Tailor-Made Detection of Individual Phosphorylated and Non-Phosphorylated EPIYA-Motifs of Helicobacter pylori Oncoprotein CagA
Abstract
:1. Introduction
2. Results
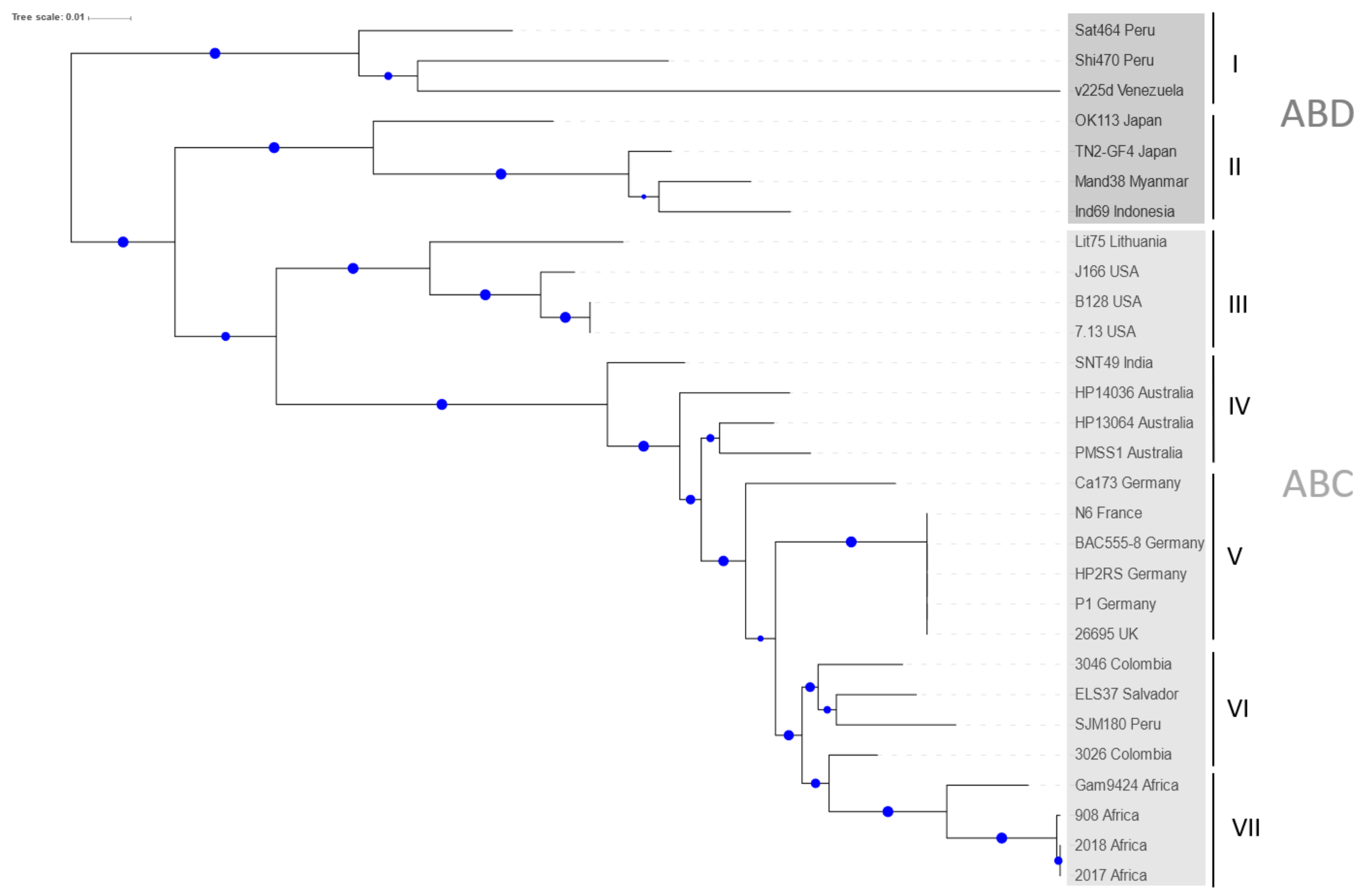
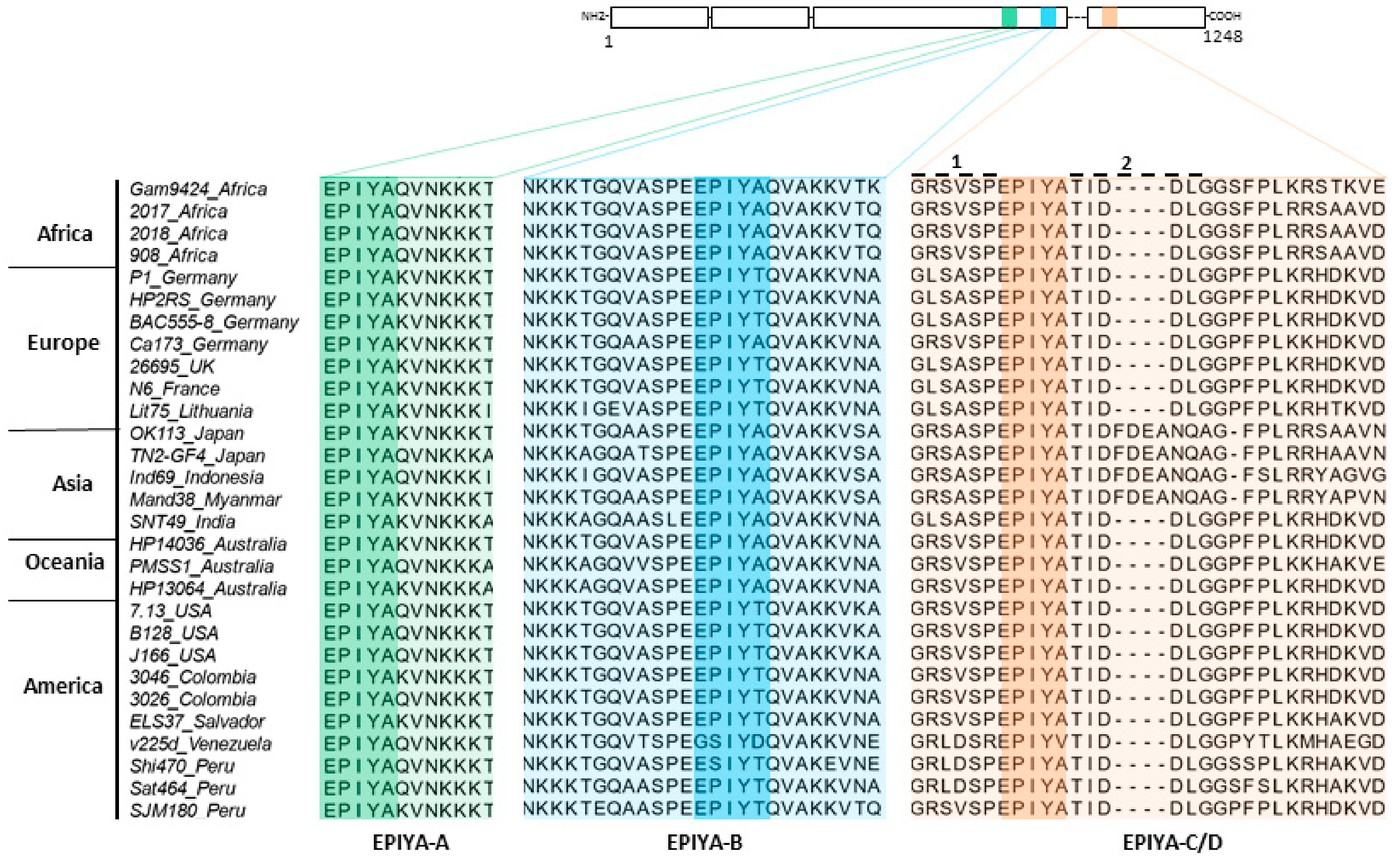
2.1. Phylogenetic Analysis of H. pylori CagA Carrying EPIYA-Motifs A, B, C and D
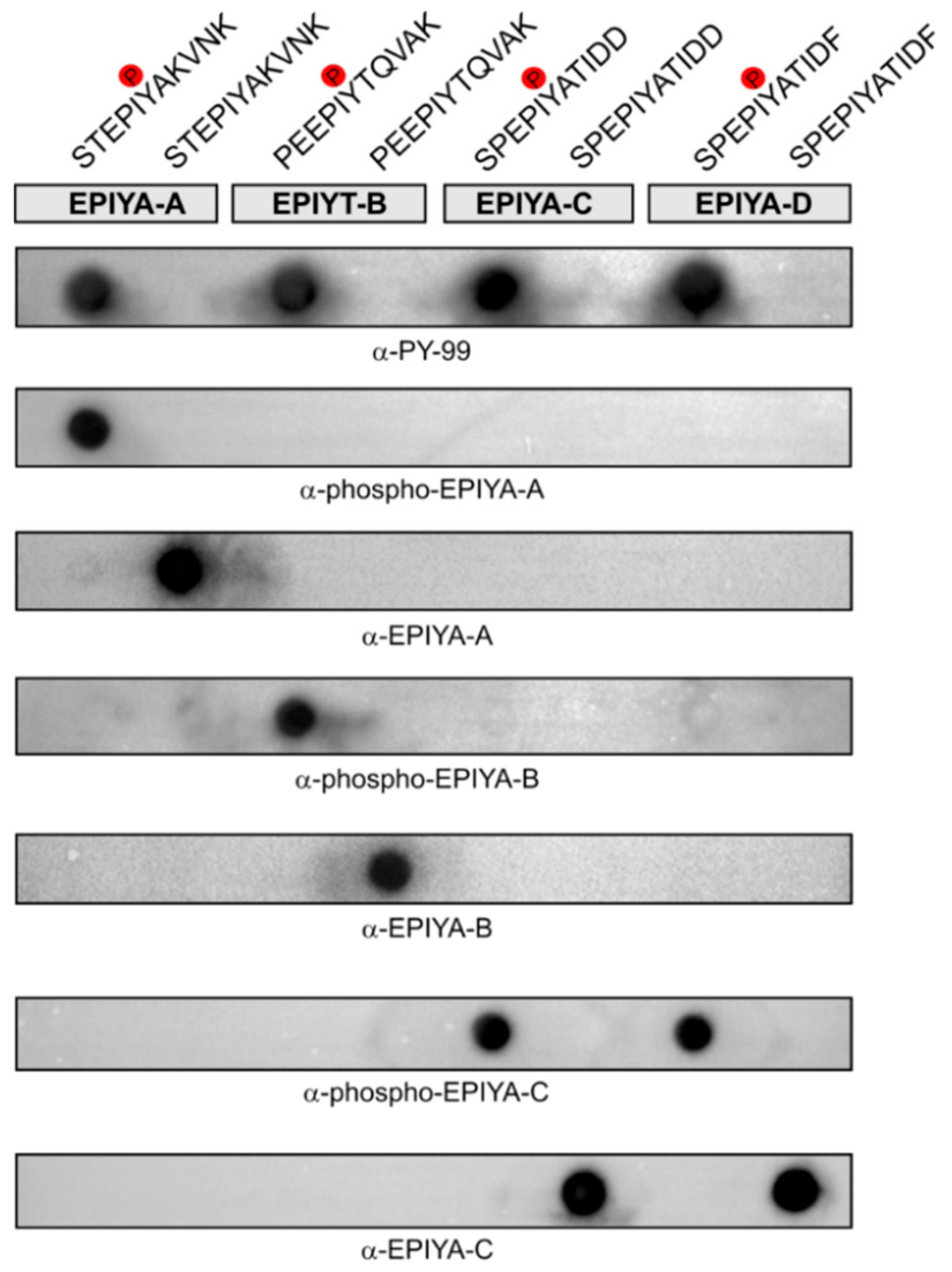
2.2. Generation of Phosphorylation-Specific Antibodies against EPIYA-Motifs A, B, and C
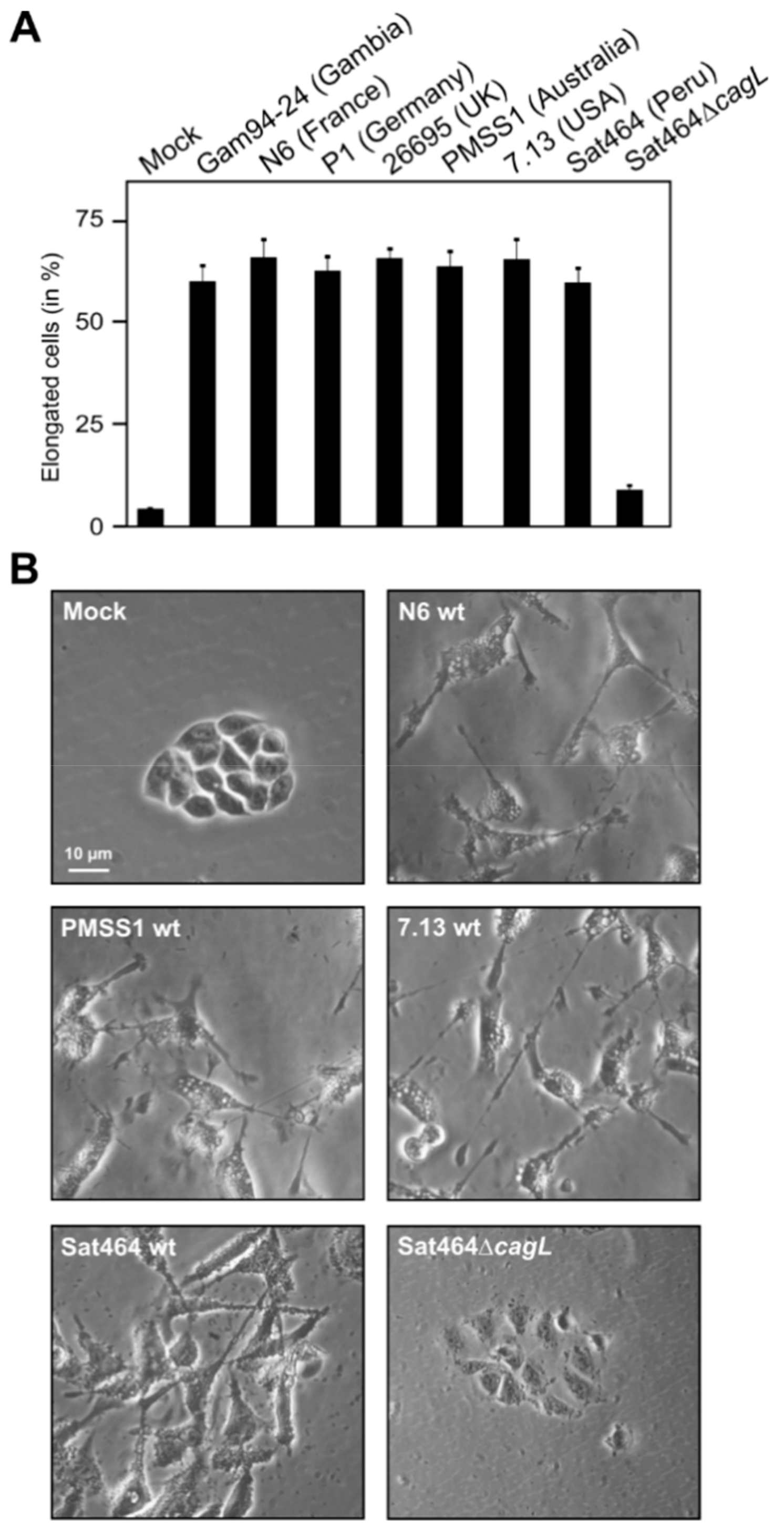
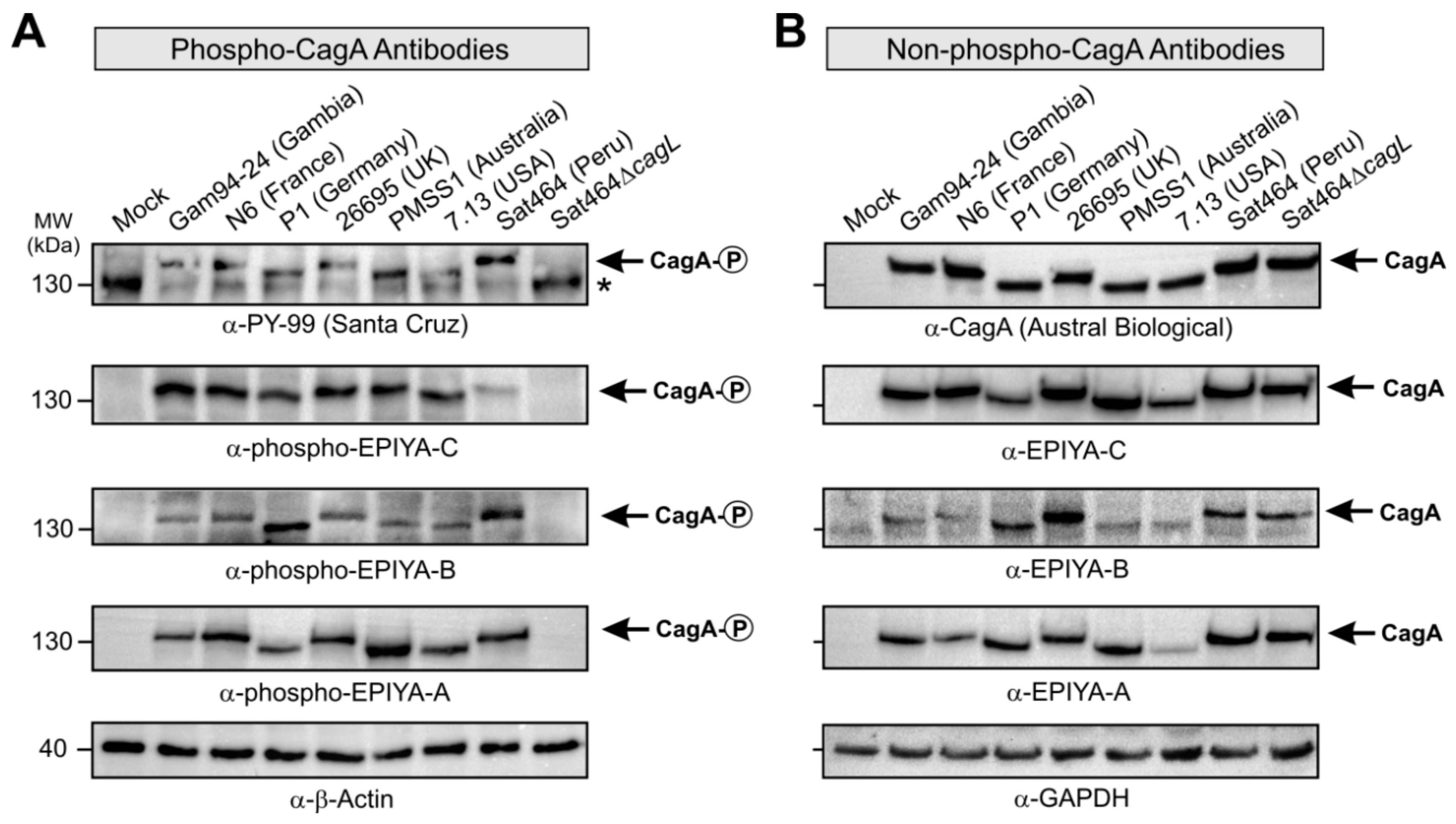
2.3. Monitoring of Phosphorylated EPIYA-Motifs in Multiple Strains Upon Infection of AGS Cells
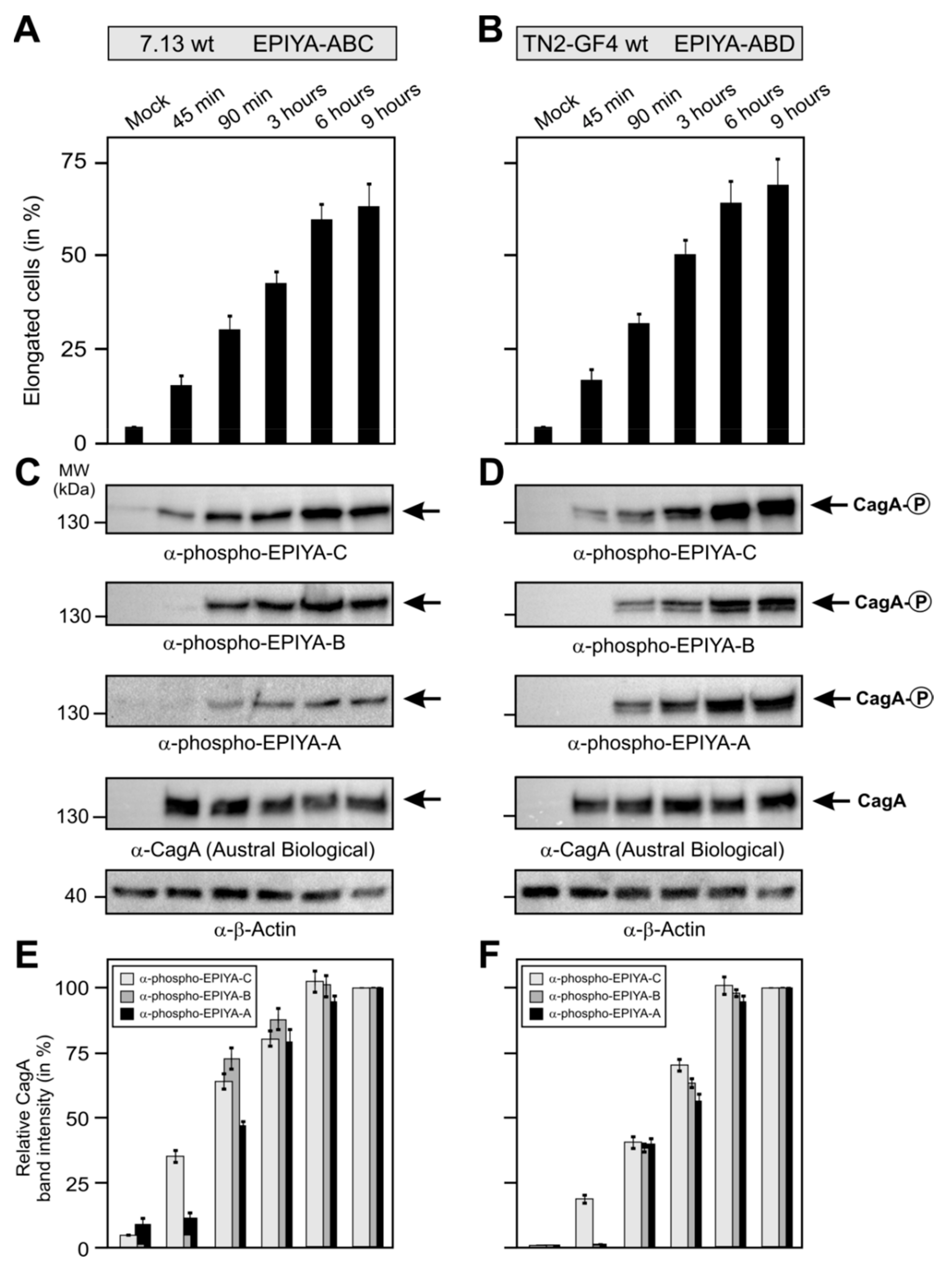
2.4. Phosphorylation of EPIYA-Motifs in ABC vs. ABD H. Pylori Strains during an Infection Time Course
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Analysis of CagA Amino Acid Sequences
4.2. H. pylori Strains and Culturing Conditions
4.3. Production of Phospho-and Non-Phospho-Specific EPIYA Antibodies
4.4. Dotblot Analysis
4.5. Host Cell Culture, Infection Assays, and Elongation Phenotype Quantitation
4.6. SDS-PAGE and Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Covacci, A.; Rappuoli, R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J. Exp. Med. 2000, 191, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Helicobacter pylori and gastric carcinogenesis. J. Gastroenterol. 2009, 44, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Haas, R.; Gerhard, M.; Naumann, M. The Helicobacter pylori Type IV Secretion System Encoded by the cag Pathogenicity Island: Architecture, Function, and Signaling. Curr. Top. Microbiol. Immunol. 2017, 413, 187–220. [Google Scholar] [PubMed]
