Heat Shock Proteins and Ovarian Cancer: Important Roles and Therapeutic Opportunities
Abstract
1. Introduction
1.1. Ovarian Cancer Is a Serious Problem in Gynaecological Oncology
1.2. Heat Shock Proteins (HSPs) Are Multifamily Chaperones Implicated in Several Malignancies
2. Biological Functions of HSPs in Healthy and Diseased Ovaries
3. Heat Shock Factor 1 (HSF1) in Ovarian Cancer
Targeting HSF1 in Ovarian Cancer
4. HSPs in Ovarian Cancer
4.1. HSPC (HSP90) Family
4.1.1. HSP90 and Therapeutic Resistance
4.1.2. Targeting HSP90 in OC
4.1.3. Diagnostic and Prognostic Value of HSP90 in OC
4.2. HSPA (HSP70) Family
4.2.1. Therapeutic Resistance and Targeting of HSP70 in OC
4.2.2. Diagnostic and Prognostic Value of HSP70 in OC
4.3. HSPD and HSPE (Chaperonin) Family
4.3.1. HSP60 and Therapeutic Resistance
4.3.2. Targeting HSP60 in OC
4.4. DNAJ (HSP40) Family
4.5. Small Heat Shock Proteins (sHSPs)
Therapeutic Resistance and Targeting of sHSPs in OC
4.6. Clusterin
4.6.1. CLU as a Prognostic Biomarker in OC
4.6.2. Targeting CLU in OC
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIF-1 | Apoptosis - inducing factor-1 |
| Akt | Serine/threonine-protein kinase |
| APC | Adenomatous polyposis coli protein |
| AR | Androgen receptor |
| ASK1 | Apoptotic signal-regulated kinase 1 |
| ATPase | Adenosine Triphosphatase |
| Bax | BCL2 - associated X protein |
| Cas 3 | Caspase 3 |
| CHIP | Carboxyl terminus of the heat shock cognate protein 70-interacting protein |
| c-KIT | CD117, also called KIT or C-kit receptor |
| Cyt C | Cytochrome c |
| EGFR | Epidermal growth factor receptor |
| ELISA | Enzyme-linked immunosorbent assay |
| ElK | ETS Like-1 protein |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| FADD | Fas-associated protein with death domain |
| Fas | Apoptosis antigen 1 |
| GRP94 | Glucose regulated protein |
| GSK-3β | Glycogen synthase kinase 3 |
| HSP | Heat shock protein |
| IκB kinas | Inhibitor of NFκB |
| IL | Interleukin |
| IFNγ | Interferon gamma |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal Kinase |
| LEF | Lymphoid enhancer factor |
| MEK | MAPK/ERK kinase |
| p53 | protein 53 or tumor protein 53 |
| PARP | Poly (ADP-ribose) polymerase |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| Pro-cas | Pro-caspase |
| Raf | Rapidly accelerated fibrosarcoma, retroviral oncogen |
| Ras | Rat sarcoma, cancer associated membrane protein |
| SMAC | Second mitochondria-derived activator of caspase |
| STAT3 | Signal Transducers and Activator of Transcription |
| SVV | Survivin |
| TNFα, | Tumor necrosis factor α |
| TRAIL | TNF - related apoptosis - inducing ligand |
| VEGF | Vascular endothelial growth factor receptor |
| EGFR | Epidermal growth factor receptor |
| IGFR | Insulin-like growth factor receptor |
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Boil. Med. 2017, 14, 9–32. [Google Scholar]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Bast, R.C. Minireview: Human Ovarian Cancer: Biology, Current Management, and Paths to Personalizing Therapy. Endocrinology 2012, 153, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C.; Urban, N.; Shridhar, V.; Smith, D.; Zhang, Z.; Skates, S.; Lu, K.; Liu, J.; Fishman, D.; Mills, G. Early Detection of Ovarian Cancer: Promise and Reality. Cancer Treat. Res. 2002, 107, 61–97. [Google Scholar] [PubMed]
- Stope, M.; Koensgen, D.; Burchardt, M.; Concin, N.; Zygmunt, M.; Mustea, A.; Information, P.E.K.F.C. Jump in the fire—Heat shock proteins and their impact on ovarian cancer therapy. Crit. Rev. Oncol. 2016, 97, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Anastasia, P.J. Ovarian Cancer: Current Treatment and Patient Management. J. Adv. Pract. Oncol. 2016, 7, 271–273. [Google Scholar] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. ICON7 Investigators A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef]
- Mullen, M.M.; Kuroki, L.M.; Thaker, P.H. Novel treatment options in platinum-sensitive recurrent ovarian cancer: A review. Gynecol. Oncol. 2019, 152, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, X.; Li, W.; Bai, H.; Zhang, Z. PARP inhibitors in ovarian cancer: Sensitivity prediction and resistance mechanisms. J. Cell. Mol. Med. 2019, 23, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Chekerov, R.; Hilpert, F.; Mahner, S.; El-Balat, A.; Harter, P.; De Gregorio, N.; Fridrich, C.; Markmann, S.; Potenberg, J.; Lorenz, R.; et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 1247–1258. [Google Scholar] [CrossRef]
- Cohen, M.; Dromard, M.; Petignat, P. Heat shock proteins in ovarian cancer: A potential target for therapy. Gynecol. Oncol. 2010, 119, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 1996, 381, 571–580. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef]
- Mogk, A.; Bukau, B.; Kampinga, H.H. Cellular Handling of Protein Aggregates by Disaggregation Machines. Mol. Cell 2018, 69, 214–226. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Boil. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Whitesell, L.; Rogers, A.B.; Lindquist, S. Heat Shock Factor 1 Is a Powerful Multifaceted Modifier of Carcinogenesis. Cell 2007, 130, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Arrigo, A.P.; Calderwood, S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013, 87, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef]
- Jee, H. Size dependent classification of heat shock proteins: A mini-review. J. Exerc. Rehabil. 2016, 12, 255–259. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Jego, G.; Hazoume, A.; Seigneuric, R.; Garrido, C. Targeting heat shock proteins in cancer. Cancer Lett. 2013, 332, 275–285. [Google Scholar] [CrossRef]
- Samali, A.; Cai, J.; Zhivotovsky, B.; Jones, D.P.; Orrenius, S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999, 18, 2040–2048. [Google Scholar] [CrossRef]
- Xanthoudakis, S.; Roy, S.; Rasper, D.; Hennessey, T.; Aubin, Y.; Cassady, R.; Tawa, P.; Ruel, R.; Rosen, A.; Nicholson, D.W. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999, 18, 2049–2056. [Google Scholar] [CrossRef]
- Kennedy, D.; Jäger, R.; Mosser, D.D.; Samali, A. Regulation of apoptosis by heat shock proteins. IUBMB Life 2014, 66, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Li, G.; Hideshima, T.; Podar, K.; Mitsiades, C.; Mitsiades, N.; Catley, L.; Tai, Y.T.; Hayashi, T.; Shringarpure, R.; et al. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood 2003, 102, 3379–3386. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat Shock Proteins 27 and 70: Anti-Apoptotic Proteins with Tumorigenic Properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.-P.; Gibert, B. HspB1 dynamic phospho-oligomeric structure dependent interactome as cancer therapeutic target. Curr. Mol. Med. 2012, 12, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, E.M.; Marchenko, N.D. Mutant p53—Heat Shock Response Oncogenic Cooperation: A New Mechanism of Cancer Cell Survival. Front. Endocrinol. 2015, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Iwakuma, T. Regulators of Oncogenic Mutant TP53 Gain of Function. Cancers 2018, 11, 4. [Google Scholar] [CrossRef]
