An Outcome Assessment of a Single Institution’s Longitudinal Experience with Uveal Melanoma Patients with Liver Metastasis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Groups Generation and Data Collection
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L. Intraocular Tumors: An. Atlas and Textbook; Wolters Kluwer Health: Philadelphia, PA, USA, 2016. [Google Scholar]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973–2013). Ocul. Oncol. Pathol. 2018, 4, 145–151. [Google Scholar] [CrossRef]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmolg Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Hawkins, B.S.; Hayman, J.A.; Jaiyesimi, I.; Jampol, L.M.; et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch. Ophthalmol. 2005, 123, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, J.J.; Correa, Z.M.; Shaikh, A.H. Effectiveness of treatments for metastatic uveal melanoma. Am. J. Ophthalmol. 2009, 148, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kuk, D.; Shoushtari, A.N.; Barker, C.A.; Panageas, K.S.; Munhoz, R.R.; Momtaz, P.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bogatch, K.; et al. Prognosis of Mucosal, Uveal, Acral, Nonacral Cutaneous, and Unknown Primary Melanoma from the Time of First Metastasis. Oncologist 2016, 21, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Survival Rates in Patients After Treatment for Metastasis from Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981–986. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology 2013, 120, 2066–2071. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer Classification of Uveal Melanoma (Anatomic Stage) Predicts Prognosis in 7731 Patients: The 2013 Zimmerman Lecture. Ophthalmology 2015, 122, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L. Management of posterior uveal melanoma: Past, present, and future: The 2014 Charles L. Schepens lecture. Ophthalmology 2015, 122, 414–428. [Google Scholar] [CrossRef]
- Bedikian, A.Y.; Legha, S.S.; Mavligit, G.; Carrasco, C.H.; Khorana, S.; Plager, C.; Papadopoulos, N.; Benjamin, R.S. Treatment of uveal melanoma metastatic to the liver: A review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer 1995, 76, 1665–1670. [Google Scholar] [CrossRef]
- Bedikian, A.Y. Metastatic uveal melanoma therapy: Current options. Int. Ophthalmol. Clin. 2006, 46, 151–166. [Google Scholar] [CrossRef]
- Schank, T.E.; Hassel, J.C. Immunotherapies for the Treatment of Uveal Melanoma-History and Future. Cancers 2019, 11, 1048. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Sosman, J.A.; Quevedo, J.F.; Milhem, M.M.; Joshua, A.M.; Kudchadkar, R.R.; Linette, G.P.; Gajewski, T.F.; Lutzky, J.; Lawson, D.H.; et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. JAMA 2014, 311, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Buder, K.; Gesierich, A.; Gelbrich, G.; Goebeler, M. Systemic treatment of metastatic uveal melanoma: Review of literature and future perspectives. Cancer Med. 2013, 2, 674–686. [Google Scholar] [CrossRef]
- Khoja, L.; Atenafu, E.G.; Joshua, A.M.; International Rare Cancer’s Intiative-Ocular Melanoma Group. Meta-analysis of phase II trials in metastatic uveal melanoma (MUM) to determine progression-free (PFS) and overall survival (OS) benchmarks for future phase II trials: An irci-ocular melanoma initiative. J. Clin. Oncol. 2016, 34, 9567. [Google Scholar] [CrossRef]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilholz, U.; Zimmer, L.; Patel, S.P.; Piperno-Neumann, S.; et al. Meta-Analysis in Metastatic Uveal Melanoma to Determine Progression-Free and Overall Survival Benchmarks: An International Rare Cancers Initiative (IRCI) Ocular Melanoma study. Ann. Oncol. 2019. [Google Scholar] [CrossRef]
