions of gammadelta T cells. Cell Mol. Life Sci. 2018, 75, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Traxlmayr, M.W.; Wesch, D.; Dohnal, A.M.; Funovics, P.; Fischer, M.B.; Kabelitz, D.; Felzmann, T. Immune suppression by gammadelta T-cells as a potential regulatory mechanism after cancer vaccination with IL-12 secreting dendritic cells. J. Immunother. 2010, 33, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, O.; Gruenbacher, G.; Gander, H.; Komuczki, J.; Rahm, A.; Thurnher, M. Essential requirements of zoledronate-induced cytokine and gammadelta T cell proliferative responses. J. Immunol. 2013, 191, 1346–1355. [Google Scholar] [CrossRef]
- Gertner-Dardenne, J.; Fauriat, C.; Orlanducci, F.; Thibult, M.L.; Pastor, S.; Fitzgibbon, J.; Bouabdallah, R.; Xerri, L.; Olive, D. The co-receptor BTLA negatively regulates human Vgamma9Vdelta2 T-cell proliferation: A potential way of immune escape for lymphoma cells. Blood 2013, 122, 922–931. [Google Scholar] [CrossRef]
- Tsuda, J.; Li, W.; Yamanishi, H.; Yamamoto, H.; Okuda, A.; Kubo, S.; Ma, Z.; Terada, N.; Tanaka, Y.; Okamura, H. Involvement of CD56brightCD11c+ cells in IL-18-mediated expansion of human gammadelta T cells. J. Immunol. 2011, 186, 2003–2012. [Google Scholar] [CrossRef]
- Li, W.; Kubo, S.; Okuda, A.; Yamamoto, H.; Ueda, H.; Tanaka, T.; Nakamura, H.; Yamanishi, H.; Terada, N.; Okamura, H. Effect of IL-18 on expansion of gammadelta T cells stimulated by zoledronate and IL-2. J. Immunother. 2010, 33, 287–296. [Google Scholar] [CrossRef]
- Domae, E.; Hirai, Y.; Ikeo, T.; Goda, S.; Shimizu, Y. Cytokine-mediated activation of human ex vivo-expanded Vgamma9Vdelta2 T cells. Oncotarget 2017, 8, 45928–45942. [Google Scholar] [CrossRef]
- Provine, N.M.; Binder, B.; FitzPatrick, M.E.; Schuch, A.; Garner, L.C.; Williamson, K.D.; van Wilgenburg, B.; Thimme, R.; Klenerman, P.; Hofmann, M. Unique and Common Features of Innate-Like Human Vdelta2(+) gammadeltaT Cells and Mucosal-Associated Invariant T Cells. Front. Immunol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, C.; Patzl, M.; Saalmuller, A.; Gerner, W. IL-12 and IL-18 induce interferon-gamma production and de novo CD2 expression in porcine gammadelta T cells. Dev. Comp. Immunol. 2014, 47, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Liong, K.H.; Gunalan, M.G.; Li, N.; Lim, D.S.; Fisher, D.A.; MacAry, P.A.; Leo, Y.S.; Wong, S.C.; Puan, K.J.; et al. Type I IFNs and IL-18 regulate the antiviral response of primary human gammadelta T cells against dendritic cells infected with Dengue virus. J. Immunol. 2015, 194, 3890–3900. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, W.; Pan, M.; Scully, E.; Girardi, M.; Augenlicht, L.H.; Craft, J.; Yin, Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J. Exp. Med. 2003, 198, 433–442. [Google Scholar] [CrossRef]
- Inatsuka, C.; Yang, Y.; Gad, E.; Rastetter, L.; Disis, M.L.; Lu, H. Gamma delta T cells are activated by polysaccharide K (PSK) and contribute to the anti-tumor effect of PSK. Cancer Immunol. Immunother. 2013, 62, 1335–1345. [Google Scholar] [CrossRef]
- Ramstead, A.G.; Jutila, M.A. Complex role of gammadelta T-cell-derived cytokines and growth factors in cancer. J. Interf. Cytokine Res. 2012, 32, 563–569. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, N.; Cui, L.; Ba, D.; He, W. Anti-gammadelta TCR antibody-expanded gammadelta T cells: A better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol. Immunol. 2012, 9, 34–44. [Google Scholar] [CrossRef]
