Health-Related Quality of Life during Chemoradiation in Locally Advanced Rectal Cancer: Impacts and Ethnic Disparities
Abstract
1. Introduction
- (1)
- Assess HRQoL before, during and after neoadjuvant chemoradiation.
- (2)
- Assess the association between patient-reported HRQoL domains and corresponding clinician-reported adverse events (CR-AEs) and Eastern Cooperative Oncology Group (ECOG) performance status.
- (3)
- To assess disparities between the Asian and Caucasian population.
- (4)
- To assess whether HRQoL domain scores differ by tumour response as measured by the TRG.
2. Methods
3. Statistical Methods
4. Results
4.1. Study Population
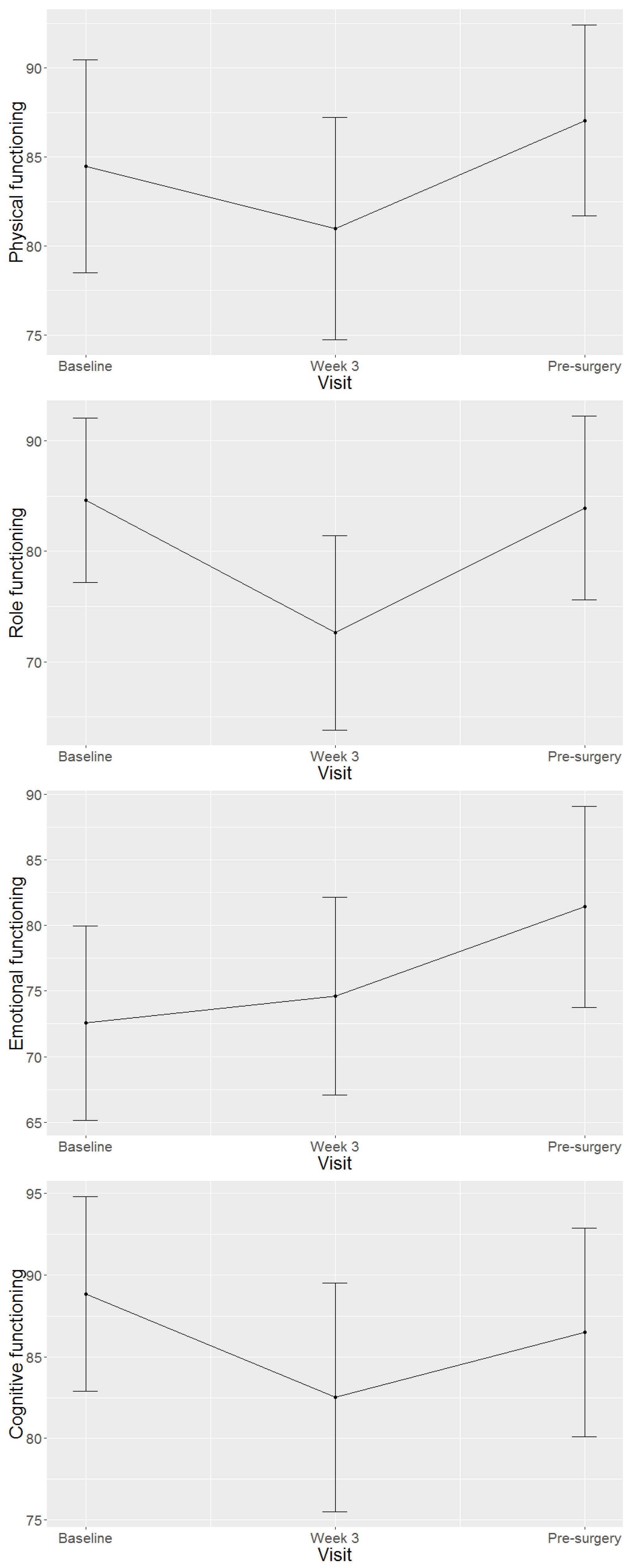
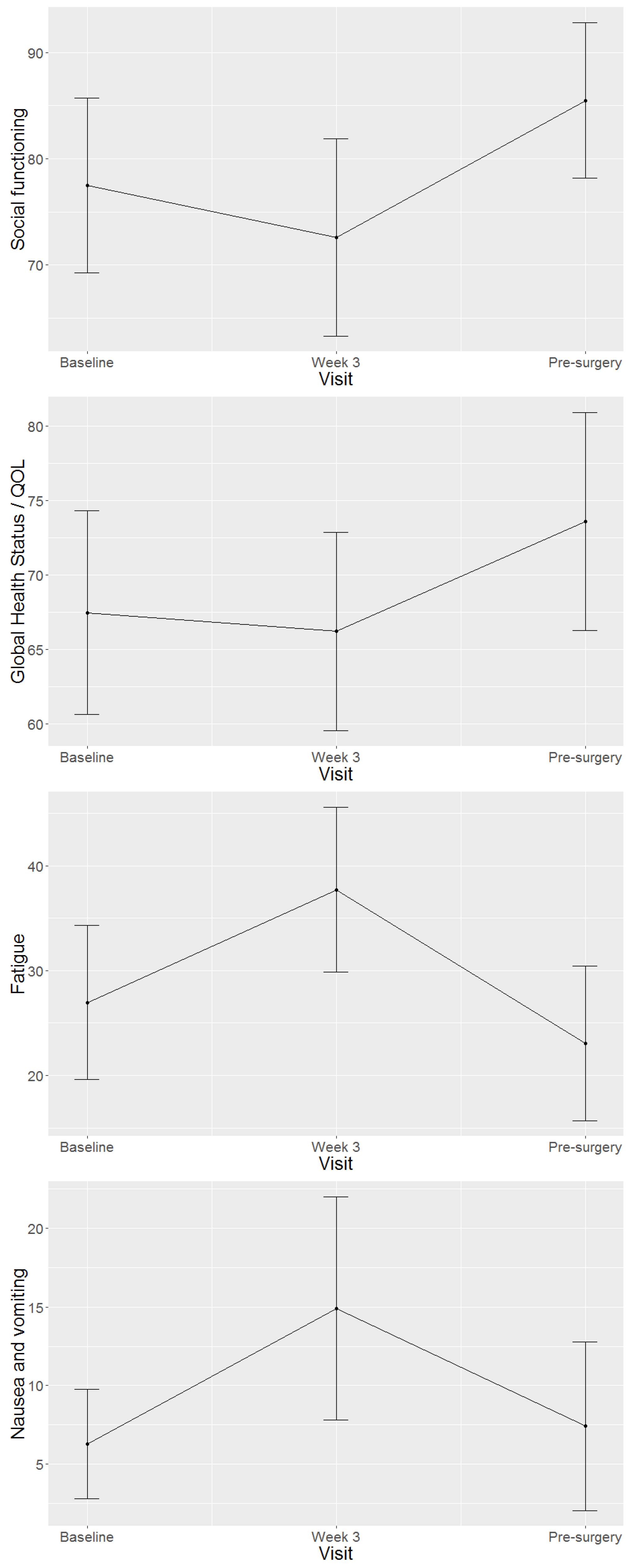
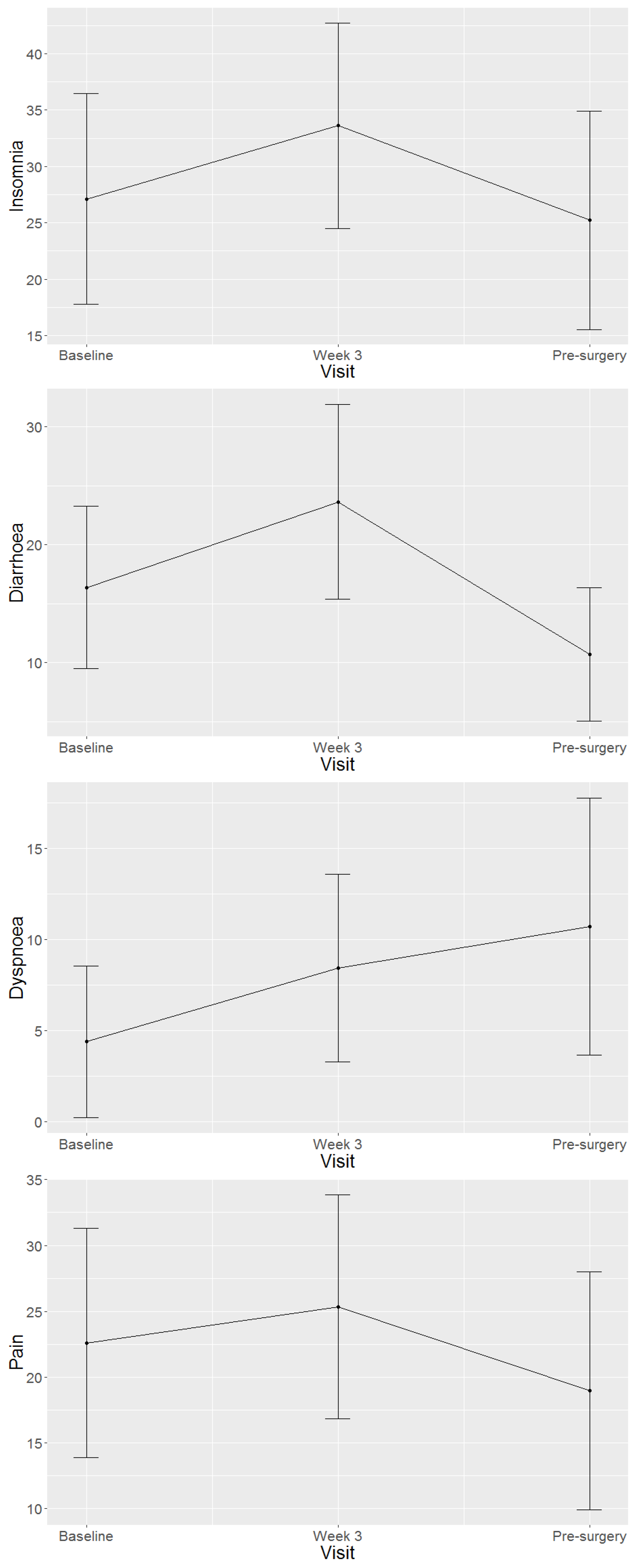
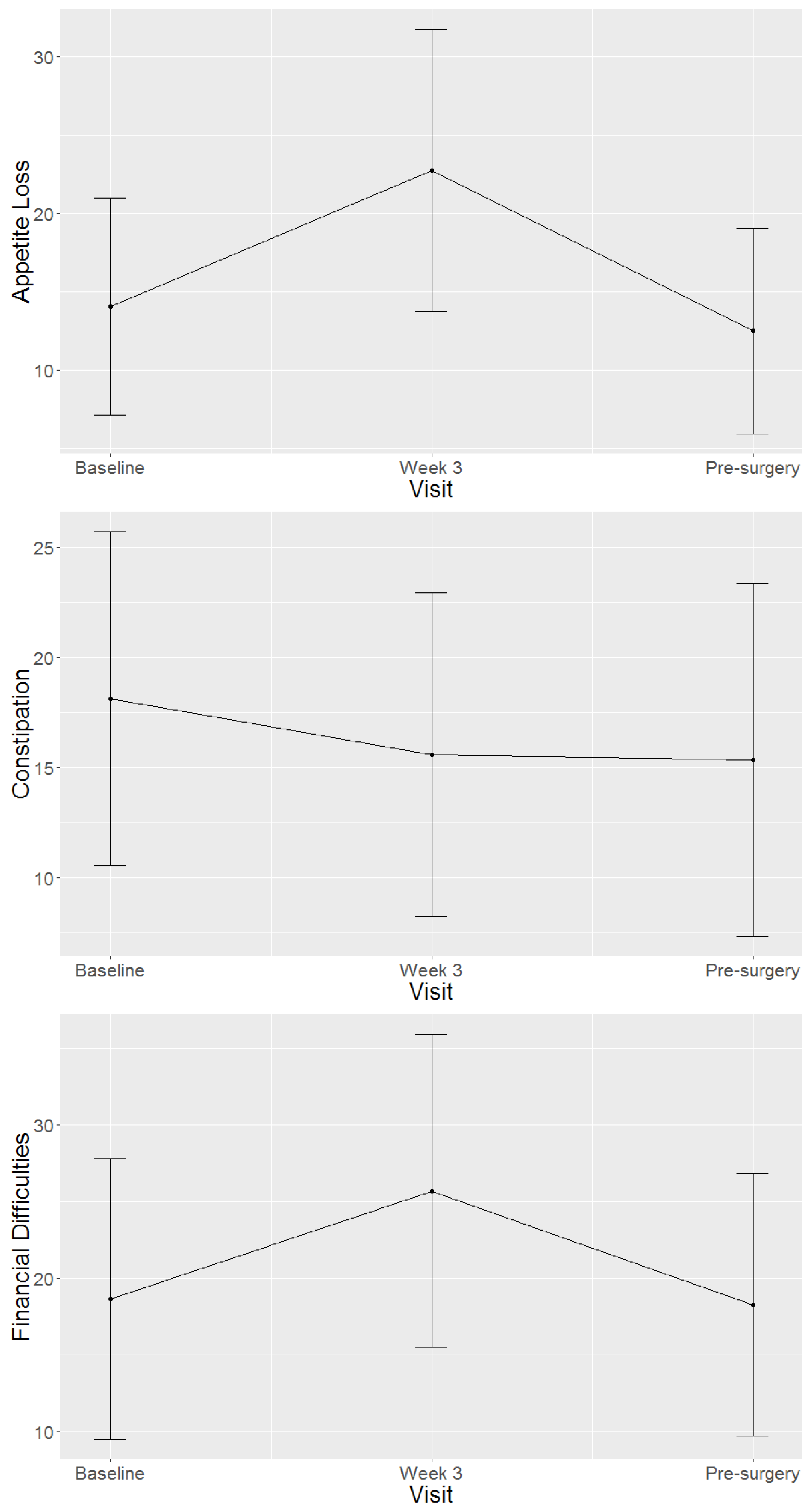
