Immune Heterogeneity Between Primary Tumors and Corresponding Metastatic Lesions and Response to Platinum Therapy in Primary Ovarian Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Immune Infiltrate in Primary Tumor
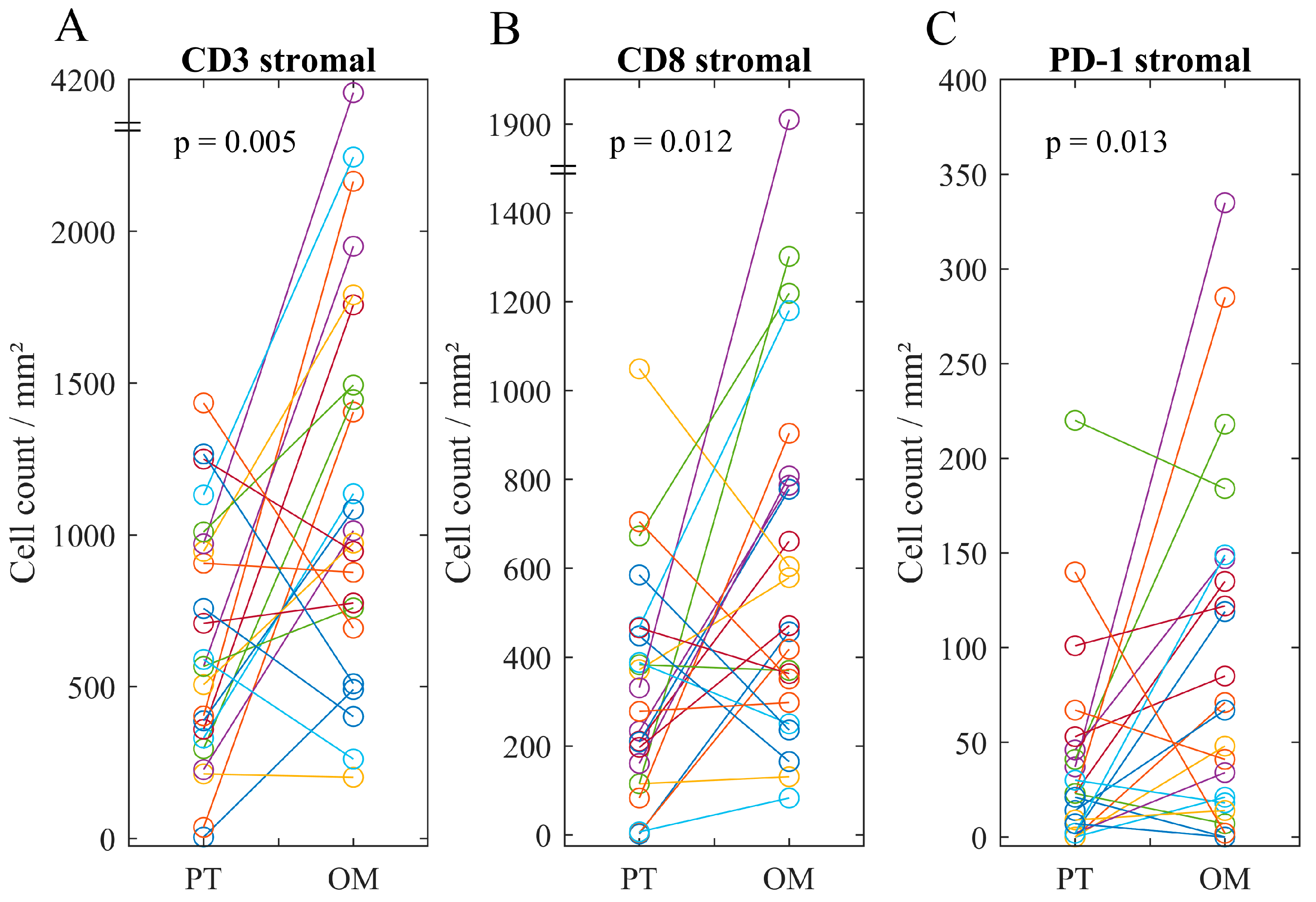
2.3. Immune Infiltrate in Metastatic Lesions
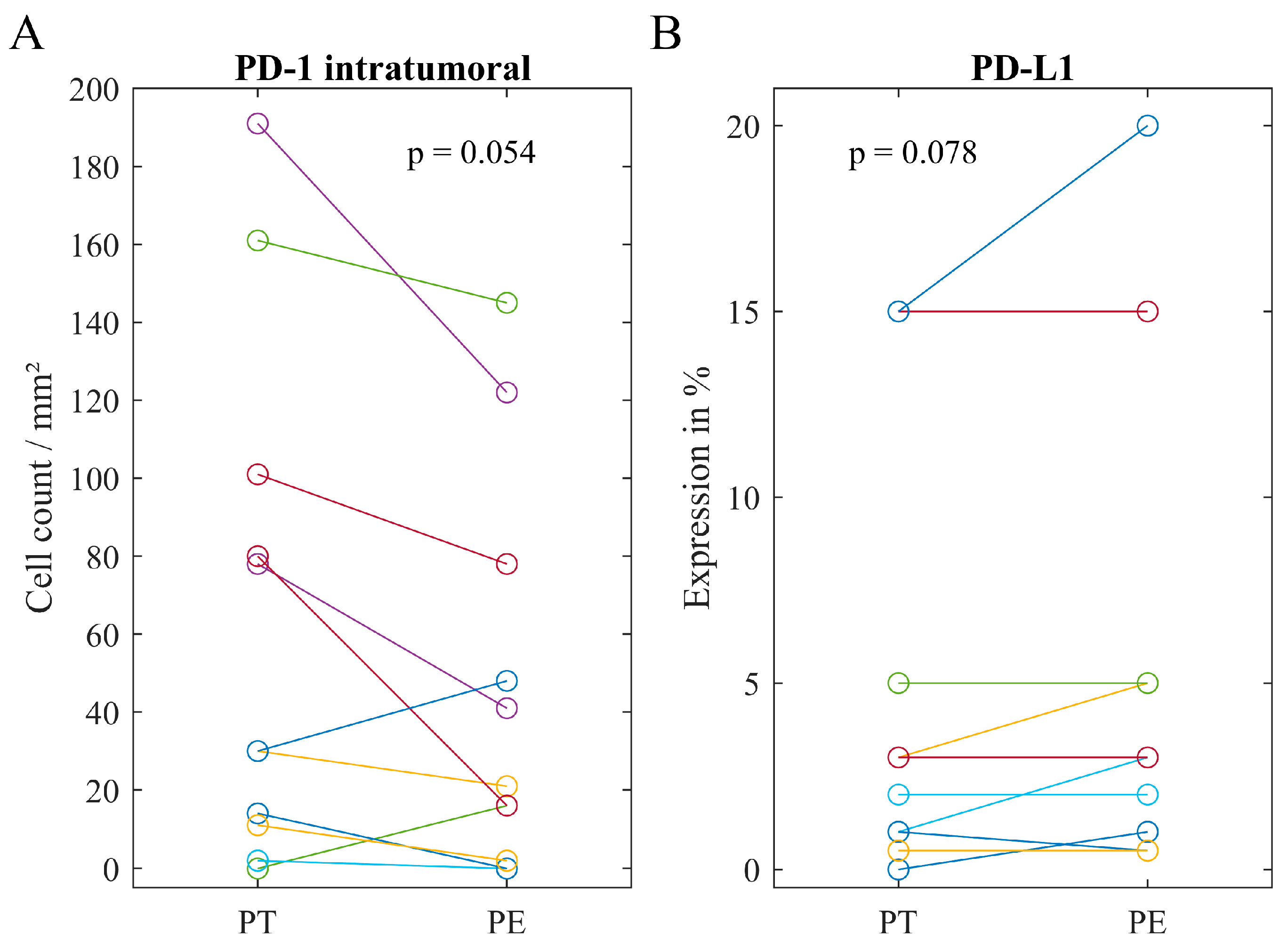
2.4. Associations of Immune Heterogeneity
3. Discussion
4. Patients and Methods
4.1. Study Population
4.2. Immunohistochemistry
4.3. Semiquantitative Analysis of the CD45+ Infiltrate
4.4. Quantitative Analysis of Immune Cells
4.5. Semiquantitative Analysis of PD-L1
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wang, J.; Chen, R.; Bai, Y.; Lu, X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017, 8, 15621–15631. [Google Scholar] [CrossRef] [PubMed]
- OTTA Consortium. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.J.; Bertelsen, K.; James, K.; Cassidy, J.; Mangioni, C.; Simonsen, E.; Stuart, G.; Kaye, S.; Vergote, I.; Blom, R.; et al. Randomized Intergroup Trial of Cisplatin-Paclitaxel Versus Cisplatin-Cyclophosphamide in Women with Advanced Epithelial Ovarian Cancer: Three-Year Results. J. Natl. Cancer Inst. 2000, 92, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Verrico, M.; Zaccarelli, E.; Papa, A.; Colonna, M.; Strudel, M.; Vici, P.; Bianco, V.; Tomao, F. Bevacizumab in ovarian cancer: A critical review of phase III studies. Oncotarget 2017, 8, 12389–12405. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C.; On behalf of the ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi24–vi32. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Topotecan, Pegylated Liposomal Doxorubicin Hydrochloride, Paclitaxel, Trabectedin and Gemcitabine for Treating Recurrent Ovarian Cancer. Available online: https://www.nice.org.uk/guidance/ta389 (accessed on 3 June 2019).
- Francis, J.; Coakley, N.; Elit, L.; Mackay, H.; The Gynecologic Cancer Disease Site Group. Systemic therapy for recurrent epithelial ovarian cancer: A clinical practice guideline. Curr. Oncol. 2017, 24, e540–e546. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Disis, M.L.; Patel, M.R.; Pant, S.; Hamilton, E.P.; Lockhart, A.C.; Kelly, K.; Beck, J.T.; Gordon, M.S.; Weiss, G.J.; Taylor, M.H.; et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. J. Clin. Oncol. 2016, 34, 5533. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Piha-Paul, S.A.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Johnson, E.A.; Cheng, J.D.; Yuan, S.; Rubin, E.H.; Matei, D.E. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J. Clin. Oncol. 2015, 33, 5510. [Google Scholar] [CrossRef]
