Clinical Relevance of Collagen Protein Degradation Markers C3M and C4M in the Serum of Breast Cancer Patients Treated with Neoadjuvant Therapy in the GeparQuinto Trial
Abstract
1. Introduction
2. Results
2.1. Serum Levels of Collagen Degradation Markers
2.2. Collagen Degradation Markers and Response to Therapy
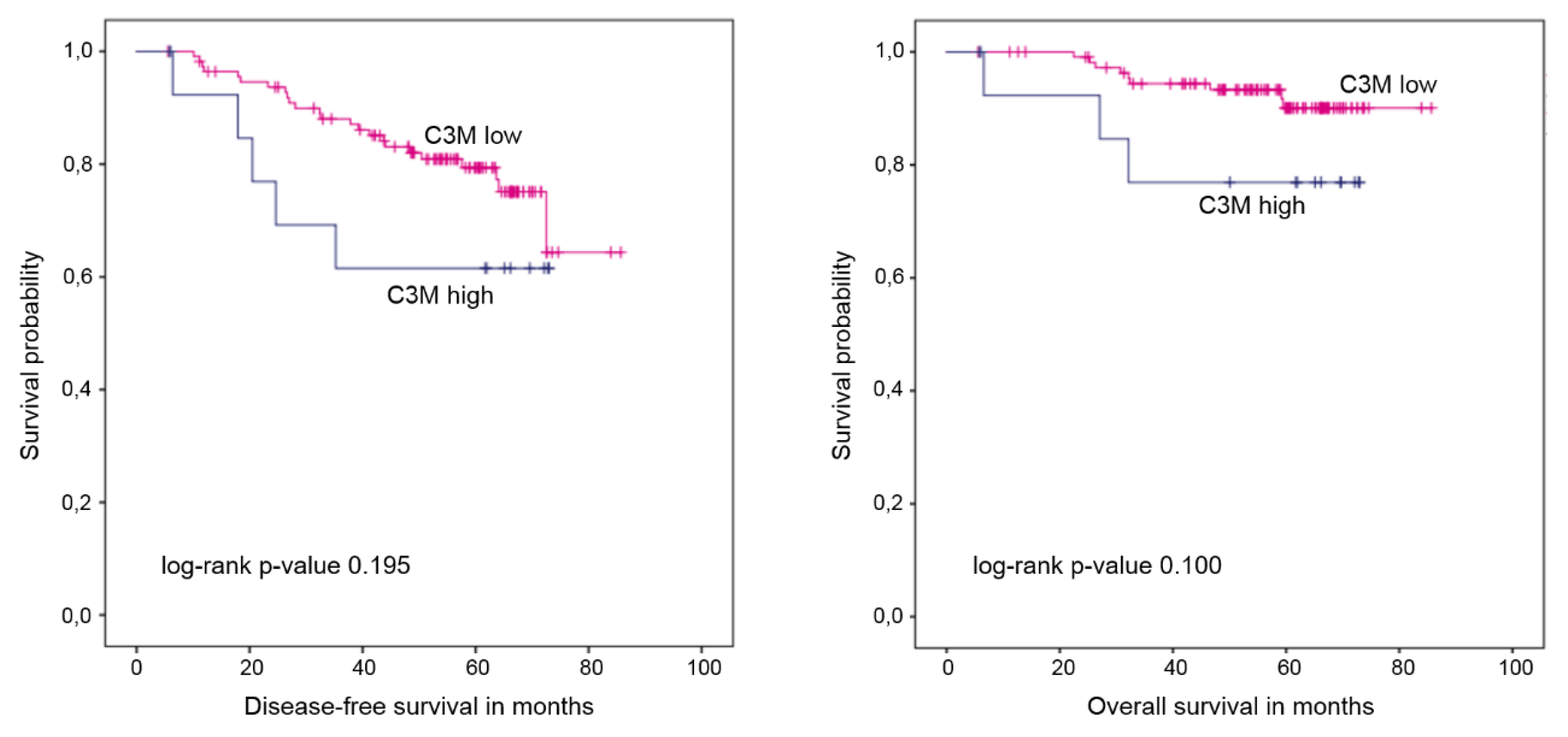
2.3. Collagen Degradation Markers and Survival
3. Discussion
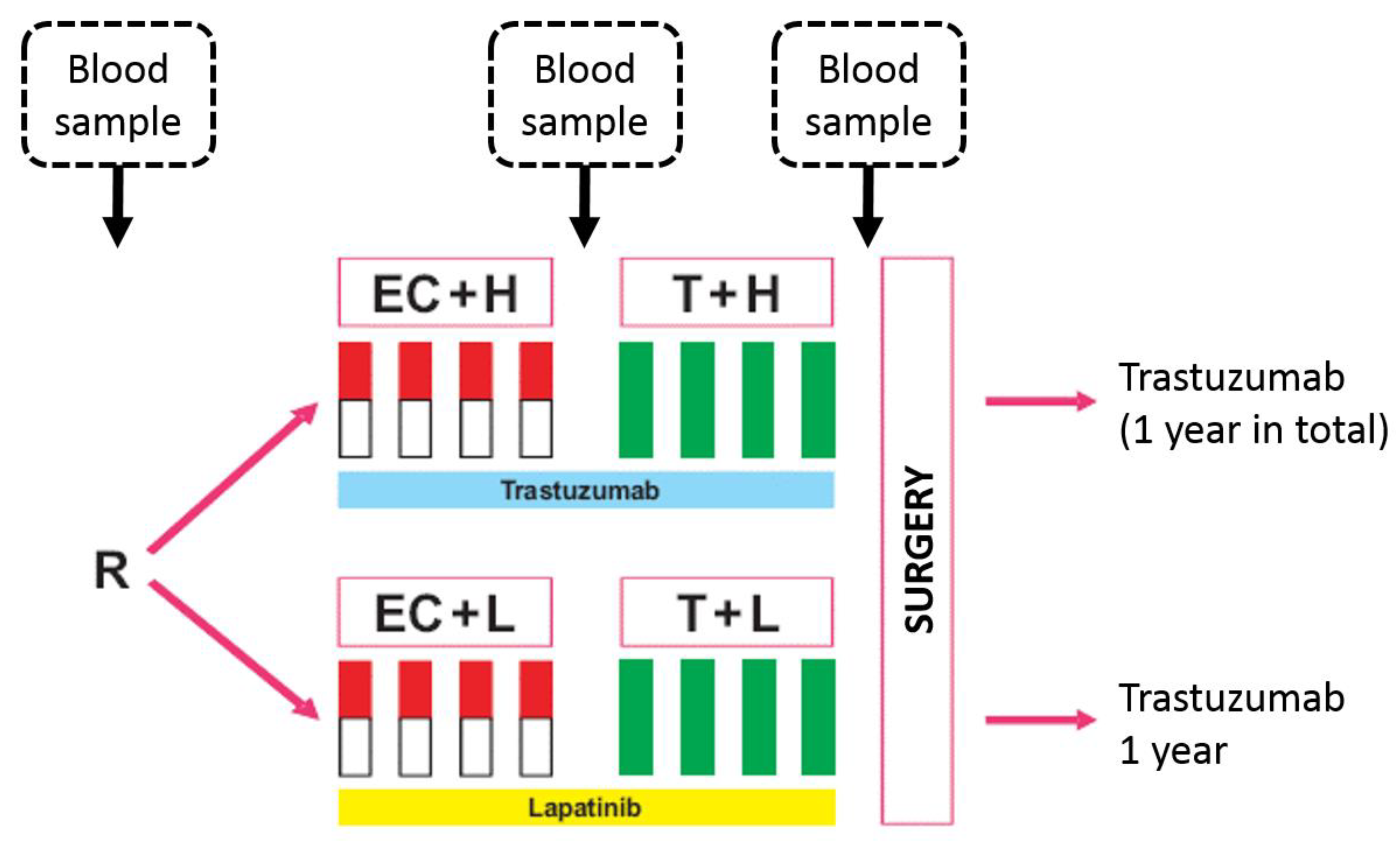
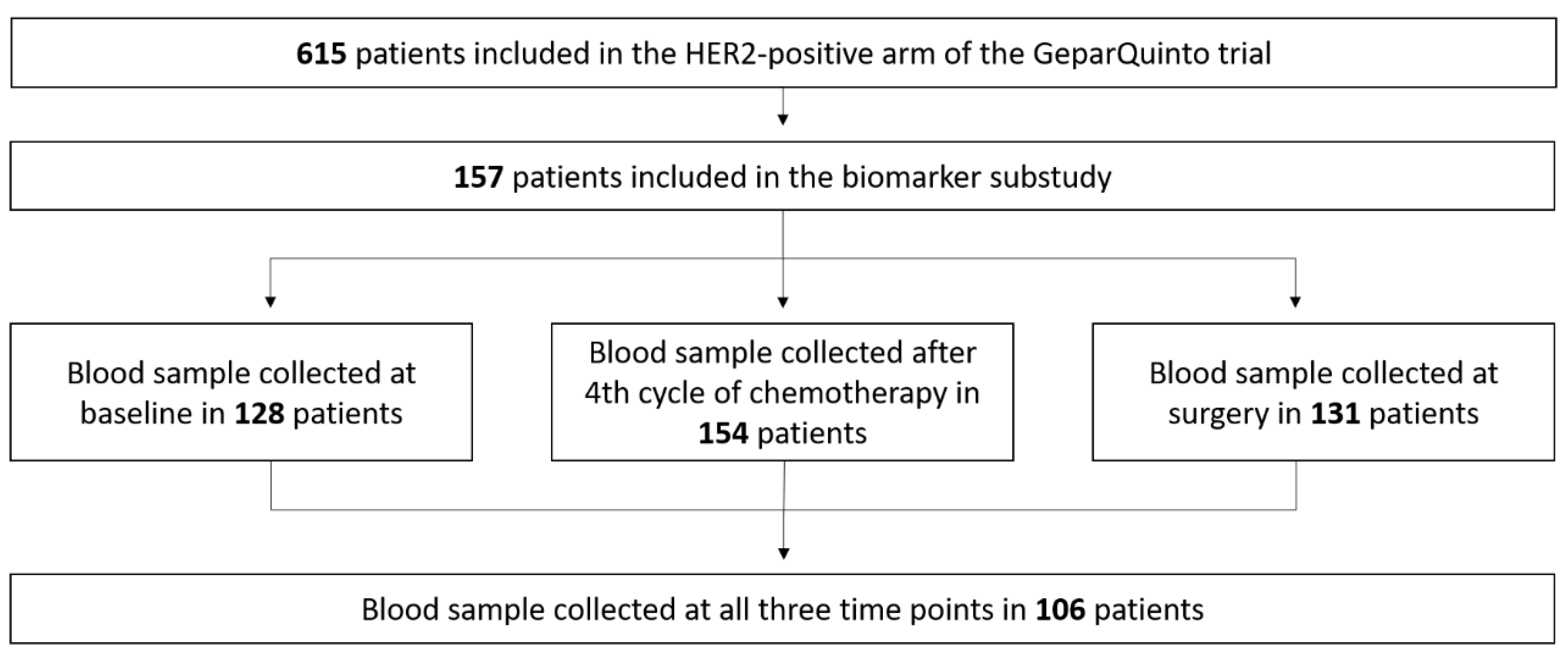
4. Materials and Methods
4.1. Quantitative Analysis of C3M and C4M
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Trastuzumab | Lapatinib | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Tumor size | ||||
| T1 | 10 | 13.0 | 8 | 10.3 |
| T2 | 39 | 50.6 | 37 | 47.4 |
| T3 | 14 | 18.2 | 23 | 29.5 |
| T4 | 14 | 18.2 | 10 | 12.8 |
| Missing | 0 | 2 | ||
| Grading | ||||
| G1 | 1 | 1.3 | 1 | 1.3 |
| G2 | 35 | 45.5 | 40 | 50.0 |
| G3 | 41 | 53.2 | 39 | 48.8 |
| Clinical nodal status | ||||
| Positive | 44 | 57.1 | 46 | 59.0 |
| Negative | 33 | 42.9 | 32 | 41.0 |
| Missing | 0 | 2 | ||
| pCR | ||||
| Yes | 23 | 29.9 | 22 | 27.5 |
| No | 54 | 70.1 | 58 | 72.5 |
| End Point | Collagen Degradation Marker | Category | 5-Year Rates (%) | HR (95% CI), p-Values | Log-Rank p-Value |
|---|---|---|---|---|---|
| DFS | C3M | No change * | 89.2 | reference | |
| Increase | 80.9 | 1.30 (0.46–3.65) | 0.005 | ||
| ≥20% | p = 0.620 | ||||
| Decrease | 53.0 | 3.55 (1.51–8.31) | |||
| ≥20% | p = 0.004 | ||||
| OS | No change * | 96.5 | reference | ||
| Increase | 83.8 | 3.76 (0.69–20.50) | 0.037 | ||
| ≥20% | p = 0.127 | ||||
| Decrease | 79.1 | 6.49 (1.31–32.23) | |||
| ≥20% | p = 0.022 | ||||
| DFS | C4M | No change * | 73.4 | reference | |
| Increase | 83.3 | 0.80 (0.33–1.95) | 0.872 | ||
| ≥20% | p = 0.623 | ||||
| Decrease | 78.3 | 0.86 (0.31–2.34) | |||
| ≥20% | p = 0.765 | ||||
| OS | No change * | 91.7 | reference | ||
| Increase | 83.5 | 1.54 (0.41–5.73) | 0.748 | ||