- Kwok, T.; Zabler, D.; Urman, S.; Rohde, M.; Hartig, R.; Wessler, S.; Misselwitz, R.; Berger, J.; Sewald, N.; König, W.; et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 2007, 449, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Shaffer, C.L.; Rettberg, L.A.; Ghosal, D.; Jensen, G.J. In Vivo Structures of the Helicobacter pylori cag Type IV Secretion System. Cell Rep. 2018, 23, 673–681. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N.; Selbach, M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter 2010, 15, 163–176. [Google Scholar] [CrossRef]
- Kaplan-Türköz, H.; Jiménez-Soto, L.F.; Dian, C.; Ertl, C.; Remaut, H.; Louche, A.; Tosi, T.; Haas, R.; Terradot, L. Structural insights into Helicobacter pylori oncoprotein CagA interaction with β1 integrin. Proc. Natl. Acad. Sci. USA 2012, 109, 14640–14645. [Google Scholar] [CrossRef]
- Hayashi, T.; Morohashi, H.; Hatakeyama, M. Bacterial EPIYA effectors—where do they come from? What are they? Where are they going? Cell Microbiol. 2013, 15, 377–385. [Google Scholar] [CrossRef]
- Botham, C.M.; Wandler, A.M.; Guillemin, K.A. Transgenic Drosophila model demonstrates that the Helicobacter pylori CagA protein functions as a eukaryotic Gab adaptor. PLoS Pathog. 2008, 4, e1000064. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Neddermann, M.; Asche, C.I.; Backert, S. Subversion of host kinases: A key network in cellular signaling hijacked by Helicobacter pylori CagA. Mol. Microbiol. 