- Gaillard, S.L.; Secord, A.A.; Monk, B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Oncol. Res. Pract. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, A.; Ha, G.; Tone, A.; Ding, J.; Prentice, L.M.; Roth, A.; Rosner, J.; Shumansky, K.; Kalloger, S.; Senz, J.; et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J. Pathol. 2013, 231, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Rhee, J.K.; Hur, S.Y.; Kim, M.S.; Lee, S.H.; Chung, Y.J.; Kim, T.M.; Lee, S.H. Intraindividual genomic heterogeneity of high-grade serous carcinoma of the ovary and clinical utility of ascitic cancer cells for mutation profiling. J. Pathol. 2017, 241, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraat, M.; de Pagter, M.S.; Cirkel, G.A.; van Roosmalen, M.J.; Harkins, T.T.; Duran, K.; Kreeftmeijer, J.; Renkens, I.; Witteveen, P.O.; Lee, C.C.; et al. Genomic and transcriptomic plasticity in treatment-naive ovarian cancer. Genome Res. 2014, 24, 200–211. [Google Scholar] [CrossRef]
- Köbel, M.; Turbin, D.; Kalloger, S.E.; Gao, D.; Huntsman, D.G.; Gilks, C.B. Biomarker expression in pelvic high-grade serous carcinoma: Comparison of ovarian and omental sites. Int. J. Gynecol. Pathol. 2011, 30, 366–371. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, B.; Tang, D.; Bowden, N.A. Biomarkers of platinum resistance in ovarian cancer: What can we use to improve treatment. Endocr.-Relat. Cancer 2018, 25, R303–R318. [Google Scholar] [CrossRef]
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of tumor microenvironment in ovarian cancer pathobiology. Oncotarget 2018, 9, 22832–22849. [Google Scholar] [CrossRef]
- Worzfeld, T.; Pogge von Strandmann, E.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Muller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [PubMed]