| ≥20% | p = 0.522 | ||||
| Decrease | 85.9 | 1.58 (0.38–6.62) | |||
| ≥20% | p = 0.530 |
| Value | Age (Years) | C3M (ng/mL) | C4M2 (ng/mL) |
|---|---|---|---|
| Mean ± SD | 51 ± 14.7 | 3.9 ± 1.1 | 28.2 ± 10.4 |
| Median (range) | 53 (31–78) | 3.6 (2.6–7.1) | 25.3 (18.3–58.6) |
References
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Nielsen, M.J.; Sand, J.M.; Henriksen, K.; Genovese, F.; Bay-Jensen, A.C.; Smith, V.; Adamkewicz, J.I.; Christiansen, C.; Leeming, D.J.; et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev. Technol. 2013, 11, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Bager, C.L.; Willumsen, N.; Leeming, D.J.; Smith, V.; Karsdal, M.A.; Dornan, D.; Bay-Jensen, A.C. Collagen degradation products measured in serum can separate ovarian and breast cancer patients from healthy controls: A preliminary study. Cancer Biomark. 2015, 15, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Bager, C.; Karsdal, M.A. Matrix Metalloprotease Generated Fragments of Type VI Collagen Have Serum Biomarker Potential in Cancer—A Proof of Concept Study. Transl. Oncol. 2019, 12, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, S.N.; Sanz-Pamplona, R.; Brix, S.; Leeming, D.J.; Karsdal, M.A.; Moreno, V. Excessive collagen turnover products are released during colorectal cancer progression and elevated in serum from metastatic colorectal cancer patients. Sci. Rep. 2016, 6, 30599. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Bager, C.L.; Leeming, D.J.; Smith, V.; Christiansen, C.; Karsdal, M.A.; Dornan, D.; Bay-Jensen, A.C. Serum biomarkers reflecting specific tumor tissue remodeling processes are valuable diagnostic tools for lung cancer. Cancer Med. 2014, 3, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Bager, C.L.; Leeming, D.J.; Smith, V.; Karsdal, M.A.; Dornan, D.; Bay-Jensen, A.C. Extracellular matrix specific protein fingerprints measured in serum can separate pancreatic cancer patients from healthy controls. BMC. Cancer 2013, 13, 554. [Google Scholar] [CrossRef]