2017, 105, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Moese, S.; Hauck, C.R.; Meyer, T.F.; Backert, S. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 2002, 277, 6775–6778. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Bagnoli, F.; Halenbeck, R.; Rappuoli, R.; Fantl, W.J.; Covacci, A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 2002, 43, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Feller, S.M.; Römer, G.; Wessler, S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene 2007, 26, 3462–3472. [Google Scholar] [CrossRef] [PubMed]
- Tammer, I.; Brandt, S.; Hartig, R.; König, W.; Backert, S. Activation of Abl by Helicobacter pylori: A novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology 2007, 132, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Tegtmeyer, N.; Brandt, S.; Yamaoka, Y.; De Poire, E.; Sgouras, D.; Wessler, S.; Torres, J.; Smolka, A.; Backert, S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J. Clin. Investig. 2012, 122, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, E.; Christie, P.J.; Waksman, G.; Backert, S. Type IV secretion in Gram-negative and Gram-positive bacteria. Mol. Microbiol. 2018, 107, 455–471. [Google Scholar] [CrossRef]
- Backert, S.; Moese, S.; Selbach, M.; Brinkmann, V.; Meyer, T.F. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 2001, 42, 631–644. [Google Scholar] [CrossRef]
- Mimuro, H.; Suzuki, T.; Tanaka, J.; Asahi, M.; Haas, R.; Sasakawa, C. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell. 2002, 10, 745–755. [Google Scholar] [CrossRef]
- Higashi, H.; Tsutsumi, R.; Muto, S.; Sugiyama, T.; Azuma, T.; Asaka, M.; Hatakeyama, M. SHP2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 2002, 295, 683–686. [Google Scholar] [CrossRef]
- Xia, Y.; Yamaoka, Y.; Zhu, Q.; Matha, I.; Gao, X. A comprehensive sequence and disease correlation analyses for the C-terminal region of CagA protein of Helicobacter pylori. PLoS ONE 2009, 4, e7736. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Yahara, K.; Hatakeyama, M.; Kobayashi, I. Evolution of cagA oncogene of Helicobacter pylori through recombination. PLoS ONE 2011, 6, e23499. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Müller, E.C.; Jungblut, P.R.; Meyer, T.F. Tyrosine phosphorylation patterns and size modification of the Helicobacter pylori CagA protein after translocation into gastric epithelial cells. Proteomics 2001, 1, 608–617. [Google Scholar] [CrossRef]
- Aras, R.A.; Lee, Y.; Kim, S.K.; Israel, D.; Peek, R.M., Jr.; Blaser, M.J. Natural variation in populations of persistently colonizing bacteria affect human host cell phenotype. J. Infect. Dis. 2003, 188, 486–496. [Google Scholar]
- Argent, R.H.; Zhang, Y.; Atherton, J. Simple method for determination of the number of Helicobacter pylori CagA variable-region EPIYA tyrosine phosphorylation motifs by PCR. J. Clin. Microbiol. 2005, 43, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.C.; Kim, H.K.; Blaser, M.J. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell. Microbiol. 