- Johnson, D.B.; Daniels, A.B. Continued Poor Survival in Metastatic Uveal Melanoma: Implications for Molecular Prognostication, Surveillance Imaging, Adjuvant Therapy, and Clinical Trials. JAMA Ophthalmol. 2018, 136, 986–988. [Google Scholar] [CrossRef]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Ellebaek, E.; Hoejberg, L.; Bagger, M.M.; Larsen, M.S.; Klausen, T.W.; Kohler, U.H.; Schmidt, H.; Bastholt, L.; Kiilgaard, J.F.; et al. Real-World Impact of Immune Checkpoint Inhibitors in Metastatic Uveal Melanoma. Cancers 2019, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Amaral, T.; Kahler, K.C.; Heinzerling, L.; Hassel, J.C.; Meissner, M.; Kreuzberg, N.; Loquai, C.; Reinhardt, L.; Utikal, J.; et al. Combined immune checkpoint blockade for metastatic uveal melanoma: A retrospective, multi-center study. J. Immunother. Cancer 2019, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kampgen, E.; et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.P.; Olza, M.O.D.; Codes, M.; Lopez-Martin, J.A.; Berrocal, A.; García, M.; Gurpide, A.; Homet, B.; Martin-Algarra, S. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): The GEM-1 trial. J. Clin. Oncol. 2014, 32, 9033. [Google Scholar] [CrossRef]
- Piulats Rodriguez, J.M.; De La Cruz Merino, L.; Espinosa, E.; Alonso Carrión, L.; Martin Algarra, S.; López-Castro, R.; Curiel García, M.T.; Rodriguez Abreu, D.; Rullan Iriarte, A.J.; Berrocal Jaime, A. Phase II multicenter, single arm, open label study of nivolumab in combination with ipilimumab in untreated patients with metastatic uveal melanoma (GEM1402.NCT02626962). Ann Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Croce, M.; Ferrini, S.; Pfeffer, U.; Gangemi, R. Targeted Therapy of Uveal Melanoma: Recent Failures and New Perspectives. Cancers 2019, 11, 846. [Google Scholar] [CrossRef]
- Steeb, T.; Wessely, A.; Ruzicka, T.; Heppt, M.V.; Berking, C. How to MEK the best of uveal melanoma: A systematic review on the efficacy and safety of MEK inhibitors in metastatic or unresectable uveal melanoma. Eur. J. Cancer 2018, 103, 41–51. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination with Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef]
- Damato, B.E.; Dukes, J.; Goodall, H.; Carvajal, R.D. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers 2019, 11, 971. [Google Scholar] [CrossRef]
- Middleton, M.R.; Steven, N.M.; Evans, T.J.; Infante, J.R.; Sznol, M.; Mulatero, C.; Hamid, O.; Shoushtari, A.N.; Shingler, W.; Johnson, A.; et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: Results from the FIH study in melanoma. J. Clin. Oncol. 2016, 34, 3016. [Google Scholar] [CrossRef]
- Sato, T.; Nathan, P.D.; Hernandez-Aya, L.; Sacco, J.J.; Orloff, M.M.; Visich, J.; Little, N.; Hulstine, A.M.; Coughlin, C.M.; Carvajal, R.D. Redirected T cell lysis in patients with metastatic uveal melanoma with gp100-directed TCR IMCgp100: Overall survival findings. J. Clin. Oncol. 2018, 36, 9521. [Google Scholar] [CrossRef]