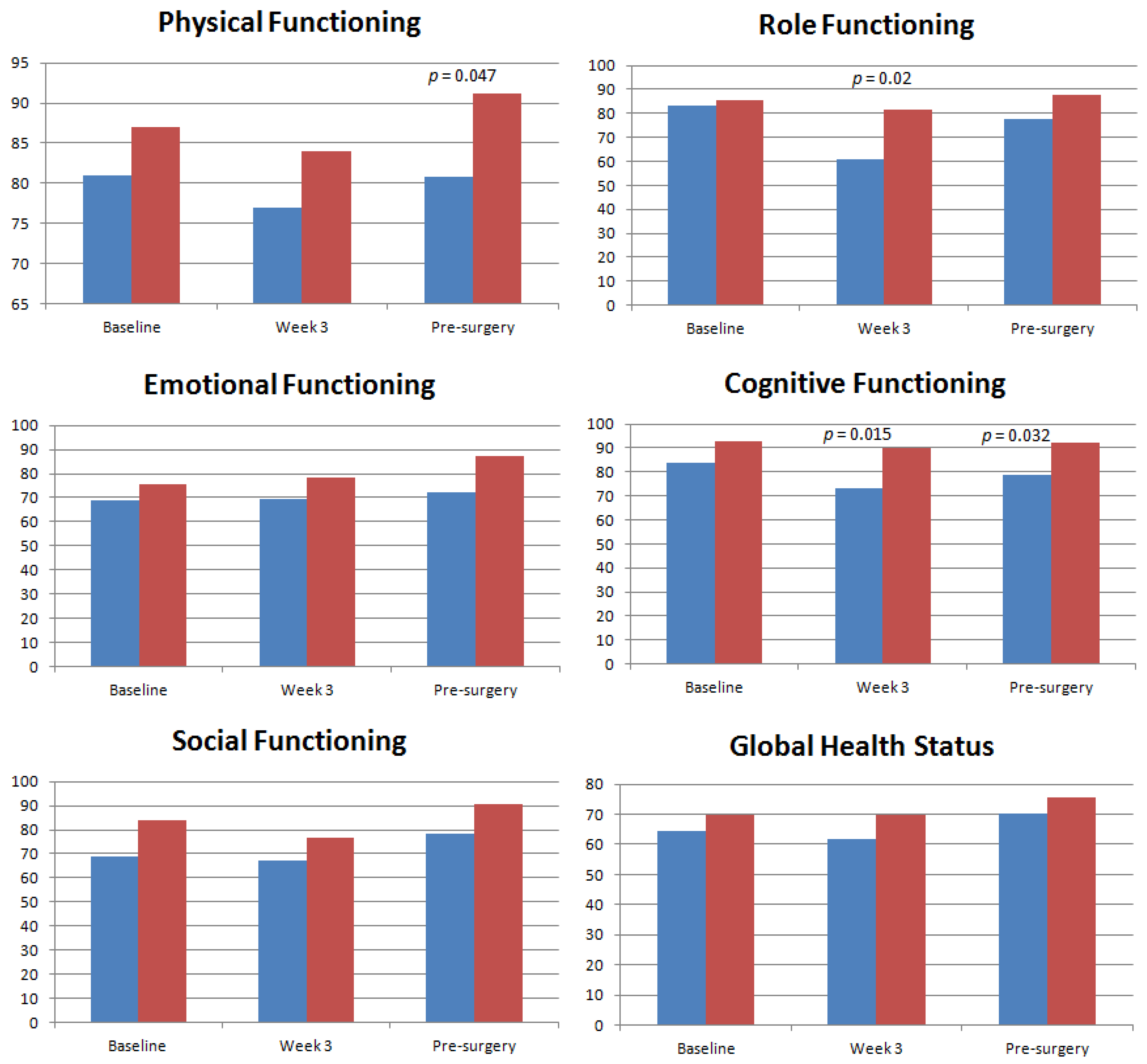
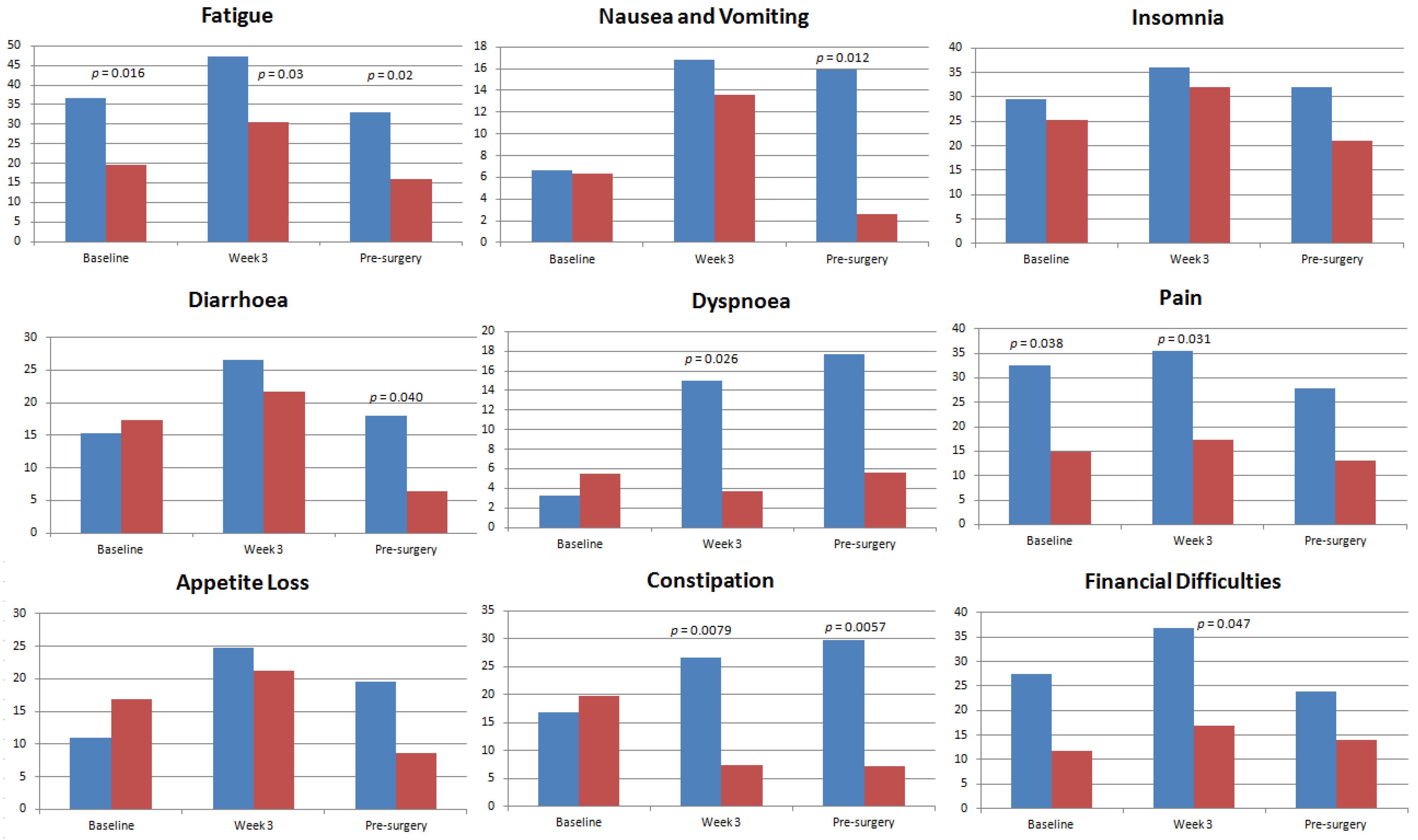
4.2. Health-Related Quality of Life Domains and Symptom Scales of Study Population
4.3. Health-Related Quality of Life Domains and Symptom Scales in Asian and Caucasian Populations
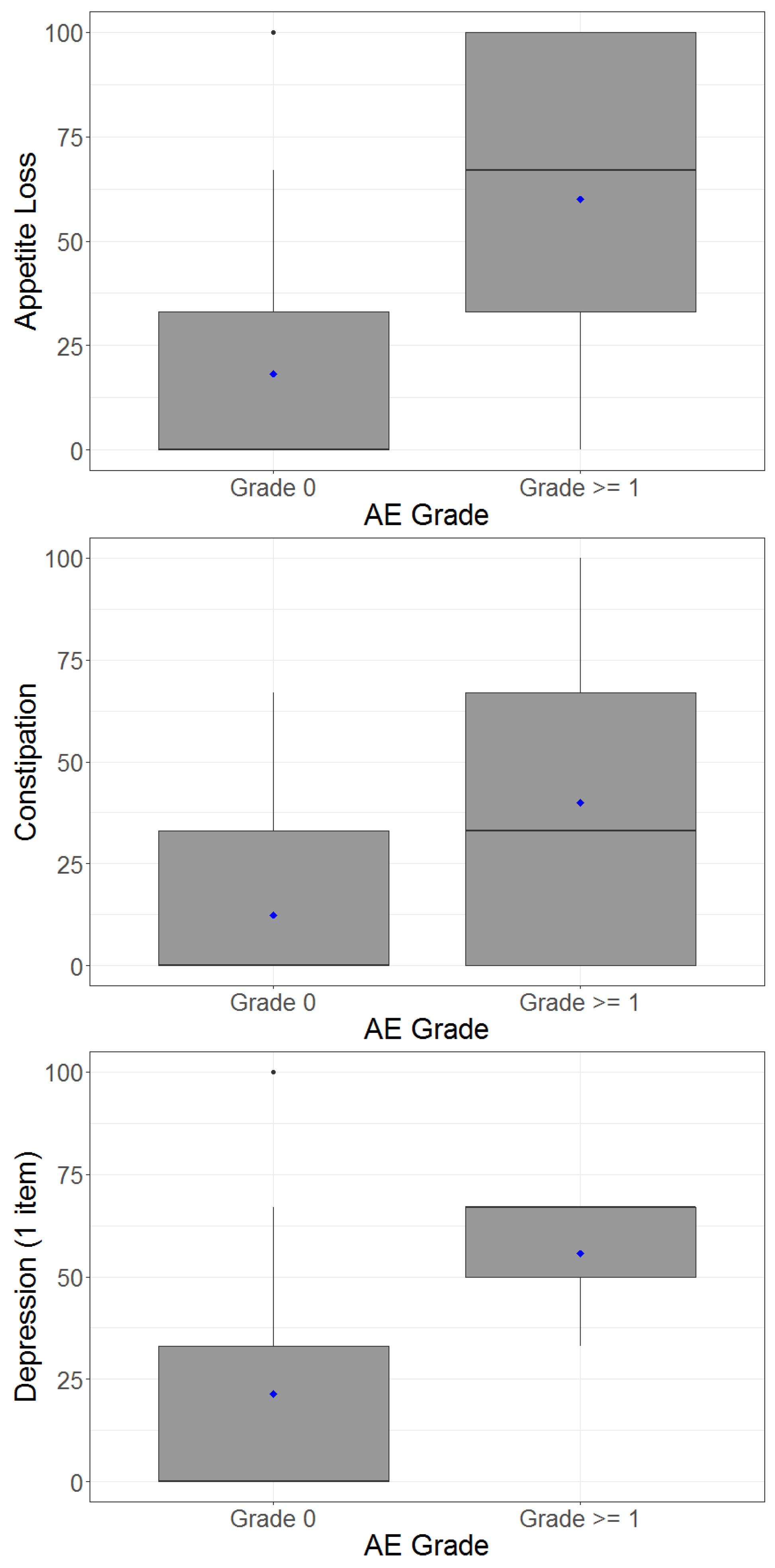
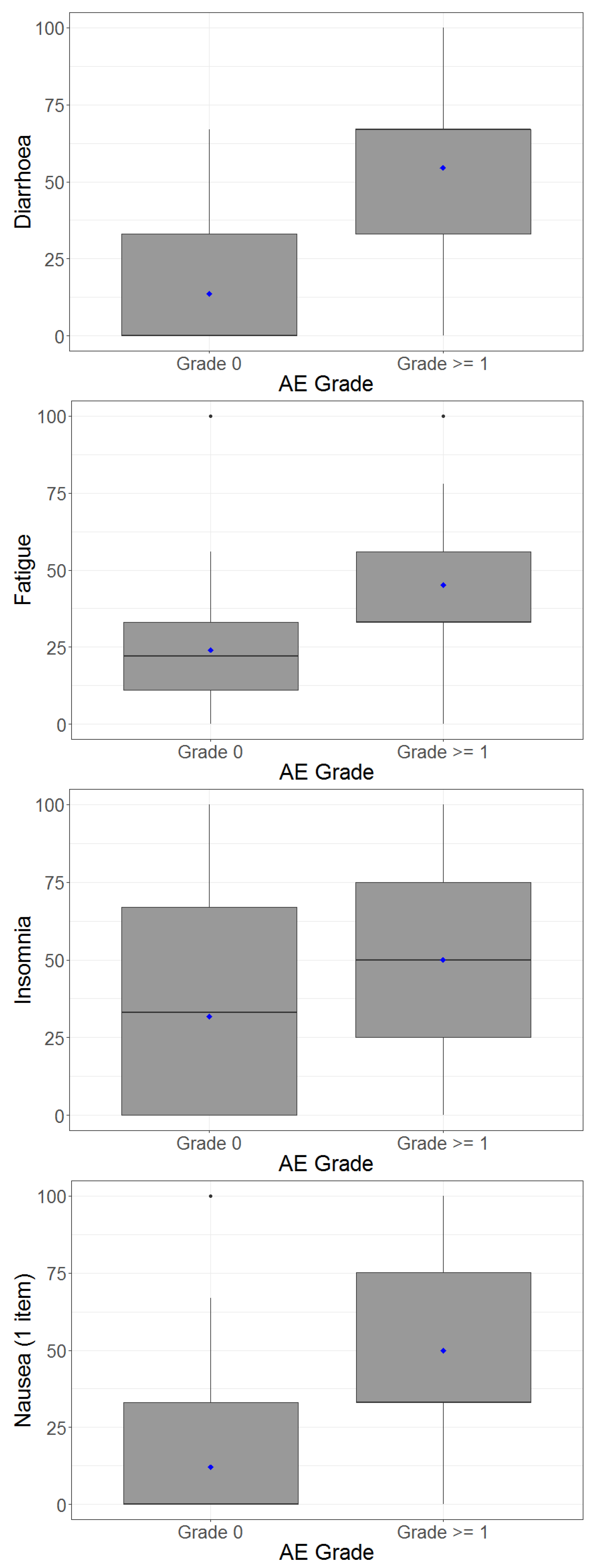
4.4. Adverse Events and Correlation between Patient-Reported Outcomes and Clinician-Reported Outcomes
4.5. Health-Related Quality of Life Domains and Tumour Response
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, S.; Del Bianco, P.; Efficace, F.; Serpentini, S.; Capirci, C.; De Paoli, A.; Amato, A.; Cuicchi, D.; Nitti, D. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: A multicenter prospective observational study. Ann. Surg. 2011, 253, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Guren, M.G.; Dueland, S.; Skovlund, E.; Fosså, S.D.; Poulsen, J.P.; Tveit, K.M. Quality of life during radiotherapy for rectal cancer. Eur. J. Cancer 2003, 39, 587–594. [Google Scholar] [CrossRef]
- Pucciarelli, S.; Del Bianco, P.; Efficace, F.; Toppan, P.; Serpentini, S.; Friso, M.L.; Lonardi, S.; De Salvo, G.L.; Nitti, D. Health-related quality of life, faecal continence and bowel function in rectal cancer patients after chemoradiotherapy followed by radical surgery. Support. Care Cancer 2010, 18, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Hagedoorn, M.; Sneeuw, K.C.; Aaronson, N.K. Changes in physical functioning and quality of life in patients with cancer: Response shift and relative evaluation of one’s condition. J. Clin. Epidemiol. 2002, 55, 176–183. [Google Scholar] [CrossRef]
- Herman, J.M.; Narang, A.K.; Griffith, K.A.; Zalupski, M.M.; Reese, J.B.; Gearhart, S.L.; Azad, N.S.; Chan, J.; Olsen, L.; Efron, J.E.; et al. The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, e15–e19. [Google Scholar] [CrossRef]
- Di Maio, M.; Gallo, C.; Leighl, N.B.; Piccirillo, M.C.; Daniele, G.; Nuzzo, F.; Gridelli, C.; Gebbia, V.; Ciardiello, F.; De Placido, S.; et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 2015, 33, 910–915. [Google Scholar] [CrossRef]
- Chen, C.H.; Tang, S.T.; Chen, C.H. Meta-analysis of cultural differences in Western and Asian patient-perceived barriers to managing cancer pain. Palliat. Med. 2012, 26, 206–221. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Dolan, M.E. Cancer pharmacoethnicity: Ethnic differences in susceptibility to the effects of