- Lipton, A.; Leitzel, K.; Ali, S.M.; Polimera, H.V.; Nagabhairu, V.; Marks, E.; Richardson, A.E.; Krecko, L.; Ali, A.; Koestler, W.; et al. High turnover of extracellular matrix reflected by specific protein fragments measured in serum is associated with poor outcomes in two metastatic breast cancer cohorts. Int. J. Cancer 2018, 143, 3027–3034. [Google Scholar] [CrossRef]
- Willumsen, N.; Bager, C.L.; Kehlet, S.N.; Dragsbaek, K.; Neergaard, J.S.; Hansen, H.B.; Bay-Jensen, A.C.; Leeming, D.J.; Lipton, A.; Christiansen, C.; et al. Excessive matrix metalloprotease-mediated degradation of interstitial tissue (type I collagen) independently predicts short-term survival in an observational study of postmenopausal women diagnosed with cancer. Oncotarget 2017, 8, 52501–52510. [Google Scholar] [CrossRef]
- Kauppila, S.; Stenback, F.; Risteli, J.; Jukkola, A.; Risteli, L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J. Pathol. 1998, 186, 262–268. [Google Scholar] [CrossRef]
- Allinen, M.; Beroukhim, R.; Cai, L.; Brennan, C.; Lahti-Domenici, J.; Huang, H.; Porter, D.; Hu, M.; Chin, L.; Richardson, A.; et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, A.; Tagliabue, E.; Sorlie, T.; Naume, B.; Triulzi, T.; Orlandi, R.; Russnes, H.G.; Nesland, J.M.; Tammi, R.; Auvinen, P.; et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J. Pathol. 2008, 214, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Fu, H.J.; Jia, L.T.; Zhang, Y.; Li, W.; Jin, B.Q.; Yao, L.B.; Chen, S.Y.; Yang, A.G. HER2-mediated upregulation of MMP-1 is involved in gastric cancer cell invasion. Arch. Biochem. Biophys. 2010, 499, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Fatunmbi, M.; Shelton, J.; Aronica, S.M. MMP-9 increases HER2/neu expression and alters apoptosis levels in human mammary epithelial cells (HMEC). Breast Cancer Res. Treat 2012, 135, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Nami, B.; Wang, Z. HER2 in Breast Cancer Stemness: A Negative Feedback Loop towards Trastuzumab Resistance. Cancers (Basel) 2017, 9, 40. [Google Scholar] [CrossRef]