2006, 8, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Yamazaki, T.; Tsutsumi, R.; Higashi, H.; Onoe, K.; Yamazaki, S.; Azuma, T.; Hatakeyama, M. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology 2006, 130, 1181–1190. [Google Scholar] [CrossRef]
- Panayotopoulou, E.G.; Sgouras, D.N.; Papadakos, K.; Kalliaropoulos, A.; Papatheodoridis, G.; Mentis, A.F.; Archimandritis, A.J. Strategy to characterize the number and type of repeating EPIYA phosphorylation motifs in the carboxyl terminus of CagA protein in Helicobacter pylori clinical isolates. J. Clin. Microbiol. 2007, 45, 488–495. [Google Scholar] [CrossRef]
- Basso, D.; Zambon, C.F.; Letley, D.P.; Stranges, A.; Marchet, A.; Rhead, J.L.; Schiavon, S.; Guariso, G.; Ceroti, M.; Nitti, D.; et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology 2008, 135, 91–99. [Google Scholar] [CrossRef]
- Schmidt, H.M.; Goh, K.L.; Fock, K.M.; Hilmi, I.; Dhamodaran, S.; Forman, D.; Mitchell, H. Distinct cagA EPIYA motifs are associated with ethnic diversity in Malaysia and Singapore. Helicobacter 2009, 14, 256–263. [Google Scholar] [CrossRef]
- Miura, M.; Ohnishi, N.; Tanaka, S.; Yanagiya, K.; Hatakeyama, M. Differential oncogenic potential of geographically distinct Helicobacter pylori CagA isoforms in mice. Int. J. Cancer 2009, 125, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Truong, B.X.; Mai, V.T.; Tanaka, H.; Ly, L.T.; Thong, T.M.; Hai, H.H.; Van Long, D.; Furumatsu, K.; Yoshida, M.; Kutsumi, H.; et al. Diverse characteristics of the CagA gene of Helicobacter pylori strains collected from patients from southern Vietnam with gastric cancer and peptic ulcer. J. Clin. Microbiol. 