- Wawrzynow, B.; Zylicz, A.; Zylicz, M. Chaperoning the guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2018, 1869, 161–174. [Google Scholar] [CrossRef]
- Azad, A.A.; Zoubeidi, A.; Gleave, M.E.; Chi, K.N. Targeting heat shock proteins in metastatic castration-resistant prostate cancer. Nat. Rev. Urol. 2015, 12, 26–36. [Google Scholar] [CrossRef]
- Hoter, A.; Rizk, S.; Naim, H.Y. The Multiple Roles and Therapeutic Potential of Molecular Chaperones in Prostate Cancer. Cancers 2019, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Guo, K.; Wang, N.; Jin, H.; Liu, Y.; Qin, W. Heat shock proteins in hepatocellular carcinoma: Molecular mechanism and therapeutic potential. Int. J. Cancer 2016, 138, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Dingemans, A.-M.C. Heat shock protein antagonists in early stage clinical trials for NSCLC. Expert Opin. Investig. Drugs 2017, 26, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Ogata, K.; Altan, B.; Yokobori, T.; Mochiki, E.; Yanai, M.; Kogure, N.; Yanoma, T.; Suzuki, M.; Bai, T.; et al. Nuclear heat shock protein 110 expression is associated with poor prognosis and hyperthermo-chemotherapy resistance in gastric cancer patients with peritoneal metastasis. World J. Gastroenterol. 2017, 23, 7541–7550. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, H.; Burrows, F.; Shores, C.G. Potent Activity of a Novel Dimeric Heat Shock Protein 90 Inhibitor against Head and Neck Squamous Cell Carcinoma In vitro and In vivo. Clin. Cancer Res. 2005, 11, 3889–3896. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.C.; Dohi, T.; Kang, B.H.; Altieri, D.C. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008, 283, 5188–5194. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperon 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Wang, J.; Cui, S.; Zhang, X.; Wu, Y.; Tang, H. High Expression of Heat Shock Protein 90 Is Associated with Tumor Aggressiveness and Poor Prognosis in Patients with Advanced Gastric Cancer. PLoS ONE 2013, 8, e62876. [Google Scholar] [CrossRef]
- Narayanankutty, V.; Narayanankutty, A.; Nair, A. Heat Shock Proteins (HSPs): A Novel Target for Cancer Metastasis Prevention. Curr. Drug Targets 2019, 20, 727–737. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Bolhassani, A. Heat-shock proteins in diagnosis and treatment: An overview of different biochemical and immunological functions. Immunotherapy 2019, 11, 215–239. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Bauer, M. Heat shock proteins in porcine ovary: Synthesis, accumulation and regulation by stress and hormones. Cell Stress Chaperones 2011, 16, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Maizels, E.T.; Cottom, J.; Jones, J.C.R.; Hunzicker-Dunn, M. Follicle Stimulating Hormone (FSH) Activates the p38 Mitogen-Activated Protein Kinase Pathway, Inducing Small Heat Shock Protein Phosphorylation and Cell Rounding in Immature Rat Ovarian Granulosa Cells. Endocrinology 1998, 139, 3353–3356. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, N.R.; Mazzini, R.A.; Taboada, A.F.; Ortega, H.H.; Acosta, J.C.; Gimeno, E.J.; Müller, L.A. Estrogen Receptors α and β and Progesterone Receptors in Normal Bovine Ovarian Follicles and Cystic Ovarian Disease. Veter.-Pathol. 2007, 44, 373–378. [Google Scholar] [CrossRef] [PubMed]
- D’Haeseleer, M.; Van Poucke, M.; Broeck, W.V.D. Cell-specific Localization of Oestrogen Receptor beta (ESR2) mRNA within Various Bovine Ovarian Cell Types Using In situ Hybridization. Anat. Histol. Embryol. 2005, 34, 265–272. [Google Scholar] [CrossRef]
- Stope, M.B.; Sauermann, A.; Rönnau, C.; Zimmermann, U.; Walther, R.; Burchardt, M. Androgen receptor and heat shock proteins in progression of prostate cancer cells. Int. J. Clin. Pharmacol. Ther. 2012, 50, 65–67. [Google Scholar] [CrossRef]
- Salvetti, N.; Baravalle, C.; Mira, G.; Gimeno, E.; Dallard, B.; Rey, F.; Ortega, H. Heat Shock Protein 70 and Sex Steroid Receptors in the Follicular Structures of Induced Ovarian Cysts. Reprod. Domest. Anim. 2009, 44, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Boil. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef]
- Khanna, A.; Aten, R.F.; Behrman, H.R. Heat shock protein induction blocks hormone-sensitive steroidogenesis in rat luteal cells. Steroids 1994, 59, 4–9. [Google Scholar] [CrossRef]
- Khanna, A.; Aten, R.F.; Behrman, H.R. Physiological and pharmacological inhibitors of luteinizing hormone-dependent steroidogenesis induce heat shock protein-70 in rat luteal cells. Endocrinology 1995, 136, 1775–1781. [Google Scholar] [CrossRef]
- Koshiyama, M.; Konishi, I.; Nanbu, K.; Nanbu, Y.; Mandai, M.; Komatsu, T.; Yamamoto, S.; Mori, T.; Fujii, S. Immunohistochemical localization of heat shock proteins HSP70 and HSP90 in the human endometrium: Correlation with sex steroid receptors and Ki-67 antigen expression. J. Clin. Endocrinol. Metab. 1995, 80, 1106–1112. [Google Scholar]
- Isobe, N.; Yoshimura, Y. Deficient proliferation and apoptosis in the granulosa and theca interna cells of the bovine cystic follicle. J. Reprod. Dev. 2007, 53, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Konishi, I.; Mandai, M.; Komatsu, T.; Yamamoto, S.; Nanbu, K.; Mori, T. Immunohistochemical analysis of p53 protein and 72 kDa heat shock protein (HSP72) expression in ovarian carcinomas. Virchows Arch. 1995, 425, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.M.; Alfaro, N.S.; Dupuy, C.R.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Heat shock protein patterns in the bovine ovary and relation with cystic ovarian disease. Anim. Reprod. Sci. 2010, 118, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, M.M.; Alfaro, N.S.; Salvetti, N.R.; Stangaferro, M.L.; Rey, F.; Panzani, C.G.; Ortega, H.H. Levels of heat shock protein transcripts in normal follicles and ovarian follicular cysts. Reprod. Boil. 2011, 11, 276–283. [Google Scholar] [CrossRef]
- Juliani, C.; Silva-Zacarin, E.; Santos, D.; Boer, P. Effects of atrazine on female Wistar rats: Morphological alterations in ovarian follicles and immunocytochemical labeling of 90kDa heat shock protein. Micron 2008, 39, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Cockrem, J.F.; Han, K.-H.; Kim, D.-H.; Jung, M.-H.; Chu, J.-P. Stress-induced activation of ovarian heat shock protein 90 in a rat model of polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2012, 38, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mo, H.; Zhang, J.; Zhou, Y.; Peng, X.; Luo, X. The Role of Heat Shock Protein 90B1 in Patients with Polycystic Ovary Syndrome. PLoS ONE 2016, 11, e0152837. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Dai, S.; Cao, J. Proteotoxic stress of cancer: Implication of the heat-shock response in oncogenesis. J. Cell. Physiol. 2012, 227, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Dai, C. The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: From proteomic stability to oncogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef]
- Jiang, S.; Tu, K.; Fu, Q.; Schmitt, D.C.; Zhou, L.; Lu, N.; Zhao, Y. Multifaceted roles of HSF1 in cancer. Tumor Boil. 2015, 36, 4923–4931. [Google Scholar] [CrossRef]