- Giussani, M.; Merlino, G.; Cappelletti, V.; Tagliabue, E.; Daidone, M.G. Tumor-extracellular matrix interactions: Identification of tools associated with breast cancer progression. Semin. Cancer Biol. 2015, 35, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Leyh, B. The impact of tumor stroma on drug response in breast cancer. Semin. Cancer Biol. 2015, 31, 3–15. [Google Scholar] [CrossRef]
- Jansen, M.P.; Foekens, J.A.; van Staveren, I.L.; Dirkzwager-Kiel, M.M.; Ritstier, K.; Look, M.P.; Meijer-van Gelder, M.E.; Sieuwerts, A.M.; Portengen, H.; Dorssers, L.C.; et al. Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J. Clin. Oncol. 2005, 23, 732–740. [Google Scholar] [CrossRef]
- Farmer, P.; Bonnefoi, H.; Anderle, P.; Cameron, D.; Wirapati, P.; Becette, V.; Andre, S.; Piccart, M.; Campone, M.; Brain, E.; et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med. 2009, 15, 68–74. [Google Scholar] [CrossRef]
- Hess, K.R.; Anderson, K.; Symmans, W.F.; Valero, V.; Ibrahim, N.; Mejia, J.A.; Booser, D.; Theriault, R.L.; Buzdar, A.U.; Dempsey, P.J.; et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J. Clin. Oncol. 2006, 24, 4236–4244. [Google Scholar] [CrossRef]
- Mazouni, C.; Arun, B.; Andre, F.; Ayers, M.; Krishnamurthy, S.; Wang, B.; Hortobagyi, G.N.; Buzdar, A.U.; Pusztai, L. Collagen IV levels are elevated in the serum of patients with primary breast cancer compared to healthy volunteers. Br. J. Cancer 2008, 99, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Revert, F.; Revert-Ros, F.; Blasco, R.; Artigot, A.; Lopez-Pascual, E.; Gozalbo-Rovira, R.; Ventura, I.; Gutierrez-Carbonell, E.; Roda, N.; Ruiz-Sanchis, D.; et al. Selective targeting of collagen IV in the cancer cell microenvironment reduces tumor burden. Oncotarget 2018, 9, 11020–11045. [Google Scholar] [CrossRef] [PubMed]