2009, 47, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Joo, Y.M.; Jang, S.; Yoo, Y.J.; Lee, H.S.; Chung, I.S.; Olsen, C.H.; Whitmire, J.M.; Merrell, D.S.; Cha, J.H. Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. J. Clin. Microbiol. 2009, 47, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.D.; Cha, J.; Lo, J.; Falkow, S.; Tompkins, L.S. Altered states: Involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 1999, 96, 14559–14564. [Google Scholar] [CrossRef] [PubMed]
- Churin, Y.; Al-Ghoul, L.; Keep, O.; Meyer, T.F.; Birchmeier, W.; Naumann, M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell. Biol. 2003, 161, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Moese, S.; Meyer, T.F.; Backert, S. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 2002, 70, 6656–6671. [Google Scholar] [CrossRef]
- Selbach, M.; Moese, S.; Hurwitz, R.; Hauck, C.R.; Meyer, T.F.; Backert, S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003, 22, 515–528. [Google Scholar] [CrossRef]
- Naumann, M.; Sokolova, O.; Tegtmeyer, N.; Backert, S. Helicobacter pylori: A Paradigm Pathogen for Subverting Host Cell Signal Transmission. Trends Microbiol. 2017, 25, 316–328. [Google Scholar] [CrossRef]
- Odenbreit, S.; Püls, J.; Sedlmaier, B.; Gerland, E.; Fischer, W.; Haas, R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000, 287, 1497–1500. [Google Scholar] [CrossRef]