- Mendillo, M.L.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 2012, 150, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Home, T.; Jensen, R.A.; Rao, R. Heat Shock Factor 1 in Protein Homeostasis and Oncogenic Signal Integration. Cancer Res. 2015, 75, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chang, R.; Yang, L. Heat shock factor 1 promotes invasion and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer 2012, 118, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.D.; Paullin, T.R.; Aoisa, C.; Menzie, C.J.; Ubaldini, A.; Westerheide, S.D. The Heat Shock Transcription Factor HSF1 Induces Ovarian Cancer Epithelial-Mesenchymal Transition in a 3D Spheroid Growth Model. PLoS ONE 2016, 11, e0168389. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Wang, S.-Y.; Wang, L.; Chen, Y.-F.; Zheng, J.; Liu, T. Targeting HSF1 leads to an antitumor effect in human epithelial ovarian cancer. Int. J. Mol. Med. 2017, 39, 1564–1570. [Google Scholar]
- Chen, Y.-F.; Dong, Z.; Xia, Y.; Tang, J.; Peng, L.; Wang, S.; Lai, D. Nucleoside analog inhibits microRNA-214 through targeting heat-shock factor 1 in human epithelial ovarian cancer. Cancer Sci. 2013, 104, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Elpek, G.O.; Karaveli, S.; Simşek, T.; Keles, N.; Aksoy, N.H. Expression of heat-shock proteins hsp27, hsp70 and hsp90 in malignant epithelial tumour of the ovaries. APMIS 2003, 111, 523–530. [Google Scholar] [CrossRef]
- Mileo, A.M.; Fanuele, M.; Battaglia, F.; Scambia, G.; Benedetti-Panici, P.; Mancuso, S.; Ferrini, U. Selective over-expression of mRNA coding for 90 KDa stress-protein in human ovarian cancer. Anticancer Res. 1990, 10, 903–906. [Google Scholar]
- Luo, L.-Y.; Herrera, I.; Soosaipillai, A.; Diamandis, E.P. Identification of heat shock protein 90 and other proteins as tumour antigens by serological screening of an ovarian carcinoma expression library. Br. J. Cancer 2002, 87, 339–343. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Beebe, K.; Neckers, L. Impact of heat-shock protein 90 on cancer metastasis. Futur. Oncol. 2009, 5, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Koga, F.; Kihara, K.; Neckers, L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer Res. 2009, 29, 797–807. [Google Scholar] [PubMed]
- Maloney, A.; Clarke, P.A.; Naaby-Hansen, S.; Stein, R.; Koopman, J.-O.; Akpan, A.; Yang, A.; Zvelebil, M.; Cramer, R.; Stimson, L.; et al. Gene and Protein Expression Profiling of Human Ovarian Cancer Cells Treated with the Heat Shock Protein 90 Inhibitor 17-Allylamino-17-Demethoxygeldanamycin. Cancer Res. 2007, 67, 3239–3253. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.R.; Matassa, D.S.; Sisinni, L.; Lettini, G.; Landriscina, M.; Esposito, F. TRAP1 revisited: Novel localizations and functions of a “next-generation” biomarker (Review). Int. J. Oncol. 2014, 45, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Matassa, D.S.; Agliarulo, I.; Avolio, R.; Landriscina, M.; Esposito, F. TRAP1 Regulation of Cancer Metabolism: Dual Role as Oncogene or Tumor Suppressor. Genes 2018, 9, 195. [Google Scholar] [CrossRef]
- Lettini, G.; Maddalena, F.; Sisinni, L.; Condelli, V.; Matassa, D.S.; Costi, M.P.; Simoni, D.; Esposito, F.; Landriscina, M. TRAP1: A viable therapeutic target for future cancer treatments? Expert Opin. Ther. Targets 2017, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matassa, D.S.; Amoroso, M.R.; Lu, H.; Avolio, R.; Arzeni, D.; Procaccini, C.; Faicchia, D.; Maddalena, F.; Simeon, V.; Agliarulo, I.; et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016, 23, 1542–1554. [Google Scholar] [CrossRef]
- Aust, S.; Bachmayr-Heyda, A.; Pateisky, P.; Tong, D.; Darb-Esfahani, S.; Denkert, C.; Chekerov, R.; Sehouli, J.; Mahner, S.; Van Gorp, T.; et al. Role of TRAP1 and estrogen receptor alpha in patients with ovarian cancer—A study of the OVCAD consortium. Mol. Cancer 2012, 11, 69. [Google Scholar] [CrossRef]
- Amoroso, M.R.; Matassa, D.S.; Agliarulo, I.; Avolio, R.; Lu, H.; Sisinni, L.; Lettini, G.; Gabra, H.; Landriscina, M.; Esposito, F. TRAP1 downregulation in human ovarian cancer enhances invasion and epithelial–mesenchymal transition. Cell Death Dis. 2016, 7, e2522. [Google Scholar] [CrossRef]
- Amoroso, M.R.; Matassa, D.S.; Agliarulo, I.; Avolio, R.; Maddalena, F.; Condelli, V.; Landriscina, M.; Esposito, F. Stress-Adaptive Response in Ovarian Cancer Drug Resistance: Role of TRAP1 in Oxidative Metabolism-Driven Inflammation. Adv. Protein Chem. Struct. Biol. 2017, 108, 163–198. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Alvero, A.B.; Chen, R.; Fu, H.-H.; Montagna, M.; Schwartz, P.E.; Rutherford, T.; Silasi, D.-A.; Steffensen, K.D.; Waldstrom, M.; Visintin, I.; et al. Molecular phenotyping of human ovarian cancer stem cells unravel the mechanisms for repair and chemo-resistance. Cell Cycle 2009, 8, 158–166. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, K.; Mullen, P.; Sewell, J.; Rabiasz, G.; Lawrie, S.; Miller, E.; Smyth, J.F.; Langdon, S.P. Altered ErbB Receptor Signaling and Gene Expression in Cisplatin-Resistant Ovarian Cancer. Cancer Res. 2005, 65, 6789–6800. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Amoroso, M.R.; Piscazzi, A.; Esposito, F. Heat shock proteins, cell survival and drug resistance: The mitochondrial chaperone TRAP1, a potential novel target for ovarian cancer therapy. Gynecol. Oncol. 2010, 117, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, F.; Serebriiskii, I.G.; O’Brien, S.W.; Maglaty, M.A.; Astsaturov, I.; Litwin, S.; Martin, L.P.; Proia, D.A.; Golemis, E.A.; et al. Network analysis identifies an HSP90-central hub susceptible in ovarian cancer. Clin. Cancer Res. 2013, 19, 5053–5067. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, S.A.; Myung, S.C.; Kim, W.; Lee, C.S. Radicicol, an inhibitor of Hsp90, enhances TRAIL-induced apoptosis in human epithelial ovarian carcinoma cells by promoting activation of apoptosis-related proteins. Mol. Cell. Biochem. 2012, 359, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Marchenko, N.D.; Moll, U.M. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ. 2011, 18, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Marchenko, N.D.; Schulz, R.; Fischer, V.; Velasco-Hernandez, T.; Talos, F.; Moll, U.M. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol. Cancer Res. 2011, 9, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [CrossRef]
- Jiao, Y.; Ou, W.; Meng, F.; Zhou, H.; Wang, A. Targeting HSP90 in ovarian cancers with multiple receptor tyrosine kinase coactivation. Mol. Cancer 2011, 10, 125. [Google Scholar] [CrossRef]
- Abbasi, F.; Marchion, D.; Xiong, Y.; Bush, S.; Al Sawah, E.; Al Rubaish, S.; Ramirez, I.; Zgheib, N.B.; Wenham, R.; Lancaster, J. HSP90 inhibition decreases ovarian cancer cell proliferation and potentiates platinum sensitivity. Gynecol. Oncol. 2014, 133, 122–123. [Google Scholar] [CrossRef]