- Harisi, R.; Jeney, A. Extracellular matrix as target for antitumor therapy. Onco. Targets Ther. 2015, 8, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Witzel, I.; Loibl, S.; von Minckwitz, G.; Eidtmann, H.; Fehm, T.; Khandan, F.; Schmatloch, S.; Hauschild, M.; Bischoff, J.; Fasching, P.A.; et al. Predictive value of HER2 serum levels in patients treated with lapatinib or trastuzumab—A translational project in the neoadjuvant GeparQuinto trial. Br. J. Cancer 2012, 107, 956–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Untch, M.; Loibl, S.; Bischoff, J.; Eidtmann, H.; Kaufmann, M.; Blohmer, J.U.; Hilfrich, J.; Strumberg, D.; Fasching, P.A.; Kreienberg, R.; et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): A randomised phase 3 trial. Lancet. Oncol. 2012, 13, 135–144. [Google Scholar] [CrossRef]
- Untch, M.; von Minckwitz, G.; Gerber, B.; Schem, C.; Rezai, M.; Fasching, P.A.; Tesch, H.; Eggemann, H.; Hanusch, C.; Huober, J.; et al. Survival Analysis After Neoadjuvant Chemotherapy With Trastuzumab or Lapatinib in Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer in the GeparQuinto (G5) Study (GBG 44). J. Clin. Oncol. 2018, 36, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Dam, E.B.; Byrjalsen, I.; Karsdal, M.A.; Qvist, P.; Christiansen, C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthr. Cartil. 2009, 17, 384–389. [Google Scholar] [CrossRef]
- Veidal, S.S.; Vassiliadis, E.; Barascuk, N.; Zhang, C.; Segovia-Silvestre, T.; Klickstein, L.; Larsen, M.R.; Qvist, P.; Christiansen, C.; Vainer, B.; et al. Matrix metalloproteinase-9-mediated type III collagen degradation as a novel serological biochemical marker for liver fibrogenesis. Liver Int. 2010, 30, 1293–1304. [Google Scholar] [CrossRef]
- Veidal, S.S.; Karsdal, M.A.; Nawrocki, A.; Larsen, M.R.; Dai, Y.; Zheng, Q.; Hagglund, P.; Vainer, B.; Skjot-Arkil, H.; Leeming, D.J. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair 2011, 4, 22. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef]