- Todaro, M.; Meraviglia, S.; Caccamo, N.; Stassi, G.; Dieli, F. Combining conventional chemotherapy and gammadelta T cell-based immunotherapy to target cancer-initiating cells. Oncoimmunology 2013, 2, e25821. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, B.L.; Gehrs, B.C.; Nan, L.; Lopez, R.D. Ex vivo expanded human Vgamma9Vdelta2+ gammadelta-T cells mediate innate antitumor activity against human prostate cancer cells in vitro. J. Urol. 2005, 173, 1552–1556. [Google Scholar] [CrossRef]
- Chen, L.; He, W.; Kim, S.T.; Tao, J.; Gao, Y.; Chi, H.; Intlekofer, A.M.; Harvey, B.; Reiner, S.L.; Yin, Z.; et al. Epigenetic and transcriptional programs lead to default IFN-gamma production by gammadelta T cells. J. Immunol. 2007, 178, 2730–2736. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, C.; Szabo, S.J.; Glimcher, L.H.; Ray, A.; Craft, J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J. Immunol. 2002, 168, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Kannan, Y.; Yu, J.; Raices, R.M.; Seshadri, S.; Wei, M.; Caligiuri, M.A.; Wewers, M.D. IkappaBzeta augments IL-12- and IL-18-mediated IFN-gamma production in human NK cells. Blood 2011, 117, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Satoh, T.; Kato, H.; Matsushita, K.; Kumagai, Y.; Vandenbon, A.; Tani, T.; Muta, T.; Akira, S.; Takeuchi, O. IkappaBzeta is essential for natural killer cell activation in response to IL-12 and IL-18. Proc. Natl. Acad. Sci. USA 2010, 107, 17680–17685. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Cao, W.; Xi, X.; Ma, C.; Cui, L.; He, W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood 2009, 114, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.T.; Ribot, J.C.; Silva-Santos, B. Five Layers of Receptor Signaling in gammadelta T-Cell Differentiation and Activation. Front. Immunol. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Rhinehart, R.; Randolph-Habecker, J.; Topp, M.S.; Riddell, S.R.; Spies, T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001, 2, 255–260. [Google Scholar] [CrossRef]
- Rincon-Orozco, B.; Kunzmann, V.; Wrobel, P.; Kabelitz, D.; Steinle, A.; Herrmann, T. Activation of V gamma 9V delta 2 T cells by NKG2D. J. Immunol. 2005, 175, 2144–2151. [Google Scholar] [CrossRef]
- Woo, S.R.; Corrales, L.; Gajewski, T.F. Innate immune recognition of cancer. Annu. Rev. Immunol. 2015, 33, 445–474. [Google Scholar] [CrossRef]
- Honda, S.-I.; Sakamoto, Y.; Fujime, M.; Kitagawa, R. Immunohistochemical Study of Tumor-Infiltrating Lymphocytes Before and After Intravesical Bacillus Calmette-Guérin Treatment for Superficial Bladder Cancer. Int. J. Urol. 1997, 4, 68–73. [Google Scholar] [CrossRef]
- Braumüller, H.; Wieder, T.; Brenner, E.; Aßmann, S.; Hahn, M.; Alkhaled, M.; Schilbach, K.; Essmann, F.; Kneilling, M.; Griessinger, C.; et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013, 494, 361–365. [Google Scholar] [CrossRef]
- Hidalgo, J.V.; Bronsert, P.; Orlowska-Volk, M.; Diaz, L.B.; Stickeler, E.; Werner, M.; Schmitt-Graeff, A.; Kayser, G.; Malkovsky, M.; Fisch, P. Histological Analysis of gammadelta T Lymphocytes Infiltrating Human Triple-Negative Breast Carcinomas. Front. Immunol. 2014, 5, 632. [Google Scholar] [CrossRef] [PubMed]
- Meraviglia, S.; Lo Presti, E.; Tosolini, M.; La Mendola, C.; Orlando, V.; Todaro, M.; Catalano, V.; Stassi, G.; Cicero, G.; Vieni, S.; et al. Distinctive features of tumor-infiltrating gammadelta T lymphocytes in human colorectal cancer. Oncoimmunology 2017, 6, e1347742. [Google Scholar] [CrossRef] [PubMed]