- Sato, T.; Eschelman, D.J.; Gonsalves, C.F.; Terai, M.; Chervoneva, I.; McCue, P.A.; Shields, J.A.; Shields, C.L.; Yamamoto, A.; Berd, D.; et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J. Clin. Oncol. 2008, 26, 5436–5442. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Chervoneva, I.; Sullivan, K.L.; Eschelman, D.J.; Gonsalves, C.F.; Mastrangelo, M.J.; Berd, D.; Shields, J.A.; Shields, C.L.; Terai, M.; et al. High-dose immunoembolization: Survival benefit in patients with hepatic metastases from uveal melanoma. Radiology 2009, 252, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.E.; Terai, M.; Eschelman, D.J.; Gonsalves, C.F.; Chervoneva, I.; Shields, J.A.; Shields, C.L.; Yamamoto, A.; Sullivan, K.L.; Laudadio, M.; et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J. Vasc. Interv. Radiol. 2015, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Sullivan, K.; Berd, D.; Mastrangelo, M.J.; Shields, C.L.; Shields, J.A.; Sato, T. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: Results of a phase II study. Melanoma Res. 2005, 15, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, C.F.; Eschelman, D.J.; Thornburg, B.; Frangos, A.; Sato, T. Uveal Melanoma Metastatic to the Liver: Chemoembolization With 1,3-Bis-(2-Chloroethyl)-1-Nitrosourea. Am. J. Roentgenol. 2015, 205, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, C.F.; Eschelman, D.J.; Sullivan, K.L.; Anne, P.R.; Doyle, L.; Sato, T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: A single-institution experience. Am. J. Roentgenol. 2011, 196, 468–473. [Google Scholar] [CrossRef]
- Rostas, J.; Tam, A.; Sato, T.; Kelly, L.; Tatum, C.; Scoggins, C.; McMasters, K.; Martin, R.C. Image-Guided Transarterial Chemoembolization with Drug-Eluting Beads Loaded with Doxorubicin (DEBDOX) for Unresectable Hepatic Metastases from Melanoma: Technique and Outcomes. Cardiovasc. Interv. Radiol. 2017, 40, 1392–1400. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.; Kivela, T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 561–568. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Egan, K.M.; Seddon, J.M.; Glynn, R.J.; Walsh, S.M.; Finn, S.M.; Munzenrider, J.E.; Spar, M.D. Survival of patients with metastases from uveal melanoma. Ophthalmology 1991, 98, 383–389. [Google Scholar] [CrossRef]
- Jochems, A.; van der Kooij, M.K.; Fiocco, M.; Schouwenburg, M.G.; Aarts, M.J.; van Akkooi, A.C.; van den Berkmortel, F.; Blank, C.U.; van den Eertwegh, A.J.M.; Franken, M.G.; et al. Metastatic Uveal Melanoma: Treatment Strategies and Survival-Results from the Dutch Melanoma Treatment Registry. Cancers 2019, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.C.; Pulido, J.S.; Dronca, R.S.; McWilliams, R.R.; Markovic, S.N.; Mansfield, A.S. The Mayo Clinic experience with the use of kinase inhibitors, ipilimumab, bevacizumab, and local therapies in the treatment of metastatic uveal melanoma. Melanoma Res. 2015, 25, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Itchins, M.; Ascierto, P.A.; Menzies, A.M.; Oatley, M.; Lo, S.; Douraghi-Zadeh, D.; Harrington, T.; Maher, R.; Grimaldi, A.M.; Guminski, A. A multireferral centre retrospective cohort analysis on the experience in treatment of metastatic uveal melanoma and utilization of sequential liver-directed treatment and immunotherapy. Melanoma Res. 2017, 27, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Babazono, A.; Shields, J.A.; Shields, C.L.; De Potter, P.; Mastrangelo, M.J. Time to systemic metastases in patients with posterior uveal melanoma. Cancer Investig. 1997, 15, 98–105. [Google Scholar] [CrossRef]
- Mavligit, G.M.; Charnsangavej, C.; Carrasco, C.H.; Patt, Y.Z.; Benjamin, R.S.; Wallace, S. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA 1988, 260, 974–976. [Google Scholar] [CrossRef]
- Martinussen, T.; Scheike, T.H. Dynamic Regression Models for Survival Data; Springer: New York, NY, USA, 2006; p. 205. [Google Scholar]
- The R Foundation. The R Project for Statistical Computing. Available online: http://www.R-project.org (accessed on 30 December 2019).