- Hudis, C.A.; Barlow, W.E.; Costantino, J.P.; Gray, R.J.; Pritchard, K.I.; Chapman, J.A.; Sparano, J.A.; Hunsberger, S.; Enos, R.A.; Gelber, R.D.; et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J. Clin. Oncol. 2007, 25, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Total | C3M | C4M | ||||
|---|---|---|---|---|---|---|---|
| High C3M n (%) | Low C3M n (%) | p-Value 1 | High C4M n (%) | Low C4M n (%) | p-Value 1 | ||
| Overall | 128 | 15 (11.7%) | 113 (88.3%) | 19 (14.8%) | 109 (85.2%) | ||
| Age | |||||||
| >50 years | 66 | 8 (12.1%) | 58 (87.9%) | 1.000 | 10 (15.2%) | 56 (84.8%) | 1.000 |
| ≤50 years | 62 | 7 (11.3%) | 55 (88.7%) | 9 (14.5%) | 53 (85.5%) | ||
| cT stage | |||||||
| cT1/2 | 75 | 9 (12.0%) | 66 (88.0%) | 1.000 | 10 (13.3%) | 65 (86.7%) | 0.616 |
| cT3/4 | 52 | 6 (11.5%) | 46 (88.5%) | 9 (17.3%) | 43 (82.7%) | ||
| cN stage | |||||||
| cN0 | 51 | 6 (11.8%) | 45 (88.2%) | 6 (11.8%) | 45 (88.2%) | ||
| cN+ | 75 | 9 (12.0%) | 66 (88.0%) | 1.000 | 13 (17.3%) | 62 (82.7%) | 0.455 |
| Estrogen receptor status | |||||||
| Positive | 64 | 5 (7.8%) | 59 (92.2%) | 0.271 | 8 (12.5%) | 56 (87.5%) | 0.620 |
| Negative | 64 | 10 (15.6%) | 54 (84.4%) | 11 (17.2%) | 53 (82.8%) | ||
| Progesterone receptor status | |||||||
| Positive | 53 | 3 (5.7%) | 50 (94.3%) | 7 (13.2%) | 46 (86.8%) | ||
| Negative | 75 | 12 (16.0%) | 63 (84.0%) | 0.096 | 12 (16.0%) | 63 (84.0%) | 0.802 |
| Grading | |||||||
| G1–2 | 65 | 5 (7.7%) | 60 (92.3%) | 0.177 | 7 (10.8%) | 58 (89.2%) | 0.220 |
| G3 | 63 | 10 (15.9%) | 53 (84.1%) | 12 (19.0%) | 51 (81.0%) | ||
| Anti-HER2 therapy | |||||||
| Lapatinib | 63 | 6 (9.5%) | 57 (90.5%) | 0.585 | 9 (14.3%) | 54 (85.7%) | 1.000 |
| Trastuzumab | 65 | 9 (13.8%) | 56 (86.2%) | 10 (15.4%) | 55 (84.6%) | ||
| pCR | |||||||
| Yes | 39 | 10 (25.6%) | 29 (74.4%) | 0.002 | 10 (25.6%) | 29 (74.4%) | 0.031 |
| No | 89 | 5 (5.6%) | 84 (94.4%) | 9 (10.1%) | 80 (89.9%) | ||