chemotherapy. Clin. Cancer Res. 2009, 15, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor Regression Grading After Preoperative Chemoradiotherapy for Locally Advanced Rectal Carcinoma Revisited: Updated Results of the CAO/ARO/AIO-94 Trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Earlam, S.; Glover, C.; Fordy, C.; Burke, D.; Allen-Mersh, T.G. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J. Clin. Oncol. 1996, 14, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Bottomley, A.; Coens, C.; Van Steen, K.; Conroy, T.; et al. Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur. J. Cancer 2006, 42, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lis, C.G.; Gupta, D.; Granick, J.; Grutsch, J.F. Can patient satisfaction with quality of life predict survival in advanced colorectal cancer? Support. Care Cancer 2006, 14, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Maisey, N.R.; Norman, A.; Watson, M.; Allen, M.J.; Hill, M.E.; Cunningham, D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur. J. Cancer 2002, 38, 1351–1357. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Smith, A.B.; Cocks, K.; Parry, D.; Taylor, M. Reporting of health-related quality of life (HRQOL) data in oncology trials: A comparison of the European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ-C30) and the Functional Assessment of Cancer Therapy-General (FACT-G). Qual. Life Res. 2014, 23, 971–976. [Google Scholar] [CrossRef]
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001; Available online: https://abdn.pure.elsevier.com/en/publications/eortc-qlq-c30-scoring-manual-3rd-edition (accessed on 18 December 2017).