| Characteristic | Cohort 1 (1971–1993) | Cohort 2 (1998–2007) | Cohort 3 (2008–2017) | p-Value | ||||
| N | % | N | % | N | % | |||
| Gender | Male | 41 | 51% | 94 | 47% | 250 | 55% | 0.176 |
| Female | 39 | 49% | 104 | 53% | 202 | 45% | ||
| AJCC Classification by T category | T 1 | 12 | 15% | 19 | 10% | 57 | 13% | 0.283 |
| T 2 | 22 | 28% | 36 | 18% | 108 | 24% | ||
| T 3 | 25 | 31% | 51 | 26% | 151 | 33% | ||
| T 4 | 7 | 9% | 12 | 6% | 73 | 16% | ||
| Unknown | 14 | 18% | 80 | 40% | 63 | 14% | ||
| Tumor location | Choroid | 56 | 70% | 89 | 45% | 286 | 63% | 0.764 |
| Ciliary | 23 | 29% | 33 | 17% | 110 | 24% | ||
| Iris | 1 | 1% | 0 | 0% | 2 | 0% | ||
| Unknown | 0 | 0% | 76 | 38% | 54 | 12% | ||
| First Treatment for Eye Tumor | Enucleation | 46 | 58% | 52 | 26% | 104 | 23% | <0.001 |
| Radioactive plaque | 25 | 31% | 114 | 58% | 302 | 67% | ||
| Other | 9 | 11% | 31 | 16% | 43 | 10% | ||
| Unknown | 0 | 0% | 1 | 1% | 3 | 1% | ||
| Adjuvant Treatment | No | 75 | 94% | 193 | 97% | 385 | 85% | <0.001 |
| Yes (not sutent) | 5 | 6% | 5 | 3% | 17 | 4% | ||
| Yes (sutent) | 0 | 0% | 0 | 0% | 50 | 11% | ||
| Treatment for Liver Metastasis (Treatment 1+2) | Systemic alone | 56 | 70% | 4 | 2% | 9 | 2% | <0.001 |
| Liver-directed alone | 16 | 20% | 139 | 70% | 251 | 56% | ||
| Liver-directed + Systemic | 8 | 10% | 55 | 28% | 192 | 42% | ||
| Characteristic | Min. | 1st Qu. | Median | Mean | 3rd Qu | Max. | p-Value | |
| Age at Eye Diagnosis | Cohort 1 | 22 | 51 | 60 | 57 | 66 | 84 | 0.005 |
| Cohort 2 | 19 | 44 | 53 | 53 | 62 | 85 | ||
| Cohort 3 | 18 | 46 | 57 | 55 | 65 | 88 | ||
| Months from Diagnosis to Metastasis | Cohort 1 | 1.0 | 24.6 | 35.9 | 46.9 | 61.3 | 211.2 | 0.005 |
| Cohort 2 | 2.0 | 23.3 | 41.7 | 62.8 | 87.3 | 309.2 | ||
| Cohort 3 | 0.3 | 15.7 | 35.6 | 48.9 | 65.6 | 330.7 | ||
| Treatment Group | Treatment 1 | Treatment 2 | |
|---|---|---|---|
| Cohort 1 (N = 80) | Systemic alone | 56 | 11 |
| Liver-directed alone | 24 | 0 | |
| L+S Concurrent | 0 | 0 | |
| Cohort 2 (N = 198) | Systemic alone | 19 | 39 |
| Liver-directed alone | 178 | 105 | |
| L+S Concurrent | 1 | 2 | |