| Time Point of Blood Sampling | Value | C3M (ng/mL) | C4M (ng/mL) | ||||
|---|---|---|---|---|---|---|---|
| Total | Trastuzumab Arm | Lapatinib Arm | Total | TRASTUZUMAB ARM | Lapatinib Arm | ||
| Baseline | Median | 6.364 | 6.316 | 6.636 | 31.940 | 32.180 | 31.724 |
| Mean | 6.597 | 6.588 | 6.606 | 33.148 | 34.058 | 32.209 | |
| Range | 1.640–17.236 | 3.128–17.236 | 1.640–12.264 | 15.940–71.684 | 15.940–71.684 | 17.816–69.192 | |
| After 4 cycles of neoadjuvant therapy | Median | 6.100 | 6.036 | 6.172 | 33.664 | 33.856 | 33.304 |
| Mean | 6.295 | 6.148 | 6.435 | 34.500 | 34.246 | 34.740 | |
| Range | 3.128–12.012 | 3.128–11.388 | 3.624–12.012 | 14.020–87.176 | 15.680–67.700 | 14.020–87.176 | |
| At time of surgery | Median | 5.352 | 5.352 | 5.290 | 30.604 | 30.742 | 29.814 |
| Mean | 5.747 | 5.826 | 5.675 | 32.760 | 33.632 | 31.952 | |
| Range | 1.724–14.316 | 1.724–13.132 | 2.496–14.316 | 14.784–78.996 | 18.416–70.060 | 14.784–78.996 | |
| Changes in Serum Levels | C3M n (%) | C4M n (%) | ||||
|---|---|---|---|---|---|---|
| Total | Trastuzumab | Lapatinib | Total | Trastuzumab | Lapatinib | |
| Increase ≥20% | 24 (22.6%) | 12 (23.1%) | 12 (22.2%) | 25 (23.6%) | 12 (22.6%) | 13 (24.5%) |
| No change 1 | 43 (40.6%) | 20 (38.5%) | 23 (42.6%) | 58 (54.7%) | 29 (54.7%) | 29 (54.7%) |
| Decrease ≥20% | 39 (36.8%) | 20 (38.5%) | 19 (35.2%) | 23 (21.7%) | 12 (22.6%) | 11 (20.8%) |
| Endpoint | C3M High vs. Low | C4M High vs. Low | |
|---|---|---|---|
| Disease-free survival | Median | Not reached vs. not reached | 63.7 months vs. not reached |
| Mean | 53.0 (95%-CI 38.9–67.1) vs. 72.2 (95%-CI 67.2–77.2) months | 49.5 (95%-CI 37.9–61.0) vs. 74.0 (95%-CI 69.2–8.8) months | |
| Log rank p-value | 0.195 | 0.001 | |
| 5-year DFS | 61.5% vs. 79.4% | 53.1% vs. 81.6% | |
| HR (95% CI), p-value | 1.88 (0.71–4.95), p = 0.202 | 3.39 (1.54–7.45), p = 0.002 | |
| Overall survival | Median | Not reached vs. not reached | Not reached vs. not reached |