- National Cancer Institute, Common Terminology Criteria for Adverse Events v4.0, NCI, NIH, DHHS. 29 May 2009. NIH publication # 09-7473. Available online: https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf (accessed on 18 December 2017).
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.T.; Velikova, G.; Fayers, P.M.; Brown, J.M. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. Eur. J. Cancer 2008, 44, 1793–1798. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.B.; Hansen, J.A.; Moskowitz, M.; Todd, B.L.; Feuerstein, M. It’s not over when it’s over: Long-term symptoms in cancer survivors--a systematic review. Int. J. Psychiatry Med. 2010, 40, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.; Moore, M.M.; McLachlan, A.J.; Piquette-Miller, M.; Xu, H.; Clarke, S.J. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin. Drug Metab. Toxicol. 2009, 5, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Reigner, B.; Watanabe, T.; Schuller, J.; Lucraft, H.; et al. Pharmacokinetics of capecitabine (Xeloda) in Japanese and Caucasian patients with breast cancer. Cancer Chemother. Pharmacol. 2003, 52, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Crossley-May, H.; Vigneau, F.D.; Brown, K.; Banerjee, M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control 2003, 14, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.M.; Bruner, D.W.; Mitchell, S.A.; Minasian, L.M.; Basch, E.; Dueck, A.C.; Cella, D.; Reeve, B.B. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support. Care Cancer 2013, 21, 1525–1550. [Google Scholar] [CrossRef] [PubMed]
- Fromme, E.K.; Eilers, K.M.; Mori, M.; Hsieh, Y.C.; Beer, T.M. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J. Clin. Oncol. 2004, 22, 3485–3490. [Google Scholar] [CrossRef]
- Flores, L.T.; Bennett, A.V.; Law, E.B.; Hajj, C.; Griffith, M.P.; Goodman, K.A. Patient-Reported Outcomes vs. Clinician Symptom Reporting During Chemoradiation for Rectal Cancer. Gastrointest. Cancer Res. 2012, 5, 119–124. [Google Scholar]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring with Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef]
- Basch, E.; Abernethy, A.P.; Mullins, C.D.; Reeve, B.B.; Smith, M.L.; Coons, S.J.; Sloan, J.; Wenzel, K.; Chauhan, C.; Eppard, W.; et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J. Clin. Oncol. 2012, 30, 4249–4255. [Google Scholar] [CrossRef]
| Variable | n | Percent (%) |
|---|---|---|
| Age ˂65 ≥65 | 29 22 | 57 43 |
| Gender Male Female | 38 13 | 75 25 |
| Ethnicity Caucasian Asian | 36 15 | 71 29 |
| Tumour stage T2 T3 T4 | 4 42 5 | 8 82 10 |
| Nodal stage N0 N1 N2 | 7 20 24 | 14 39 47 |
| Smoking status Yes No | 21 30 | 41 59 |