- Wieser, V.; Gaugg, I.; Fleischer, M.; Shivalingaiah, G.; Wenzel, S.; Sprung, S.; Lax, S.F.; Zeimet, A.G.; Fiegl, H.; Marth, C. BRCA1/2 and TP53 mutation status associates with PD-1 and PD-L1 expression in ovarian cancer. Oncotarget 2018, 9, 17501–17511. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Bosmuller, H.; Haitchi-Petnehazy, S.; Webersinke, G.; Marschon, R.; Roithmeier, F.; Stummvoll, W.; Fehm, T.; Klier-Richter, M.; Bonzheim, I.; Staebler, A.; et al. Intratumoral lymphocyte density in serous ovarian carcinoma is superior to ERCC1 expression for predicting response to platinum-based therapy. Virchows Arch. 2011, 459, 183–191. [Google Scholar] [CrossRef] [PubMed]
- de Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin. Cancer Res. 2014, 20, 5384–5391. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, A.S.; Genestie, C.; Auguste, A.; Leary, A. Impact of neoadjuvant chemotherapy on the immune microenvironment in advanced epithelial ovarian cancer: Prognostic and therapeutic implications. Int. J. Cancer 2018, 143, 8–15. [Google Scholar] [CrossRef]
- Chang, C.L.; Hsu, Y.T.; Wu, C.C.; Lai, Y.Z.; Wang, C.; Yang, Y.C.; Wu, T.C.; Hung, C.F. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013, 73, 119–127. [Google Scholar] [CrossRef]
- Chatterjee, J.; Dai, W.; Aziz, N.H.A.; Teo, P.Y.; Wahba, J.; Phelps, D.L.; Maine, C.J.; Whilding, L.M.; Dina, R.; Trevisan, G.; et al. Clinical Use of Programmed Cell Death-1 and Its Ligand Expression as Discriminatory and Predictive Markers in Ovarian Cancer. Clin. Cancer Res. 2017, 23, 3453–3460. [Google Scholar] [CrossRef]
- Drakes, M.L.; Mehrotra, S.; Aldulescu, M.; Potkul, R.K.; Liu, Y.; Grisoli, A.; Joyce, C.; O’Brien, T.E.; Stack, M.S.; Stiff, P.J. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J. Ovarian Res. 2018, 11, 43. [Google Scholar] [CrossRef]
- Heintz, A.P.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Benedet, J.L.; Creasman, W.T.; Ngan, H.Y.; Pecorelli, S.; Beller, U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S161–S192. [Google Scholar] [CrossRef]
- Meza-Perez, S.; Randall, T.D. Immunological Functions of the Omentum. Trends Immunol. 2017, 38, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Krishnan, V.; Schoof, M.; Rodriguez, I.; Theriault, B.; Chekmareva, M.; Rinker-Schaeffer, C. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am. J. Pathol. 2013, 183, 576–591. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, C.W.; Rodriguez, G.M.; Galpin, K.J.C.; Vanderhyden, B.C. Ovarian Cancer Immunotherapy: Preclinical Models and Emerging Therapeutics. Cancers 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.X.; Chan, S.; Kwek, S.; Lewis, J.; Dao, V.; Zhang, L.; Cooperberg, M.R.; Ryan, C.J.; Lin, A.M.; Friedlander, T.W.; et al. Systemic GM-CSF Recruits Effector T Cells into the Tumor Microenvironment in Localized Prostate Cancer. Cancer Immunol. Res. 2016, 4, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, M.; Wang, C.; Xu, Y.; Gao, W.Q.; Zhang, Y. CCL5-deficiency enhances intratumoral infiltration of CD8(+) T cells in colorectal cancer. Cell Death Dis. 2018, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Heindl, A.; Lan, C.; Rodrigues, D.N.; Koelble, K.; Yuan, Y. Similarity and diversity of the tumor microenvironment in multiple metastases: Critical implications for overall and progression-free survival of high-grade serous ovarian cancer. Oncotarget 2016, 7, 71123–71135. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, A.; Memon, D.; Pourpe, S.; Veeraraghavan, H.; Li, Y.; Vargas, H.A.; Gill, M.B.; Park, K.J.; Zivanovic, O.; Konner, J.; et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell 2017, 170, 927–938. [Google Scholar] [CrossRef]
- du Bois, A.; Kristensen, G.; Ray-Coquard, I.; Reuss, A.; Pignata, S.; Colombo, N.; Denison, U.; Vergote, I.; Del Campo, J.M.; Ottevanger, P.; et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016, 17, 78–89. [Google Scholar] [CrossRef]
- Selle, F.; Colombo, N.; Korach, J.; Mendiola, C.; Cardona, A.; Ghazi, Y.; Oza, A.M. Safety and Efficacy of Extended Bevacizumab Therapy in Elderly (>/=70 Years) Versus Younger Patients Treated for Newly Diagnosed Ovarian Cancer in the International ROSiA Study. Int. J. Gynecol. Cancer 2018, 28, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Java, J.J.; Eskander, R.N.; Monk, B.J.; Burger, R.A. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Ann. Oncol. 2016, 27, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, D.K., AWMF). S3-Leitlinie Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren, Langversion 3.0. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/ovarialkarzinom/ (accessed on 3 June 2019).