- Kramer, D.; Stark, N.; Schulz-Heddergott, R.; Erytch, N.; Edmunds, S.; Roßmann, L.; Bastians, H.; Concin, N.; Moll, U.M.; Dobbelstein, M. Strong antitumor synergy between DNA crosslinking and HSP90 inhibition causes massive premitotic DNA fragmentation in ovarian cancer cells. Cell Death Differ. 2017, 24, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Braicu, I.; Berger, R.; Mahner, S.; Sehouli, J.; Zeimet, A.G.; Pujade-Lauraine, E.; Cassier, P.A.; Moll, U.M.; Ulmer, H.; et al. Phase I results of GANNET53: A multicenter phase I/II trial of the Hsp90 inhibitor ganetespib (G) combined with weekly paclitaxel (P) in women with high-grade serous, high-grade endometrioid, or undifferentiated, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer. J. Clin. Oncol. 2016, 34, e17038. [Google Scholar]
- Concin, N.; Braicu, I.; Combe, P.; Ray-Coquard, I.L.; Joly, F.; Harter, P.; Wimberger, P.; Lotz, J.-P.; Ignatov, A.; Schmalfeldt, B.; et al. Phase II results of GANNET53: A European multicenter phase I/randomized II trial of the Hsp90 inhibitor Ganetespib (G) combined with weekly Paclitaxel (P) in women with high-grade serous, high-grade endometrioid, or undifferentiated, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer. J. Clin. Oncol. 2018, 36, 5567. [Google Scholar]
- Nanbu, K.; Konishi, I.; Komatsu, T.; Mandai, M.; Yamamoto, S.; Kuroda, H.; Koshiyama, M.; Mori, T. Expression of heat shock proteins HSP70 and HSP90 in endometrial carcinomas: Correlation with clinicopathology, sex steroid receptor status, and p53 protein expression. Cancer 1996, 77, 330–338. [Google Scholar] [CrossRef]
- Block, M.S.; Maurer, M.J.; Goergen, K.; Kalli, K.R.; Erskine, C.L.; Behrens, M.D.; Oberg, A.L.; Knutson, K.L. Plasma immune analytes in patients with epithelial ovarian cancer. Cytokine 2015, 73, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Niloff, J.M.; Knapp, R.C.; Schaetzl, E.; Reynolds, C.; Bast, R.C. CA125 antigen levels in obstetric and gynecologic patients. Obstet. Gynecol. 1984, 64, 703–707. [Google Scholar]
- Wang, Y.; Cheon, D.-J.; Lu, Z.; Cunningham, S.L.; Chen, C.-M.; Luo, R.Z.; Xing, D.; Orsulic, S.; Bast, R.C.; Behringer, R.R. MUC16 expression during embryogenesis, in adult tissues, and ovarian cancer in the mouse. Differentiation 2008, 76, 1081–1092. [Google Scholar] [CrossRef]
- Canney, P.A.; Moore, M.; Wilkinson, P.M.; James, R.D. Ovarian cancer antigen CA125: A prospective clinical assessment of its role as a tumour marker. Br. J. Cancer 1984, 50, 765–769. [Google Scholar] [CrossRef]
- Elstrand, M.B.; Stavnes, H.T.; Trope, C.G.; Davidson, B. Heat shock protein 90 is a putative therapeutic target in patients with recurrent advanced-stage ovarian carcinoma with serous effusions. Hum. Pathol. 2012, 43, 529–535. [Google Scholar] [CrossRef]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, C.M.; Kleinberg, L.; Davidson, B.; Berner, A.; Gius, D.; Tchabo, N.; Steinberg, S.M.; Kohn, E.C. BAG-4/SODD and Associated Antiapoptotic Proteins Are Linked to Aggressiveness of Epithelial Ovarian Cancer. Clin. Cancer Res. 2007, 13, 6585–6592. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Jagadish, N.; Surolia, A.; Suri, A. Heat shock protein 70-2 (HSP70-2) a novel cancer testis antigen that promotes growth of ovarian cancer. Am. J. Cancer Res. 2017, 7, 1252–1269. [Google Scholar] [PubMed]
- Elstrand, M.B.; Kleinberg, L.; Kohn, E.C.; Trope, C.G.; Davidson, B. Expression and Clinical Role of Antiapoptotic Proteins of the Bag, Heat Shock, and Bcl-2 Families in Effusions, Primary Tumors, and Solid Metastases in Ovarian Carcinoma. Int. J. Gynecol. Pathol. 2009, 28, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.C.; Deocaris, C.C.; Wadhwa, R. Three faces of mortalin: A housekeeper, guardian and killer. Exp. Gerontol. 2007, 42, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, H.; Jiang, Y.; Zuo, J.; Liu, W. Inhibition of mortalin expression reverses cisplatin resistance and attenuates growth of ovarian cancer cells. Cancer Lett. 2013, 336, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.C.; Aida, S.; Yaguchi, T.; Kaur, K.; Wadhwa, R. Activation of Wild Type p53 Function by Its Mortalin-binding, Cytoplasmically Localizing Carboxyl Terminus Peptides. J. Boil. Chem. 2005, 280, 39373–39379. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, L.; Yang, Y.; Han, Y.; Wang, Y.; Liu, W.; Zuo, J. Oncogenic role of mortalin contributes to ovarian tumorigenesis by activating the MAPK–ERK pathway. J. Cell. Mol. Med. 2016, 20, 2111–2121. [Google Scholar] [CrossRef]
- Xu, M.; Jin, T.; Chen, L.; Zhang, X.; Zhu, G.; Wang, Q.; Lin, Z. Mortalin is a distinct bio-marker and prognostic factor in serous ovarian carcinoma. Gene 2019, 696, 63–71. [Google Scholar] [CrossRef]
- Li, S.; Lv, M.; Qiu, S.; Meng, J.; Liu, W.; Zuo, J.; Yang, L. NF-κB p65 promotes ovarian cancer cell proliferation and migration via regulating mortalin. J. Cell. Mol. Med. 2019, 23, 4338–4348. [Google Scholar] [CrossRef]
- Delie, F.; Petignat, P.; Cohen, M. GRP78 Protein Expression in Ovarian Cancer Patients and Perspectives for a Drug-Targeting Approach. J. Oncol. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chinni, S.R.; Falchetto, R.; Gercel-Taylor, C.; Shabanowitz, J.; Hunt, D.F.; Taylor, D.D. Humoral immune responses to cathepsin D and glucose-regulated protein 78 in ovarian cancer patients. Clin. Cancer Res. 1997, 3, 1557–1564. [Google Scholar] [PubMed]
- Shin, B.K.; Wang, H.; Yim, A.M.; Le Naour, F.; Brichory, F.; Jang, J.H.; Zhao, R.; Puravs, E.; Tra, J.; Michael, C.W.; et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J. Biol. Chem. 2003, 278, 7607–7616. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Gercel-Taylor, C.; Parker, L.P. Patient-derived tumor reactive antibodies as diagnostic markers for ovarian cancer. Gynecol. Oncol. 2009, 115, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Kuhn, E.; Bristow, R.E.; Giuntoli, R.L.; Kjær, S.K.; Shih, I.-M.; Roden, R.B. Comparison of Candidate Serologic Markers for Type I and Type II Ovarian Cancer. Gynecol. Oncol. 2011, 122, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Van Hoesen, K.; Meynier, S.; Ribaux, P.; Petignat, P.; Delie, F.; Cohen, M. Circulating GRP78 antibodies from ovarian cancer patients: A promising tool for cancer cell targeting drug delivery system? Oncotarget 2017, 8, 107176–107187. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Zhou, Y.; Wang, Y.; Wang, S.; Zhang, W. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett. 2012, 321, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, W.; Yeung, S.-C.J.; Freedman, R.S.; Kavanagh, J.J.; Verschraegen, C.F. Increased expression of heat shock protein 70 in adherent ovarian cancer and mesothelioma following treatment with manumycin, a farnesyl transferase inhibitor. Anticancer Res. 2002, 22, 665–672. [Google Scholar] [PubMed]
- Court, K.A.; Hatakeyama, H.; Wu, S.Y.; Lingegowda, M.S.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Ju-Seog, L.; Rinaldi, C.; Juan, E.J.; Sood, A.K.; et al. HSP70 inhibition synergistically enhances the effects of magnetic fluid hyperthermia in ovarian cancer. Mol. Cancer Ther. 2017, 16, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Li, P.-L.; Xu, A.; Zhang, X.-C. Involvement of GRP78 in the Resistance of Ovarian Carcinoma Cells to Paclitaxel. Asian Pac. J. Cancer Prev. 2015, 16, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, W.; Dong, H.; Li, Y.; Li, L.; Han, L.; Han, Z.; Wang, S.; Ma, D.; Wang, H. Cisplatin-induced senescence in ovarian cancer cells is mediated by GRP78. Oncol. Rep. 2014, 31, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, R.; Scholz, S.R.; Dahmen, H.; Wegener, A.; Sirrenberg, C.; Musil, D.; Bomke, J.; Eggenweiler, H.-M.; Mayer, M.P.; Bukau, B. Functional Analysis of Hsp70 Inhibitors. PLoS ONE 2013, 8, e78443. [Google Scholar] [CrossRef]