- Stein, M.; Rappuoli, R.; Covacci, A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 2000, 97, 1263–1268. [Google Scholar] [CrossRef]
- Asahi, M.; Azuma, T.; Ito, S.; Ito, Y.; Suto, H.; Nagai, Y.; Tsubokawa, M.; Tohyama, Y.; Maeda, S.; Omata, M.; et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 2000, 191, 593–602. [Google Scholar] [PubMed]
- Backert, S.; Ziska, E.; Brinkmann, V.; Zimny-Arndt, U.; Fauconnier, A.; Jungblut, P.R.; Naumann, M.; Meyer, T.F. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2000, 2, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.; Backert, S.; Pfleiderer, K.; Berg, D.E.; Yamaoka, Y.; Sticht, H.; Tegtmeyer, N. Systematic analysis of phosphotyrosine antibodies recognizing single phosphorylated EPIYA-motifs in CagA of Western-type Helicobacter pylori strains. PLoS ONE 2014, 9, e96488. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.; Backert, S.; Hoffmann, R.; Eichler, J.; Yamaoka, Y.; Perez-Perez, G.I.; Torres, J.; Sticht, H.; Tegtmeyer, N. Systematic analysis of phosphotyrosine antibodies recognizing single phosphorylated EPIYA-motifs in CagA of East Asian-type Helicobacter pylori strains. BMC Microbiol. 2016, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Asahi, M.; Tanaka, Y.; Izumi, T.; Ito, Y.; Naiki, H.; Kersulyte, D.; Tsujikawa, K.; Saito, M.; Sada, K.; Yanagi, S.; et al. Helicobacter pylori CagA containing ITAM-like sequences localized to lipid rafts negatively regulates VacA-induced signaling in vivo. Helicobacter 