| BMI ˂30 ≥30 | 37 13 | 73 25 |
| ECOG 0 1 | 36 15 | 71 29 |
| CTC-AE Specific Toxicity or ECOG * | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Diarrhoea (%) | 76 | 18 | 4 | 2 |
| Pain (%) | 90 | 8 | 2 | 0 |
| Fatigue (%) | 40 | 60 | 0 | 0 |
| Weight loss (%) | 94 | 6 | 0 | 0 |
| Nausea (%) | 76 | 22 | 2 | 0 |
| Vomiting (%) | 96 | 4 | 0 | 0 |
| Constipation (%) | 90 | 10 | 0 | 0 |
| Anorexia (%) | 88 | 10 | 2 | 0 |
| Insomnia (%) | 94 | 6 | 0 | 0 |
| Depression (%) | 92 | 8 | 0 | 0 |
| ECOG performance status (%) | 56 | 44 | 0 | 0 |
| Clinician-Rated Outcome | Patient-Reported Outcome (PRO) | Spearman’s Correlation | Mean (SD) PRO for Patients with CTC AE Toxicity Grade 0 † | Mean (SD) PRO for Patients with CTC AE Toxicity Grade ≥ 1 † |
|---|---|---|---|---|
| Diarrhoea | Diarrhoea | 0.58 | 13.7 (18.6) | 54.6 (30.8) |
| Nausea | Nausea | 0.54 | 12.1 (23.3) | 50.0 (36.2) |
| Fatigue | Fatigue | 0.44 | 24.2 (25.5) | 45.2 (24.9) |
| Anorexia | Appetite loss | 0.35 | 18.3 (26.1) | 60.0 (43.5) |
| Depression | Depression | 0.32 | 21.4 (26.4) | 55.6 (19.2) |
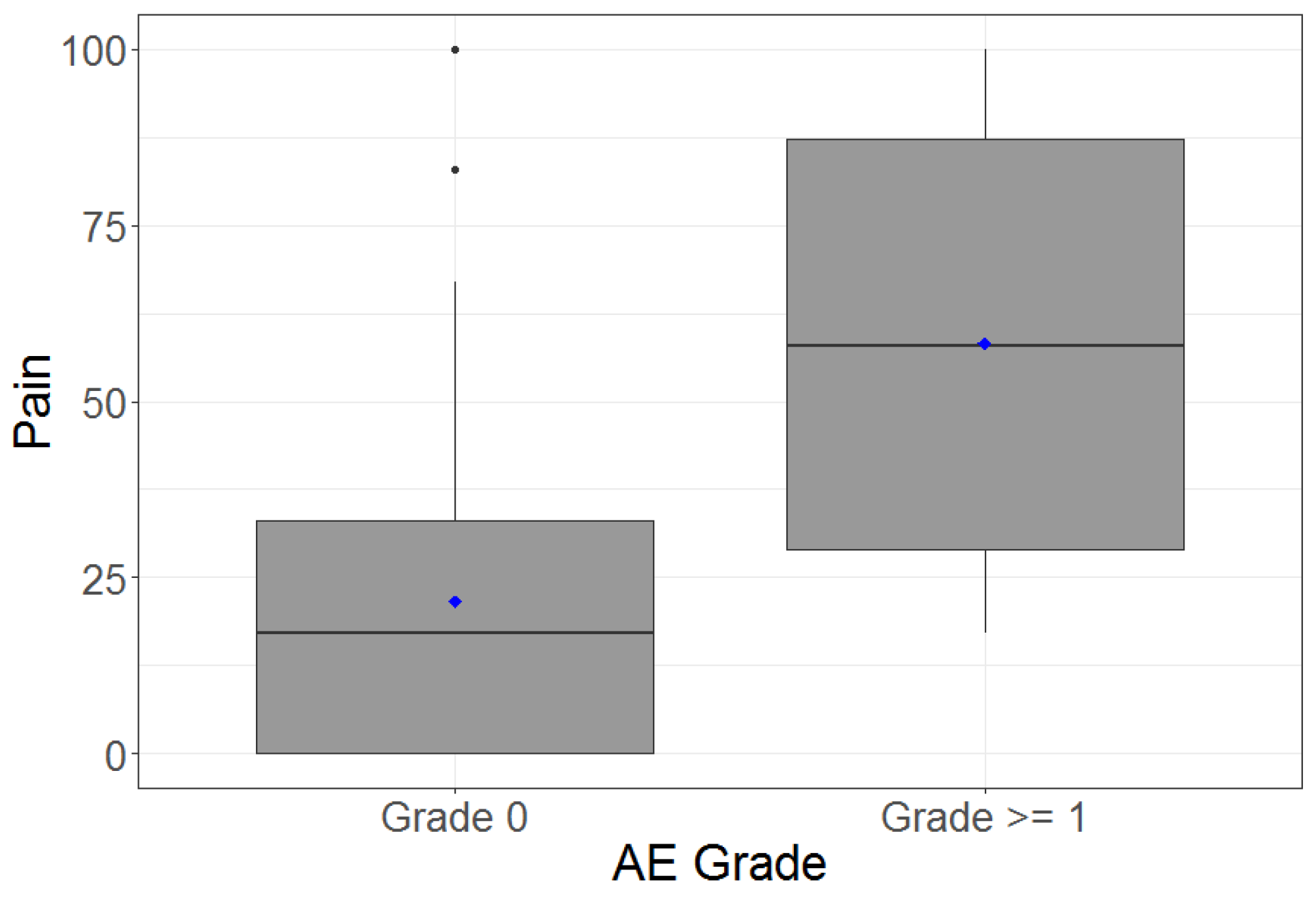
| Pain | Pain | 0.31 | 21.5 (26.2) | 58.3 (39.7) |
| Constipation | Constipation | 0.24 | 12.5 (18.0) | 40.0 (43.5) |
| Insomnia | Insomnia | 0.06 | 31.8 (31.7) | 50.0 (70.7) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.H.-S.; Ip, E.; Ng, W.; Chua, W.; Asghari, R.; Roohullah, A.; Descallar, J.; Henderson, C.; Spring, K.; de Souza, P.; et al. Health-Related Quality of Life during Chemoradiation in Locally Advanced Rectal Cancer: Impacts and Ethnic Disparities. Cancers 2019, 11, 1263. https://doi.org/10.3390/cancers11091263
Lim SH-S, Ip E, Ng W, Chua W, Asghari R, Roohullah A, Descallar J, Henderson C, Spring K, de Souza P, et al. Health-Related Quality of Life during Chemoradiation in Locally Advanced Rectal Cancer: Impacts and Ethnic Disparities. Cancers. 2019; 11(9):1263. https://doi.org/10.3390/cancers11091263
Chicago/Turabian StyleLim, Stephanie Hui-Su, Emilia Ip, Weng Ng, Wei Chua, Ray Asghari, Aflah Roohullah, Joseph Descallar, Christopher Henderson, Kevin Spring, Paul de Souza, and et al. 2019. "Health-Related Quality of Life during Chemoradiation in Locally Advanced Rectal Cancer: Impacts and Ethnic Disparities" Cancers 11, no. 9: 1263. https://doi.org/10.3390/cancers11091263
APA StyleLim, S. H.-S., Ip, E., Ng, W., Chua, W., Asghari, R., Roohullah, A., Descallar, J., Henderson, C., Spring, K., de Souza, P., & King, M. T. (2019). Health-Related Quality of Life during Chemoradiation in Locally Advanced Rectal Cancer: Impacts and Ethnic Disparities. Cancers, 11(9), 1263. https://doi.org/10.3390/cancers11091263