- Prince, T.; Ackerman, A.; Cavanaugh, A.; Schreiter, B.; Juengst, B.; Andolino, C.; Danella, J.; Chernin, M.; Williams, H. Dual targeting of HSP70 does not induce the heat shock response and synergistically reduces cell viability in muscle invasive bladder cancer. Oncotarget 2018, 9, 32702–32717. [Google Scholar] [CrossRef] [PubMed]
- Vydra, N.; Toma, A.; Widlak, W. Pleiotropic Role of HSF1 in Neoplastic Transformation. Curr. Cancer Drug Targets 2014, 14, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Coscia, F.; Watters, K.M.; Curtis, M.; Eckert, M.A.; Chiang, C.Y.; Tyanova, S.; Montag, A.; Lastra, R.R.; Lengyel, E.; Mann, M. Integrative proteomic profiling of ovarian cancer cell lines reveals precursor cell associated proteins and functional status. Nat. Commun. 2016, 7, 12645. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Moon, H.-S.; Chung, H.-W. The expression of FAS-associated factor 1 and heat shock protein 70 in ovarian cancer. Obstet. Gynecol. Sci. 2014, 57, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-X.; Ye, H.; Wang, P.; Li, L.-X.; Zhang, Y.; Zhang, J.-Y. Proteomic-based identification of HSP70 as a tumor-associated antigen in ovarian cancer. Oncol. Rep. 2017, 37, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, B.X.; Xiao, X. Toward Developing Chemical Modulators of Hsp60 as Potential Therapeutics. Front. Mol. Biosci. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Evolution of the chaperonin families (HSP60, HSP 10 and TCP-1) of proteins and the origin of eukaryotic cells. Mol. Microbiol. 1995, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Fares, M.A.; Lund, P.A. Chaperonin 60: A paradoxical, evolutionarily conserved protein family with multiple moonlighting functions. Boil. Rev. 2013, 88, 955–987. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Enns, R.E.; Alcaraz, J.E.; Arboleda, J.; Slamon, D.J.; Howell, S.B. Correlation of the survival of ovarian cancer patients with mRNA expression of the 60-kD heat-shock protein HSP-60. J. Clin. Oncol. 1993, 11, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Hjerpe, E.; Egyhazi, S.; Carlson, J.; Stolt, M.F.; Schedvins, K.; Johansson, H.; Shoshan, M.; Åvall-Lundqvist, E. HSP60 Predicts Survival in Advanced Serous Ovarian Cancer. Int. J. Gynecol. Cancer 2013, 23, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Bodzek, P.; Partyka, R.; Damasiewicz-Bodzek, A. Antibodies against Hsp60 and Hsp65 in the sera of women with ovarian cancer. J. Ovarian Res. 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Akyol, S.; Gercel-Taylor, C.; Reynolds, L.C.; Taylor, D.D. HSP-10 in ovarian cancer: Expression and suppression of T-cell signaling. Gynecol. Oncol. 2006, 101, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Têtu, B.; Popa, I.; Bairati, I.; L’Espérance, S.; Bachvarova, M.; Plante, M.; Harel, F.; Bachvarov, D. Immunohistochemical analysis of possible chemoresistance markers identified by micro-arrays on serous ovarian carcinomas. Mod. Pathol. 2008, 21, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hadid, M.; Wilkes, J.; Elakawi, Z.; Pendyala, L.; Perez, R.; Perez, R. Relationship between heat shock protein 60 (HSP60) mRNA expression and resistance to platinum analogues in human ovarian and bladder carcinoma cell lines. Cancer Lett. 1997, 119, 63–70. [Google Scholar] [CrossRef]
- Fletcher, N.; Memaj, I.; Diamond, M.; Morris, R.; Saed, G. Heat shock protein 60 (HSP60) serves as a potential target for the sensitization of chemoresistant ovarian cancer cells. Gynecol. Oncol. 2018, 149, 72–73. [Google Scholar] [CrossRef]
- Kamishima, T.; Fukuda, T.; Yoshiya, N.; Suzuki, T. Expression and intracellular localization of heat shock proteins in multidrug resistance of a cisplatin resistant human ovarian cancer cell line. Cancer Lett. 1997, 116, 205–211. [Google Scholar] [CrossRef]
- Mayer, M.P.; Gierasch, L.M. Recent advances in the structural and mechanistic aspects of Hsp70 molecular chaperones. J. Boil. Chem. 2018, 294, 2085–2097. [Google Scholar] [CrossRef]
- Laufen, T.; Mayer, M.; Beisel, C.; Klostermeier, D.; Mogk, A.; Reinstein, J.; Bukau, B. Mechanism of regulation of Hsp70 chaperones by DnaJ cochaperones. Proc. Natl. Acad. Sci. USA 1999, 96, 5452–5457. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Sato, S.-I.; Soda, H.; Fukuda, M.; Kawabata, S.; Nakatomi, K.; Shiozawa, K.; Nakamura, Y.-I.; Ohtsuka, K.; Kohno, S. Autoantibody to Heat Shock Protein Hsp40 in Sera of Lung Cancer Patients. Jpn. J. Cancer Res. 2001, 92, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Oka, M.; Yano, Y.; Kanazawa, Y.; Soda, H.; Terada, R.; Yasutake, T.; Nakayama, T.; Shikuwa, S.; Takeshima, F.; et al. Expression of heat shock protein (Hsp) 70 and Hsp 40 in gastric cancer. Cancer Lett. 2003, 198, 219–228. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Isomoto, H.; Oka, M.; Yano, Y.; Soda, H.; Shikuwa, S.; Takeshima, F.; Omagari, K.; Mizuta, Y.; Murase, K.; et al. Expression of Heat Shock Protein (Hsp) 70 and Hsp 40 in Colorectal Cancer. Med. Oncol. 2003, 20, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Ashfaq, R.; Ansari, F.; Muller, C.Y. Immunohistochemical evaluation of heat shock proteins in normal and preinvasive lesions of the cervix. Cancer Lett. 2005, 229, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, V.; Bible, K.C.; Staub, J.; Avula, R.; Lee, Y.K.; Kalli, K.; Huang, H.; Hartmann, L.C.; Kaufmann, S.H.; Smith, D.I. Loss of expression of a new member of the DNAJ protein family confers resistance to chemotherapeutic agents used in the treatment of ovarian cancer. Cancer Res. 2001, 61, 4258–4265. [Google Scholar] [PubMed]
- Basha, E.; O’Neill, H.; Vierling, E. Small heat shock proteins and α-crystallins: Dynamic proteins with flexible functions. Trends Biochem. Sci. 2012, 37, 106–117. [Google Scholar] [CrossRef]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2015, 1854, 291–319. [Google Scholar] [CrossRef]
- Eyles, S.J.; Gierasch, L.M. Nature’s molecular sponges: Small heat shock proteins grow into their chaperone roles. Proc. Natl. Acad. Sci. USA 2010, 107, 2727–2728. [Google Scholar] [CrossRef] [PubMed]
- Zoubeidi, A.; Gleave, M. Small heat shock proteins in cancer therapy and prognosis. Int. J. Biochem. Cell Boil. 2012, 44, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Van De Schootbrugge, C.; Bussink, J.; Span, P.N.; Sweep, F.C.; Grenman, R.; Stegeman, H.; Pruijn, G.J.; Kaanders, J.H.; Boelens, W.C. αB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma. BMC Cancer 2013, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Katsogiannou, M.; Andrieu, C.; Rocchi, P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front. Genet. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed]