- Mayer, B.; Funke, I.; Johnson, J.P. High expression of a Lewis(x)-related epitope in gastric carcinomas indicates metastatic potential and poor prognosis. Gastroenterology 1996, 111, 1433–1446. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Miksch, R.C.; Hao, J.; Schoenberg, M.B.; Dotzer, K.; Schluter, F.; Weniger, M.; Yin, S.; Ormanns, S.; D’Haese, J.G.; Guba, M.O.; et al. Development of a reliable and accurate algorithm to quantify the tumor immune stroma (QTiS) across tumor types. Oncotarget 2017, 8, 114935–114944. [Google Scholar] [CrossRef]
- Vayrynen, J.P.; Vornanen, J.O.; Sajanti, S.; Bohm, J.P.; Tuomisto, A.; Makinen, M.J. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch. 2012, 460, 455–465. [Google Scholar] [CrossRef]
- Miksch, R.C.; Schoenberg, M.B.; Weniger, M.; Bosch, F.; Ormanns, S.; Mayer, B.; Werner, J.; Bazhin, A.V.; D’Haese, J.G. Prognostic Impact of Tumor-Infiltrating Lymphocytes and Neutrophils on Survival of Patients with Upfront Resection of Pancreatic Cancer. Cancers 2019, 11, 39. [Google Scholar] [CrossRef]
- Schoenberg, M.B.; Hao, J.; Bucher, J.N.; Miksch, R.C.; Anger, H.J.W.; Mayer, B.; Mayerle, J.; Neumann, J.; Guba, M.O.; Werner, J.; et al. Perivascular Tumor-Infiltrating Leukocyte Scoring for Prognosis of Resected Hepatocellular Carcinoma Patients. Cancers 2018, 10, 389. [Google Scholar] [CrossRef]
- Weiss, L.; Huemer, F.; Mlineritsch, B.; Greil, R. Immune checkpoint blockade in ovarian cancer. Memo 2016, 9, 82–84. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]

| n or Value | % | ||
|---|---|---|---|
| Age | mean/median | 62/66 years | |
| range | 24–83 years | ||
| FIGO Stage | I/II | 0 | 0.0 |
| III | 35 | 71.4 | |
| IV | 14 | 28.6 | |
| pT | pT2 | 5 | 10.2 |
| pT3 | 44 | 89.8 | |
| pN | pN0 | 6 | 12.2 |
| pN1 | 32 | 65.3 | |
| Nx | 11 | 22.4 | |
| cM | cM0 | 35 | 71.4 |
| cM1 | 14 | 28.6 | |
| Primary Tumor Site | Ovarian | 39 | 79.6 |
| Fallopian Tube | 7 | 14.3 | |
| Peritoneal | 3 | 6.1 | |
| Histological Subtype | Serous | 44 | 89.8 |
| Other | 5 | 10.2 | |
| Grading | G1/G2 | 1 | 4.0 |
| G3 | 47 | 95.9 | |
| Ascites | yes | 41 | 83.7 |
| no | 8 | 16.3 | |
| Macroscopic Residual Tumor after Surgery | None | 35 | 71.4 |
| <1 cm | 8 | 16.3 | |
| >1 cm | 6 | 12.2 | |
| Lymphatic Vessel Invasion | yes | 26 | 53.1 |
| no | 21 | 42.9 | |
| missing | 2 | 4.1 | |
| Vascular Invasion | yes | 9 | 18.4 |