| Cohort 3 (N = 452) | Systemic alone | 54 | 81 |
| Liver-directed alone | 333 | 248 | |
| L+S Concurrent | 65 | 23 | |
| Overall Survival Rates from Liver Metastasis | |||||||||
| Post-Mets | Cohort 1 | Cohort 2 | Cohort 3 | ||||||
| Year | Alive | LL95% CI | UL95% CI | Alive | LL95% CI | UL95% CI | Alive | LL95% CI | UL95% CI |
| 1 | 0.23 | 0.15 | 0.34 | 0.59 | 0.52 | 0.66 | 0.67 | 0.63 | 0.72 |
| 2 | 0.08 | 0.03 | 0.16 | 0.28 | 0.22 | 0.35 | 0.35 | 0.31 | 0.40 |
| 3 | 0 | - | - | 0.14 | 0.10 | 0.19 | 0.18 | 0.14 | 0.22 |
| 4 | 0 | - | - | 0.08 | 0.05 | 0.13 | 0.11 | 0.08 | 0.15 |
| 5 | 0 | - | - | 0.05 | 0.02 | 0.09 | 0.07 | 0.04 | 0.11 |
| Overall Survival Rates from Eye Treatment | |||||||||
| Post-Tx | Cohort 1 | Cohort 2 | Cohort 3 | ||||||
| Year | Alive | LL95% CI | UL95% CI | Alive | LL95% CI | UL95% CI | Alive | LL95% CI | UL95% CI |
| 2 | 0.84 | 0.76 | 0.92 | 0.91 | 0.88 | 0.95 | 0.88 | 0.85 | 0.91 |
| 4 | 0.45 | 0.35 | 0.57 | 0.62 | 0.55 | 0.69 | 0.63 | 0.58 | 0.68 |
| 6 | 0.26 | 0.18 | 0.38 | 0.42 | 0.36 | 0.49 | 0.40 | 0.35 | 0.45 |
| 8 | 0.11 | 0.06 | 0.21 | 0.31 | 0.25 | 0.38 | 0.27 | 0.23 | 0.32 |
| 10 | 0.05 | 0.02 | 0.13 | 0.23 | 0.18 | 0.30 | 0.21 | 0.17 | 0.25 |
| Overall Survival from Liver Mets to Death | |||||
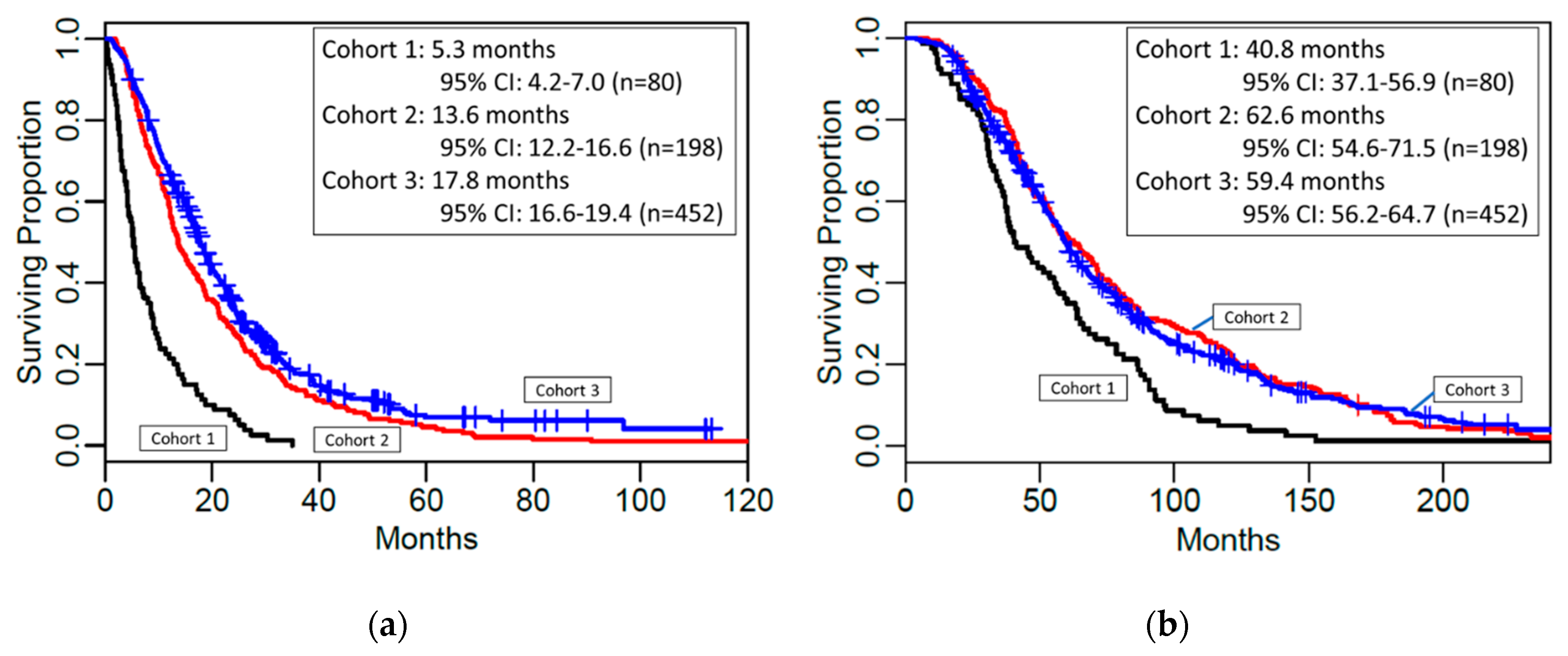
| Cohort | No. patients | No. events | Median OS | LL95% CI | UL95% CI |
| 1 | 80 | 80 | 5.3 | 4.2 | 7.0 |
| 2 | 198 | 197 | 13.6 | 12.2 | 16.6 |
| 3 | 452 | 374 | 17.8 | 16.6 | 19.4 |
| Overall Survival from Eye Tx to Death | |||||
| Cohort | No. patients | No. events | Median OS | LL95% CI | UL95% CI |
| 1 | 80 | 80 | 40.8 | 37.1 | 56.9 |
| 2 | 198 | 197 | 62.6 | 54.6 | 71.5 |
| 3 | 452 | 374 | 59.4 | 56.2 | 64.7 |
| Cohort 1 (N = 80, 80 Events) | ||||
| Comparison | Hazard Ratio (#) | LL95% CI | UL95% CI | p-value |
| Eye Treatment to Metastasis Time (*) | 0.83 | 0.70 | 0.97 | 0.022 |
| Liver-directed only vs. Systemic | 0.81 | 0.45 | 1.46 | 0.483 |
| Liver-directed + Systemic vs. Liver-directed only | 0.35 | 0.14 | 0.86 | 0.023 |
| Liver-directed + Systemic vs. Systemic | 0.28 | 0.12 | 0.65 | 0.003 |