- Bakthisaran, R.; Akula, K.K.; Tangirala, R.; Rao, C.M. Phosphorylation of αB-crystallin: Role in stress, aging and patho-physiological conditions. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Wu, J.; Li, W.; Chen, Z.; Yang, L. Progression of the role of CRYAB in signaling pathways and cancers. Onco Targets Ther. 2019, 12, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, T.-T.; Wang, H.-H.; Hong, H.-M.; Yu, A.L.; Feng, H.-P.; Chang, W.-W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res. 2011, 13, R101. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.P.; Geisler, H.E.; Tammela, J.; Miller, G.A.; Wiemann, M.C.; Zhou, Z. A Study of Heat Shock Protein 27 in Endometrial Carcinoma. Gynecol. Oncol. 1999, 72, 347–350. [Google Scholar] [CrossRef]
- Sheng, B.; Qi, C.; Liu, B.; Lin, Y.; Fu, T.; Zeng, Q. Increased HSP27 correlates with malignant biological behavior of non-small cell lung cancer and predicts patient’s survival. Sci. Rep. 2017, 7, 13807. [Google Scholar] [CrossRef]
- Eto, D.; Hisaka, T.; Horiuchi, H.; Uchida, S.; Ishikawa, H.; Kawashima, Y.; Kinugasa, T.; Nakashima, O.; Yano, H.; Okuda, K.; et al. Expression of HSP27 in Hepatocellular Carcinoma. Anticancer Res. 2016, 36, 3775–3779. [Google Scholar]
- Voll, E.A.; Ogden, I.M.; Pavese, J.M.; Huang, X.; Xu, L.; Jovanovic, B.D.; Bergan, R.C. Heat shock protein 27 regulates human prostate cancer cell motility and metastatic progression. Oncotarget 2014, 5, 2648–2663. [Google Scholar] [CrossRef]
- Arts, H.J.; Hollema, H.; Lemstra, W.; Willemse, P.H.; De Vries, E.G.; Kampinga, H.H.; Van Der Zee, A.G. Heat-shock-protein-27(HSP27) expression in ovarian carcinoma: Relation in response to chemotherapy and prognosis. Int. J. Cancer 1999, 84, 234–238. [Google Scholar] [CrossRef]
- Stope, M.B.; Wiegank, L.; Weiss, M.; Diesing, K.; Koensgen, D.; Burchardt, M.; Zygmunt, M.; Mustea, A. Drug-induced Modulation of Heat Shock Protein HSPB1 in an Ovarian Cancer Cell Model. Anticancer Res. 2016, 36, 3321–3327. [Google Scholar] [PubMed]
- Geisler, J.P.; Geisler, H.E.; Tammela, J.; Wiemann, M.C.; Zhou, Z.; Miller, G.A.; Crabtree, W. Heat Shock Protein 27: An Independent Prognostic Indicator of Survival in Patients with Epithelial Ovarian Carcinoma. Gynecol. Oncol. 1998, 69, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.P.; Tammela, J.E.; Manahan, K.J.; Geisler, H.E.; Miller, G.A.; Zhou, Z.; Wiemann, M.C. HSP27 in patients with ovarian carcinoma: Still an independent prognostic indicator at 60 months follow-up. Eur. J. Gynaecol. Oncol. 2004, 25, 165–168. [Google Scholar]
- Langdon, S.P.; Rabiasz, G.J.; Hirst, G.L.; King, R.J.; Hawkins, R.A.; Smyth, J.F.; Miller, W.R. Expression of the heat shock protein HSP27 in human ovarian cancer. Clin. Cancer Res. 1995, 1, 1603–1609. [Google Scholar]
- Schneider, J.; Jimenez, E.; Marenbach, K.; Marx, D.; Meden, H. Co-expression of the MDR1 gene and HSP27 in human ovarian cancer. Anticancer Res. 1998, 18, 2967–2971. [Google Scholar] [PubMed]
- Korneeva, I.; Caputo, T.A.; Witkin, S.S. Cell-free 27 kDa heat shock protein (hsp27) and hsp27-cytochromec complexes in the cervix of women with ovarian or endometrial cancer. Int. J. Cancer 2002, 102, 483–486. [Google Scholar] [CrossRef]
- Olejek, A.; Damasiewicz-Bodzek, A.; Bodzek, P.; Wielkoszyński, T.; Zamłyński, J.; Stołtny, P.; Skutil, M. Concentrations of Antibodies Against Heat Shock Protein 27 in the Sera of Women With Ovarian Carcinoma. Int. J. Gynecol. Cancer 2009, 19, 1516–1520. [Google Scholar] [CrossRef]
- Zhao, M.; Shen, F.; Yin, Y.X.; Yang, Y.Y.; Xiang, D.J.; Chen, Q. Increased Expression of Heat Shock Protein 27 Correlates with Peritoneal Metastasis in Epithelial Ovarian Cancer. Reprod. Sci. 2012, 19, 748–753. [Google Scholar] [CrossRef]
- Zhao, M.; Ding, J.X.; Zeng, K.; Zhao, J.; Shen, F.; Yin, Y.X.; Chen, Q. Heat shock protein 27: A potential biomarker of peritoneal metastasis in epithelial ovarian cancer? Tumour Biol. 2014, 35, 1051–1056. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar] [CrossRef] [PubMed]
- Jakob, U.; Gaestel, M.; Engel, K.; Buchner, J. Small heat shock proteins are molecular chaperones. J. Boil. Chem. 1993, 268, 1517–1520. [Google Scholar]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Large Potentials of Small Heat Shock Proteins. Physiol. Rev. 2011, 91, 1123–1159. [Google Scholar] [CrossRef]
- Qin, H.; Ni, Y.; Tong, J.; Zhao, J.; Zhou, X.; Cai, W.; Liang, J.; Yao, X. Elevated expression of CRYAB predicts unfavorable prognosis in non-small cell lung cancer. Med. Oncol. 2014, 31, 142. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, D.-W.; Lin, H.; Xiong, L.; Liu, Y.; Li, Q.-D.; Ma, J.; Cao, Q.; Chen, R.-J.; Zhu, J.; et al. Alpha B-crystallin is a new prognostic marker for laryngeal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2012, 31, 101. [Google Scholar] [CrossRef] [PubMed]
- Malin, D.; Strekalova, E.; Petrovic, V.; Deal, A.M.; Al Ahmad, A.; Adamo, B.; Miller, C.R.; Ugolkov, A.; Livasy, C.; Fritchie, K.; et al. αB-crystallin: A novel regulator of breast cancer metastasis to the brain. Clin. Cancer Res. 2014, 20, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-K.; Lui, P.C.W.; Tan, P.-H.; Yamaguchi, R.; Moriya, T.; Yu, A.M.C.; Shao, M.-M.; Hliang, T.; Wong, S.-I.; Tse, G.M. Increased alpha-B-crystallin expression in mammary metaplastic carcinomas. Histopathology 2011, 59, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; He, Z.; Hou, N.; Ni, Y.; Xiong, L.; Chen, P. Alpha B-crystallin correlates with poor survival in colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 6056–6063. [Google Scholar]
- Volkmann, J.; Reuning, U.; Rudelius, M.; Häfner, N.; Schuster, T.; Becker, V.; Ros, A.; Weimer, J.; Hilpert, F.; Kiechle, M.; et al. High expression of crystallin αB represents an independent molecular marker for unfavourable ovarian cancer patient outcome and impairs TRAIL- and cisplatin-induced apoptosis in human ovarian cancer cells. Int. J. Cancer 2013, 132, 2820–2832. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Sha, L.; Hou, N.; Zhang, M.; Ma, Q.; Shi, C. High α B-crystallin and p53 co-expression is associated with poor prognosis in ovarian cancer. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef]
- Stronach, E.A.; Sellar, G.C.; Blenkiron, C.; Rabiasz, G.J.; Taylor, K.J.; Miller, E.P.; Massie, C.E.; Al-Nafussi, A.; Smyth, J.F.; Porteous, D.J.; et al. Identification of clinically relevant genes on chromosome 11 in a functional model of ovarian cancer tumor suppression. Cancer Res. 2003, 63, 8648–8655. [Google Scholar] [PubMed]
- Arrigo, A.-P.; Gibert, B. HspB1, HspB5 and HspB4 in Human Cancers: Potent Oncogenic Role of Some of Their Client Proteins. Cancers 2014, 6, 333–365. [Google Scholar] [CrossRef] [PubMed]
- Pai, H.C.; Kumar, S.; Shen, C.-C.; Liou, J.P.; Pan, S.L.; Teng, C.M. MT-4 Suppresses Resistant Ovarian Cancer Growth through Targeting Tubulin and HSP27. PLoS ONE 2015, 10, 0123819. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Roy, G.; Lambert, H.; Chrétien, P.; Landry, J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991, 51, 5245–5252. [Google Scholar] [PubMed]