| no | 38 | 77.6 | |
| missing | 2 | 4.1 | |
| First-Line-Treatment | C | 5 | 10.2 |
| C+P | 15 | 30.6 | |
| C+P+B | 25 | 51.0 | |
| None | 4 | 8.2 | |
| Relapse after Chemotherapy | < 6 months | 2 | 4.1 |
| 6–12 months | 12 | 24.5 | |
| >12 months | 28 | 57.1 | |
| none or non-sufficient chemotherapy | 7 | 14.3 |
| PT | OM | PE | ||
|---|---|---|---|---|
| Rating | CD45 stromal | |||
| Mode (Range) | 3 (1–5) | 3 (2–5) | 3;4 (2–5) | |
| CD45 intratumoral | ||||
| Mode (Range) | 1 (0–3) | 1 (0–3) | 1 (0–3) | |
| Cell Count | CD3 stromal | |||
| Mean (Range) | 626 (5–2491) | 1241 (202–4157) | 851 (117–2766) | |
| CD3 intratumoral | ||||
| Mean (Range) | 201 (0–1134) | 212 (0–569) | 272 (0–985) | |
| CD8 stromal | ||||
| Mean (Range) | 318 (0–1049) | 623 (83–1910) | 364 (0–843) | |
| CD8 intratumoral | ||||
| Mean (Range) | 88 (0–716) | 104 (0–636) | 130 (0–494) | |
| PD-1 stromal | ||||
| Mean (Range) | 73 (0–404) | 91 (0–335) | 130 (0–601) | |
| PD-1 intratumoral | ||||
| Mean (Range) | 26 (0–191) | 21 (0–83) | 33 (0–145) | |
| Expression | PD-L1 | |||
| Median (Range) | 1% (0–20%) | 0.5% (0–20%) | 3% (0–20%) |
| PT | OM/PT | PE/PT | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platinum-Sensitivity * | Platinum-Sensitivity * | Platinum-Sensitivity * | |||||||||||||
| n | Red | Full | p# | n | Red | Full | p# | n | Red | Full | p# | ||||
| CD45 stromal | 42 | 1 | 19 | 0.350 | 12 | 0.250 | |||||||||
| Low | 4 | 8 | OM ≤ PT | 2 | 7 | PE ≤ PT | 2 | 9 | |||||||
| High | 10 | 20 | OM > PT | 5 | 5 | PE > PT | 1 | 0 | |||||||
| CD45 intratumoral | 42 | 1 | 19 | 1 | 12 | 1 | |||||||||
| Low | 8 | 15 | OM ≤ PT | 6 | 11 | PE ≤ PT | 3 | 8 | |||||||
| High | 6 | 13 | OM > PT | 1 | 1 | PE > PT | 0 | 1 | |||||||
| CD3 stromal | 42 | 1 | 19 | 1 | 12 | 0.523 | |||||||||
| Low | 8 | 16 | OM ≤ PT | 1 | 3 | PE ≤ PT | 2 | 3 | |||||||
| High | 6 | 12 | OM > PT | 6 | 9 | PE > PT | 1 | 6 | |||||||
| CD3 intratumoral | 42 | 0.057 | 19 | 0.633 | 12 | 1 | |||||||||
| Low | 11 | 13 | OM ≤ PT | 2 | 6 | PE ≤ PT | 2 | 4 | |||||||
| High | 3 | 15 | OM > PT | 5 | 6 | PE > PT | 1 | 5 | |||||||
| CD8 stromal | 42 | 0.748 | 19 | 1 | 12 | 0.523 | |||||||||
| Low | 7 | 16 | OM ≤ PT | 2 | 3 | PE ≤ PT | 1 | 6 | |||||||
| High | 7 | 12 | OM > PT | 5 | 9 | PE > PT | 2 | 3 | |||||||