- Kakimi, K.; Matsushita, H.; Murakawa, T.; Nakajima, J. Gammadelta T cell therapy for the treatment of non-small cell lung cancer. Transl. Lung Cancer Res. 2014, 3, 23–33. [Google Scholar] [PubMed]
- Cordova, A.; Toia, F.; La Mendola, C.; Orlando, V.; Meraviglia, S.; Rinaldi, G.; Todaro, M.; Cicero, G.; Zichichi, L.; Donni, P.L.; et al. Characterization of human gammadelta T lymphocytes infiltrating primary malignant melanomas. PLoS ONE 2012, 7, e49878. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Anguille, S.; Van Tendeloo, V.F.; Lion, E. Empowering gamma delta T cells with antitumor immunity by dendritic cell-based immunotherapy. Oncoimmunology 2015, 4, e1021538. [Google Scholar] [CrossRef]
- Ribot, J.C.; Ribeiro, S.T.; Correia, D.V.; Sousa, A.E.; Silva-Santos, B. Human gammadelta thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J. Immunol. 2014, 192, 2237–2243. [Google Scholar] [CrossRef]
- Gatenby, R.A. A change of strategy in the war on cancer. Nature 2009, 459, 508–509. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Althubiti, M.; Lezina, L.; Carrera, S.; Jukes-Jones, R.; Giblett, S.M.; Antonov, A.; Barlev, N.; Saldanha, G.S.; Pritchard, C.A.; Cain, K.; et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 2014, 5, e1528. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Correia, D.V.; Fernandes-Platzgummer, A.; da Silva, C.L.; da Silva, M.G.; Anjos, D.R.; Silva-Santos, B. Delta One T cells for immunotherapy of chronic lymphocytic leukemia: Clinical-grade expansion/ differentiation and preclinical proof-of-concept. Clin. Cancer Res. 2016, 22, 5795–5804. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Anderson, J. Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front. Immunol. 2018, 9, 1409. [Google Scholar] [CrossRef]
- Handgretinger, R.; Schilbach, K. The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood 2018, 131, 1063–1072. [Google Scholar] [CrossRef]
- Schilbach, K.; Krickeberg, N.; Kaißer, C.; Mingram, S.; Kind, J.; Siegers, G.M.; Hashimoto, H. The suppressive activity of Vδ2+ γδ T cells on αβ T cells is licensed by TCR signaling and correlates with signal strength. Cancer Immunol. Immunother. Accepted 28 December 2019.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilbach, K.; Welker, C.; Krickeberg, N.; Kaißer, C.; Schleicher, S.; Hashimoto, H. In the Absence of a TCR Signal IL-2/IL-12/18-Stimulated γδ T Cells Demonstrate Potent Anti-Tumoral Function Through Direct Killing and Senescence Induction in Cancer Cells. Cancers 2020, 12, 130. https://doi.org/10.3390/cancers12010130
Schilbach K, Welker C, Krickeberg N, Kaißer C, Schleicher S, Hashimoto H. In the Absence of a TCR Signal IL-2/IL-12/18-Stimulated γδ T Cells Demonstrate Potent Anti-Tumoral Function Through Direct Killing and Senescence Induction in Cancer Cells. Cancers. 2020; 12(1):130. https://doi.org/10.3390/cancers12010130
Chicago/Turabian StyleSchilbach, Karin, Christian Welker, Naomi Krickeberg, Carlotta Kaißer, Sabine Schleicher, and Hisayoshi Hashimoto. 2020. "In the Absence of a TCR Signal IL-2/IL-12/18-Stimulated γδ T Cells Demonstrate Potent Anti-Tumoral Function Through Direct Killing and Senescence Induction in Cancer Cells" Cancers 12, no. 1: 130. https://doi.org/10.3390/cancers12010130
APA StyleSchilbach, K., Welker, C., Krickeberg, N., Kaißer, C., Schleicher, S., & Hashimoto, H. (2020). In the Absence of a TCR Signal IL-2/IL-12/18-Stimulated γδ T Cells Demonstrate Potent Anti-Tumoral Function Through Direct Killing and Senescence Induction in Cancer Cells. Cancers, 12(1), 130. https://doi.org/10.3390/cancers12010130