- Ciocca, D.R.; Fuqua, S.A.; Lock-Lim, S.; Toft, D.O.; Welch, W.J.; McGuire, W.L. Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res. 1992, 52, 3648–3654. [Google Scholar] [PubMed]
- Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Davidson, M.; McGuire, W.P.; Clarke-Pearson, D.L. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar]
- Piccart, M.J.; Bertelsen, K.; James, K.; Cassidy, J.; Mangioni, C.; Simonsen, E.; Stuart, G.; Kaye, S.; Vergote, I.; Blom, R.; et al. Randomized Intergroup Trial of Cisplatin-Paclitaxel Versus Cisplatin-Cyclophosphamide in Women With Advanced Epithelial Ovarian Cancer: Three-Year Results. J. Natl. Cancer Inst. 2000, 92, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Fujiwara, K.; Tanaka, H.; Maehata, K.; Kohno, I. Paclitaxel inhibits expression of heat shock protein 27 in ovarian and uterine cancer cells. Int. J. Gynecol. Cancer 2004, 14, 616–620. [Google Scholar] [CrossRef]
- Owen, S.; Zhao, H.; Dart, A.; Wang, Y.; Ruge, F.; Gao, Y.; Wei, C.; Wu, Y.; Jiang, W.G. Heat shock protein 27 is a potential indicator for response to YangZheng XiaoJi and chemotherapy agents in cancer cells. Int. J. Oncol. 2016, 49, 1839–1847. [Google Scholar] [CrossRef]
- Bazov, J.; Higano, C.S.; Gleave, M.E.; Stewart, P.S.; Hotte, S.J.; Chi, K.N.; Yu, E.Y.; Jacobs, C.; Kollmannsberger, C.; Mukherjee, S.D. A phase I dose-escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann. Oncol. 2016, 27, 1116–1122. [Google Scholar]
- Wilson, M.R.; Easterbrook-Smith, S.B. Clusterin is a secreted mammalian chaperone. Trends Biochem. Sci. 2000, 25, 95–98. [Google Scholar] [CrossRef]
- Rohne, P.; Prochnow, H.; Koch-Brandt, C. The CLU-files: Disentanglement of a mystery. Biomol. Concepts 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Poon, S.; Treweek, T.M.; Wilson, M.R.; Easterbrook-Smith, S.B.; Carver, J.A. Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett. 2002, 513, 259–266. [Google Scholar] [CrossRef]
- Rohne, P.; Prochnow, H.; Wolf, S.; Renner, B.; Koch-Brandt, C. The Chaperone Activity of Clusterin is Dependent on Glycosylation and Redox Environment. Cell. Physiol. Biochem. 2014, 34, 1626–1639. [Google Scholar] [CrossRef] [PubMed]
- Xiu, P.; Dong, X.-F.; Li, X.-P.; Li, J. Clusterin: Review of research progress and looking ahead to direction in hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 8262–8270. [Google Scholar] [CrossRef] [PubMed]
- Choi-Miura, N.H.; Takahashi, Y.; Nakano, Y.; Tobe, T.; Tomita, M. Identification of the Disulfide Bonds in Human Plasma Protein SP-40,40 (Apolipoprotein-J)1. J. Biochem. 1992, 112, 557–561. [Google Scholar] [CrossRef]
- García-Aranda, M.; Téllez, T.; Muñoz, M.; Redondo, M. Clusterin inhibition mediates sensitivity to chemotherapy and radiotherapy in human cancer. Anti-Cancer Drugs 2017, 28, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. Clusterin: A key player in cancer chemoresistance and its inhibition. Onco Targets Ther. 2014, 7, 447–456. [Google Scholar] [CrossRef]
- Zheng, W.; Yao, M.; Qian, Q.; Sai, W.; Qiu, L.; Yang, J.; Wu, W.; Dong, Z.; Yao, D. Oncogenic secretory clusterin in hepatocellular carcinoma: Expression at early staging and emerging molecular target. Oncotarget 2017, 8, 52321–52332. [Google Scholar] [CrossRef]
- Flanagan, L.; Whyte, L.; Chatterjee, N.; Tenniswood, M. Effects of clusterin over-expression on metastatic progression and therapy in breast cancer. BMC Cancer 2010, 10, 107. [Google Scholar] [CrossRef]
- Panico, F.; Rizzi, F.; Fabbri, L.; Bettuzzi, S.; Luppi, F.; Fabbri, L. Clusterin (CLU) and Lung Cancer. Adv. Cancer Res. 2009, 105, 63–76. [Google Scholar] [PubMed]
- Muhammad, L.A.; Saad, F. The role of clusterin in prostate cancer: Treatment resistance and potential as a therapeutic target. Expert Rev. Anticancer Ther. 2015, 15, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xue, T.; Wang, J.; Chen, B.; Lei, Y.; Huang, Y.; Wang, H.; Xin, X. Roles of clusterin in progression, chemoresistance and metastasis of human ovarian cancer. Int. J. Cancer 2009, 125, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.K.; Watari, H.; Christenson, L.; Bettuzzi, S.; Sakuragi, N. Intracellular clusterin negatively regulates ovarian chemoresistance: Compromised expression sensitizes ovarian cancer cells to paclitaxel. Tumor Boil. 2011, 32, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, H.; Liu, Y.; Zhou, J.; He, W.; Quick, C.M.; Xie, D.; Smoller, B.R.; Fan, C.-Y. Epigenetic and immunohistochemical characterization of the Clusterin gene in ovarian tumors. Arch. Gynecol. Obstet. 2013, 287, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Lau, S.H.; Sham, J.S.T.; Wu, Q.-L.; Fang, Y.; Liang, L.-Z.; Che, L.-H.; Zeng, Y.-X.; Guan, X.-Y. Up-regulated expression of cytoplasmic clusterin in human ovarian carcinoma. Cancer 2005, 103, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.K. An association between clusterin over-expression and taxol-resistance in ovarian cancer. Hokkaido J. Med. Sci. 2008, 83, 335–346. [Google Scholar] [PubMed]
- Hassan, M.K.; Watari, H.; Han, Y.; Mitamura, T.; Hosaka, M.; Wang, L.; Tanaka, S.; Sakuragi, N. Clusterin is a potential molecular predictor for ovarian cancer patient’s survival: Targeting Clusterin improves response to paclitaxel. J. Exp. Clin. Cancer Res. 2011, 30, 113. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Yeo, S.G.; Wilson, M.R.; Yerbury, J.J.; Welch, W.R.; Choi, Y.K.; Birrer, M.J.; Mok, S.C.; Wong, K.-K.; Kwong, J.; et al. Clusterin Interacts with Paclitaxel and Confer Paclitaxel Resistance in Ovarian Cancer. Neoplasia 2008, 10, 964. [Google Scholar] [CrossRef]
- Fu, Y.; Lai, Y.; Liu, J.; Liu, X.; You, Z.; Yang, G. Lentivirus-mediated shRNA interference of clusterin blocks proliferation, motility, invasion and cell cycle in the ovarian cancer cells. J. Ovarian Res. 2015, 8, 277. [Google Scholar] [CrossRef][Green Version]
- Lyu, N.; Yao, H.; Xiao, T.; Gao, Y.; Wu, L. Protein levels and its clinical significance of septin-9 and clusterin in peripheral blood of epithelial ovarian cancer patients. Zhonghua Fu Chan Ke Za Zhi 2015, 50, 679–684. [Google Scholar] [PubMed]
- Yang, G.F.; Li, X.M.; Xie, D. Overexpression of Clusterin in Ovarian Cancer is Correlated with Impaired Survival. Int. J. Gynecol. Cancer 2009, 19, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lai, Y.; Wang, Q.; Liu, X.; He, W.; Zhang, H.; Fan, C.; Yang, G. Overexpression of clusterin promotes angiogenesis via the vascular endothelial growth factor in primary ovarian cancer. Mol. Med. Rep. 2013, 7, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, K.; Zhang, W.; Yang, B.; Zhao, C.; Zhang, X.; Zhang, F.; Zhao, L.; Shan, B. Postoperative recurrence of epithelial ovarian cancer patients and chemoresistance related protein analyses. J. Ovarian Res. 2019, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Higano, C.S.; Blumenstein, B.; Ferrero, J.-M.; Reeves, J.; Feyerabend, S.; Gravis, G.; Merseburger, A.S.; Stenzl, A.; Bergman, A.M.; et al. Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): A phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2017, 18, 473–485. [Google Scholar] [CrossRef]