| Cohort 2 (N = 194, Number of Events = 193, 4 Patients with Systemic only Tx Excluded) | ||||
| Comparison | Hazard Ratio (#) | LL95% CI | UL95% CI | p-value |
| Female vs. Male | 0.65 | 0.48 | 0.87 | 0.003 |
| Liver-directed + Systemic vs. Liver-directed only | 0.58 | 0.42 | 0.80 | 0.001 |
| Cohort 3 (N = 443, Number of Events = 365, 9 Patients with Systemic only Tx Excluded) | ||||
| Comparison | Hazard Ratio (#) | LL95% CI | UL95% CI | p-value |
| Female vs. Male | 0.75 | 0.61 | 0.93 | 0.003 |
| Age 60+ vs. < 60 | 1.32 | 1.07 | 1.64 | 0.003 |
| Liver-directed + Systemic vs. Liver-directed only | <0.001 (&) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seedor, R.S.; Eschelman, D.J.; Gonsalves, C.F.; Adamo, R.D.; Orloff, M.; Amjad, A.; Sharpe-Mills, E.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; et al. An Outcome Assessment of a Single Institution’s Longitudinal Experience with Uveal Melanoma Patients with Liver Metastasis. Cancers 2020, 12, 117. https://doi.org/10.3390/cancers12010117
Seedor RS, Eschelman DJ, Gonsalves CF, Adamo RD, Orloff M, Amjad A, Sharpe-Mills E, Chervoneva I, Shields CL, Shields JA, et al. An Outcome Assessment of a Single Institution’s Longitudinal Experience with Uveal Melanoma Patients with Liver Metastasis. Cancers. 2020; 12(1):117. https://doi.org/10.3390/cancers12010117
Chicago/Turabian StyleSeedor, Rino S., David J. Eschelman, Carin F. Gonsalves, Robert D. Adamo, Marlana Orloff, Anjum Amjad, Erin Sharpe-Mills, Inna Chervoneva, Carol L. Shields, Jerry A. Shields, and et al. 2020. "An Outcome Assessment of a Single Institution’s Longitudinal Experience with Uveal Melanoma Patients with Liver Metastasis" Cancers 12, no. 1: 117. https://doi.org/10.3390/cancers12010117
APA StyleSeedor, R. S., Eschelman, D. J., Gonsalves, C. F., Adamo, R. D., Orloff, M., Amjad, A., Sharpe-Mills, E., Chervoneva, I., Shields, C. L., Shields, J. A., Mastrangelo, M. J., & Sato, T. (2020). An Outcome Assessment of a Single Institution’s Longitudinal Experience with Uveal Melanoma Patients with Liver Metastasis. Cancers, 12(1), 117. https://doi.org/10.3390/cancers12010117