| Mean | 61.2 (95%-CI 49.2–73.3) vs. 81.2 (95%-CI 78.4–84.0) months | 64.0 (95%-CI 54.8–73.3) vs. 81.0 (95%-CI 78.1–84.0) months | |
| Log rank p-value | 0.100 | 0.252 | |
| 5-year OS | 76.9% vs. 90.1% | 82.6% vs. 89.8% | |
| HR (95% CI), p-value | 2.86 (0.77–10.57), p = 0.116 | 2.11 (0.57–7.80), p = 0.263 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banys-Paluchowski, M.; Loibl, S.; Witzel, I.; Mundhenke, C.; Lederer, B.; Solbach, C.; Karn, T.; Marmé, F.; Nekljudova, V.; Schem, C.; et al. Clinical Relevance of Collagen Protein Degradation Markers C3M and C4M in the Serum of Breast Cancer Patients Treated with Neoadjuvant Therapy in the GeparQuinto Trial. Cancers 2019, 11, 1186. https://doi.org/10.3390/cancers11081186
Banys-Paluchowski M, Loibl S, Witzel I, Mundhenke C, Lederer B, Solbach C, Karn T, Marmé F, Nekljudova V, Schem C, et al. Clinical Relevance of Collagen Protein Degradation Markers C3M and C4M in the Serum of Breast Cancer Patients Treated with Neoadjuvant Therapy in the GeparQuinto Trial. Cancers. 2019; 11(8):1186. https://doi.org/10.3390/cancers11081186
Chicago/Turabian StyleBanys-Paluchowski, Malgorzata, Sibylle Loibl, Isabell Witzel, Christoph Mundhenke, Bianca Lederer, Christine Solbach, Thomas Karn, Frederik Marmé, Valentina Nekljudova, Christian Schem, and et al. 2019. "Clinical Relevance of Collagen Protein Degradation Markers C3M and C4M in the Serum of Breast Cancer Patients Treated with Neoadjuvant Therapy in the GeparQuinto Trial" Cancers 11, no. 8: 1186. https://doi.org/10.3390/cancers11081186
APA StyleBanys-Paluchowski, M., Loibl, S., Witzel, I., Mundhenke, C., Lederer, B., Solbach, C., Karn, T., Marmé, F., Nekljudova, V., Schem, C., Stickeler, E., Willumsen, N., Karsdal, M. A., Untch, M., & Müller, V. (2019). Clinical Relevance of Collagen Protein Degradation Markers C3M and C4M in the Serum of Breast Cancer Patients Treated with Neoadjuvant Therapy in the GeparQuinto Trial. Cancers, 11(8), 1186. https://doi.org/10.3390/cancers11081186