- Laskin, J.J.; Nicholas, G.; Lee, C.; Gitlitz, B.; Vincent, M.; Cormier, Y.; Stephenson, J.; Ung, Y.; Sanborn, R.; Pressnail, B.; et al. Phase I/II Trial of Custirsen (OGX-011), an Inhibitor of Clusterin, in Combination with a Gemcitabine and Platinum Regimen in Patients with Previously Untreated Advanced Non-small Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Siu, L.L.; Hirte, H.; Hotte, S.J.; Knox, J.; Kollmansberger, C.; Gleave, M.; Guns, E.; Powers, J.; Walsh, W.; et al. A Phase I Study of OGX-011, a 2’-Methoxyethyl Phosphorothioate Antisense to Clusterin, in Combination with Docetaxel in Patients with Advanced Cancer. Clin. Cancer Res. 2008, 14, 833–839. [Google Scholar] [CrossRef]
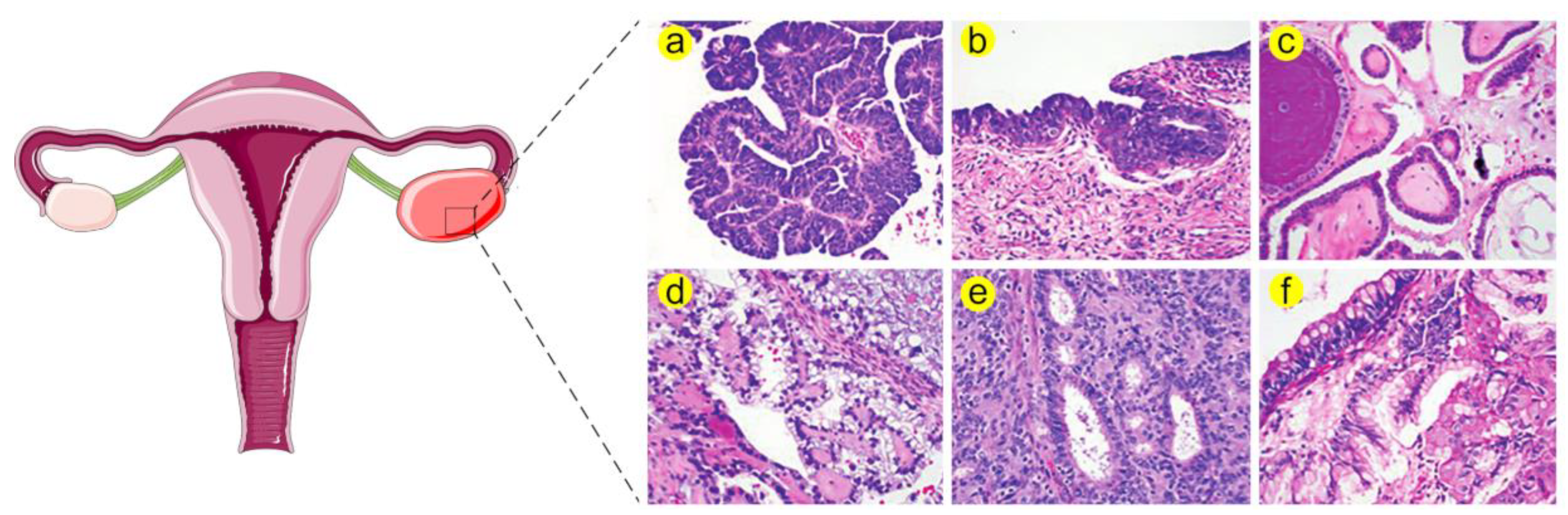
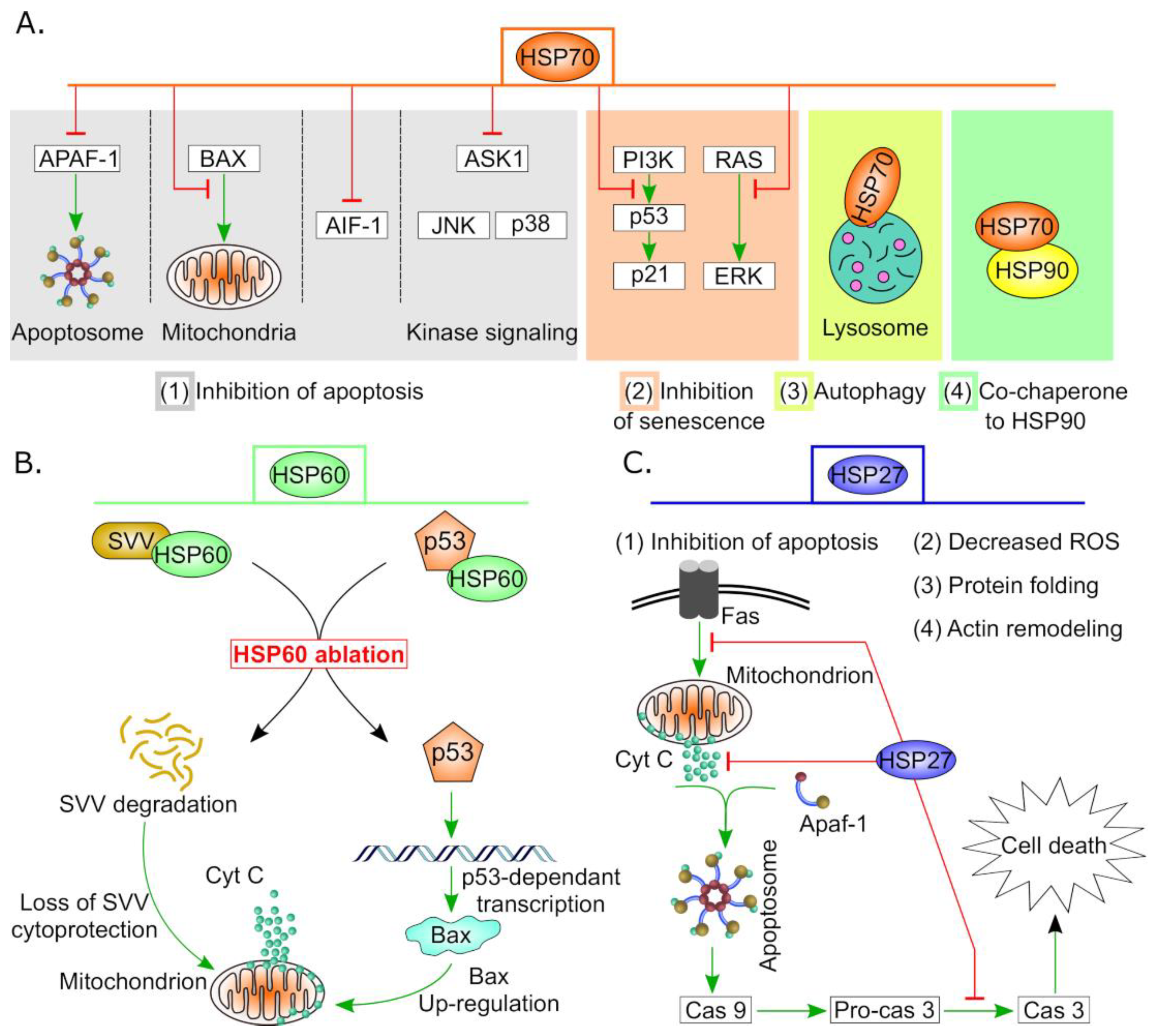
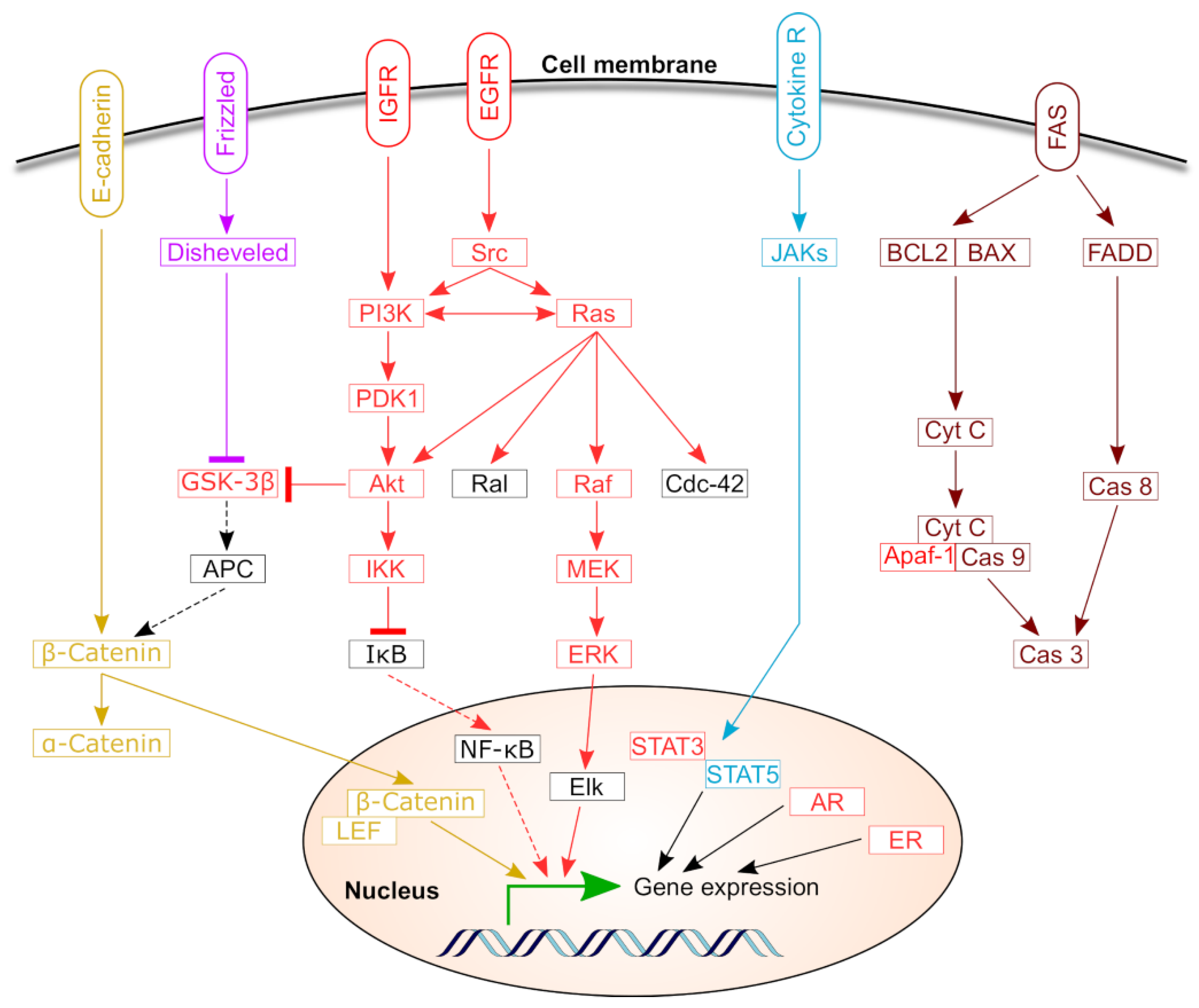
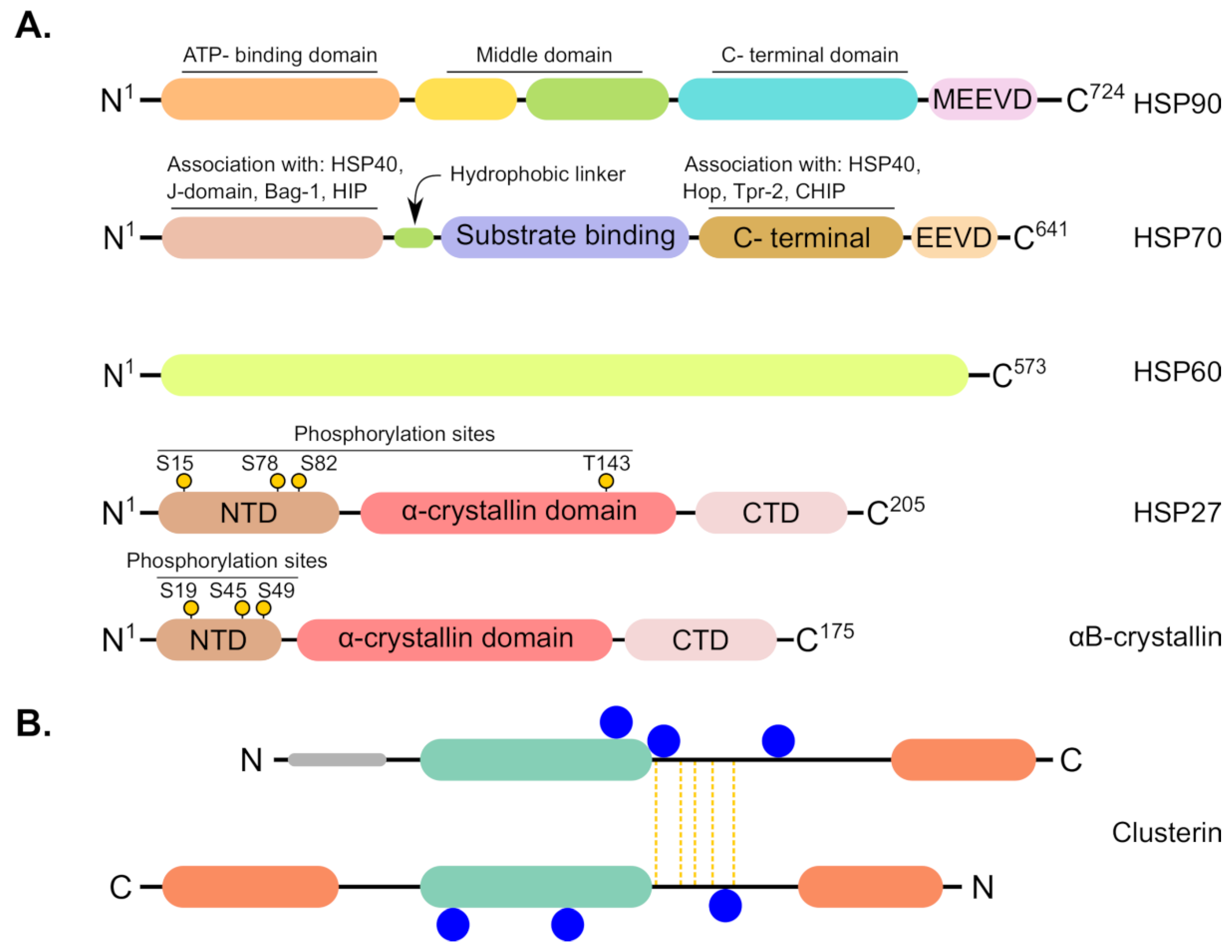
| HSP Family | Recent Name | Number of Members | Common Members and Their Alternative Names |
|---|---|---|---|
| HSP110 | HSPH | 4 | HSPH1 (HSP105) |
| HSPH2 (HSP110, HSPA4 and APG-2) | |||
| HSPH4 (HYOU1/Grp170, ORP150 and HSP12A) | |||
| HSP90 | HSPC | 5 | HSPC2 (HSP90α, HSP90AA2, HSPCA and HSPCAL3) |
| HSPC3 (HSP90β, HSP90AB1, HSPC2, HSPCB, D6S182, HSP90B, FLJ26984) | |||
| HSPC4 (GRP94, HSP90B1, GP96, ECGP, TRA1, endoplasmin) | |||
| HSPC5 (TRAP1, HSP75, HSP90L) | |||
| HSP70 | HSPA | 13 | HSPA1A (HSP70-1, HSP72 and HSPA1) |
| HSPA1B (HSP70-2) | |||
| HSPA5 (BIP, GRP78 and MIF2) | |||
| HSPA6 (Heat shock 70kD protein 6 and HSP70B′) | |||
| HSPA8 (HSC70, HSC71, HSP71 and HSP73) | |||
| HSPA9 (GRP75, HSPA9B, MOT, MOT2, PBP74 and mot-2) | |||
| Chaperonins | HSPD and HSPE | 14 | HSPD1 (HSP60 and GroEL) |
| HSPE1 (HSP10, chaperonin 10 and GroES) | |||
| HSP40 | DNAJ | 50 | DNAJA1 (DJ-2, DjA1, HDJ2, HSDJ, HSJ2, HSPF4 and hDJ-2) |
| DNAJB1 (HSPF1 and HSP40) | |||
| DNAJC1 (MTJ1, ERdj1, ERj1p and Dnajl1) | |||
| sHSPs | HSPB | 11 | HSPB1 (HSP27, HSP28, HSP25, HS.76067, DKFZp586P1322, CMT2F and HMN2B) |
| HSPB4 (CRYAA, crystallin alpha A and CRYA1) | |||
| HSPB5 (CRYAB, crystallin alpha B and CRYA2) |
| Compound Used for HSP90 Targeting | Effect/Mechanism | Reference |
|---|---|---|
| Ganetespib (small molecule inhibitor of HSP90) | -Cell-cycle arrest and induction of apoptosis in vitro. -Delayed growth of orthotopic xenografts and spontaneous ovarian tumors in transgenic mice. -Downregulation and suppression of numerous proteins associated with EOC progression | [95] |
| Ganetespib + paclitaxel | -Paclitaxel potentiated the activity of ganetespib both in cultured cells and tumors. | [95] |
| Ganetespib + siRNAs | -Synergistic effect | [95] |
| Radicicol | -Increased enhanced TRAIL-induced apoptosis-related protein activation, nuclear damage and apoptosis | [96] |
| Suberoylanilide hydroxamic acid (SAHA) | -SAHA is histone deacetylase inhibitor (HDACi) which targets the HSP90/mutant p53 protein complex and liberates mutP35 from the complex leading to its degradation | [97,98] |
| 17-AAG or 17AAG + tyrosine kinase inhibitors | -Marked apoptotic effect was observed in SKOV3, OVCA429 and ES2 cells after using of 17-AAG alone or in combination compared to single tyrosine kinase inhibitor | [100] |
| AUY922 or AUY922 + carboplatin | -The HSP90 inhibitor AUY922 suppressed proliferation of OC cells and decreased carboplatin IC50 | [101] |
| Ganetespib + carboplatin | -Marked synergistic action in terms of cytotoxicity in ovarian tumor cells lacking wild-type p53 | [102] |
| Ganetespib + other anticancer drugs including niraparib, carboplatin, paclitaxel, gemcitabine (ongoing phase II trial) | -This study is known as European Trial on Enhanced DNA Repair Inhibition in Ovarian Cancer (EUDARIO) -It includes women with variant stages of ovarian cancer, fallopian Tube Cancer and primary Peritoneal Carcinoma -The trial started in 30 November 2018 and completion date are expected to be in 30 June 2022 | ClinicalTrials.gov Identifier: NCT03783949 |
| Ganetespib + paclitaxel (GANNET53, completed phase I and phase II trials) | -The addition of ganetespib, HSP90 inhibitor besides weekly paclitaxel did not improve survival in platinum-resistant epithelial ovarian cancer (PROC) patients | ClinicalTrials.gov Identifier: NCT02012192 [103,104] |
| AT13387 + talazoparib (phase I) | -AT13387 is an HSP90 Inhibitor, while talazoparib is a PARP inhibitor | ClinicalTrials.gov Identifier: NCT02627430 |
| Onalespib (AT13387) + olaparib (ongoing phase I trial) | -AT13387 is an HSP90 Inhibitor, whereas olaparib is a PARP inhibitor -The trial started in 19 May 2017 and completion date is expected to be in 1 June 2020 | ClinicalTrials.gov Identifier: NCT02898207 |
| HSP 70 Member | Targeting Approach | Effect | Used Cell Line/Model | Reference |
|---|---|---|---|---|
| HSPA5 (GRP78) | siRNA + paclitaxel | Marked reduction in cell viability | HO-8910 | [130] |
| Knocking down | Rescues senescence sensitivity to cisplatin through P21 and CDC2 | C13K cells | [131] | |
| HSPA6 (HSP70B’) | siRNA and 2-phenylethyenesulfonamide (PES) | Reduction of cell viability following exposure to magnetic fluid hyperthermia (MFH) | A2780 cp20 and HeyA8 | [129] |
| HSPA9 (GRP75 or Mortalin) | shRNA + cisplatin | Decreased cell proliferation, potentiation of cisplatin-induced apoptosis and lowering cell invasion potential | A2780 and A2780 cisplatin resistant cells | [116] |
| sHSP | Targeting Compound | Effect or Mechanism | Used Cell Line/Model | Reference |
|---|---|---|---|---|
| HSP27 | Paclitaxel | Suppression of HSP27 expression concomitant with cell growth inhibition | BG-1 ovarian cancer cells and HeLa uterine cancer cells | [198] |
| Apatorsen (OGX-427) | The OGX-427, antisense inhibitor targeting HSP 27, caused marked reduction of CA-125 in a dose dependent manner | Phase I trial (OC patients) | [200] | |
| YangZheng XiaoJi (traditional Chinese herbal medicine) | Increasing sensitivity of cancer cells to chemotherapeutics via modulating phospho-HSP27 levels | A2780 and A2780-CP70, SKOV3 and COV504 | [199] | |
| MT-4 | Inhibition of tubulin polymerization and induction of apoptosis via hindering HSP27/caspase 3 interaction | A2780 and multidrug- resistant NCI-ADR/res human OC cell lines | [193] | |
| CRYAB | None (CRYAB effect was proofed in vitro via overexpression) | Resistance of TRAIL- and cisplatin-induced apoptosis | OV-MZ-6 and HEY cells as well as tumor tissues from patients with OC | [189] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoter, A.; Naim, H.Y. Heat Shock Proteins and Ovarian Cancer: Important Roles and Therapeutic Opportunities. Cancers 2019, 11, 1389. https://doi.org/10.3390/cancers11091389
Hoter A, Naim HY. Heat Shock Proteins and Ovarian Cancer: Important Roles and Therapeutic Opportunities. Cancers. 2019; 11(9):1389. https://doi.org/10.3390/cancers11091389
Chicago/Turabian StyleHoter, Abdullah, and Hassan Y. Naim. 2019. "Heat Shock Proteins and Ovarian Cancer: Important Roles and Therapeutic Opportunities" Cancers 11, no. 9: 1389. https://doi.org/10.3390/cancers11091389
APA StyleHoter, A., & Naim, H. Y. (2019). Heat Shock Proteins and Ovarian Cancer: Important Roles and Therapeutic Opportunities. Cancers, 11(9), 1389. https://doi.org/10.3390/cancers11091389