| CD8 intratumoral | 42 | 1 | 19 | 0.656 | 12 | 0.045 | |||||||||
| Low | 9 | 18 | OM ≤ PT | 2 | 5 | PE ≤ PT | 3 | 2 | |||||||
| High | 5 | 10 | OM > PT | 5 | 7 | PE > PT | 0 | 7 | |||||||
| PD-1 stromal | 42 | 0.283 | 19 | 1 | 12 | 0.045 | |||||||||
| Low | 12 | 19 | OM ≤ PT | 2 | 4 | PE ≤ PT | 0 | 7 | |||||||
| High | 2 | 9 | OM > PT | 5 | 8 | PE > PT | 3 | 2 | |||||||
| PD-1 intratumoral | 42 | 0.738 | 19 | 1 | 12 | 1 | |||||||||
| Low | 10 | 18 | OM ≤ PT | 5 | 8 | PE ≤ PT | 3 | 7 | |||||||
| High | 4 | 10 | OM > PT | 2 | 4 | PE > PT | 0 | 2 | |||||||
| PD-L1 Positivity | 42 | 1 | 19 | 0.603 | 12 | 1 | |||||||||
| No | 5 | 10 | OM ≤ PT | 5 | 10 | PE ≤ PT | 2 | 7 | |||||||
| Yes | 9 | 18 | OM > PT | 2 | 2 | PE > PT | 1 | 2 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dötzer, K.; Schlüter, F.; Schoenberg, M.B.; Bazhin, A.V.; Edler von Koch, F.; Schnelzer, A.; Anthuber, S.; Grab, D.; Czogalla, B.; Burges, A.; et al. Immune Heterogeneity Between Primary Tumors and Corresponding Metastatic Lesions and Response to Platinum Therapy in Primary Ovarian Cancer. Cancers 2019, 11, 1250. https://doi.org/10.3390/cancers11091250
Dötzer K, Schlüter F, Schoenberg MB, Bazhin AV, Edler von Koch F, Schnelzer A, Anthuber S, Grab D, Czogalla B, Burges A, et al. Immune Heterogeneity Between Primary Tumors and Corresponding Metastatic Lesions and Response to Platinum Therapy in Primary Ovarian Cancer. Cancers. 2019; 11(9):1250. https://doi.org/10.3390/cancers11091250
Chicago/Turabian StyleDötzer, Katharina, Friederike Schlüter, Markus Bo Schoenberg, Alexandr V. Bazhin, Franz Edler von Koch, Andreas Schnelzer, Sabine Anthuber, Dieter Grab, Bastian Czogalla, Alexander Burges, and et al. 2019. "Immune Heterogeneity Between Primary Tumors and Corresponding Metastatic Lesions and Response to Platinum Therapy in Primary Ovarian Cancer" Cancers 11, no. 9: 1250. https://doi.org/10.3390/cancers11091250
APA StyleDötzer, K., Schlüter, F., Schoenberg, M. B., Bazhin, A. V., Edler von Koch, F., Schnelzer, A., Anthuber, S., Grab, D., Czogalla, B., Burges, A., Werner, J., Mahner, S., & Mayer, B. (2019). Immune Heterogeneity Between Primary Tumors and Corresponding Metastatic Lesions and Response to Platinum Therapy in Primary Ovarian Cancer. Cancers, 11(9), 1250. https://doi.org/10.3390/cancers11091250