2003, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Tegtmeyer, N.; Traube, L.; Jindal, S.; Perez-Perez, G.; Sticht, H.; Backert, S.; Blaser, M.J. A specific A/T polymorphism in Western tyrosine phosphorylation B-motifs regulates Helicobacter pylori CagA epithelial cell interactions. PLoS Pathog. 2015, 11, e1004621. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Linz, B. Helicobacter pylori Sequences Reflect Past Human Migrations. Genome Dyn. 2009, 6, 62–74. [Google Scholar]
- Blaydes, J.P.; Vojtesek, B.; Bloomberg, G.B.; Hupp, T.R. The development and use of phospho-specific antibodies to study protein phosphorylation. Methods Mol. Biol. 2000, 99, 177–189. [Google Scholar]
- Houseman, B.T.; Huh, J.H.; Kron, S.J.; Mrksich, M. Peptide chips for the quantitative evaluation of protein kinase activity. Nat. Biotechnol. 2002, 20, 270–274. [Google Scholar] [CrossRef]
- Kim, M.; Shin, D.S.; Kim, J.; Lee, Y.S. Substrate screening of protein kinases: Detection methods and combinatorial peptide libraries. Biopolymers 2010, 94, 753–762. [Google Scholar] [CrossRef]
- Tinti, M.; Nardozza, A.P.; Ferrari, E.; Sacco, F.; Corallino, S.; Cesareni, G. The 4G10, pY20 and p-TYR-100 antibody specificity: Profiling by peptide microarrays. Nat. Biotechnol. 2012, 29, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Schwarz, T.; Miehlke, S.; Kirsch, C.; Sommer, C.; Kwok, T.; Gerhard, M.; Goebel, U.B.; Lehn, N.; Koenig, W.; et al. Functional analysis of the cag pathogenicity island in Helicobacter pylori isolates from patients with gastritis, peptic ulcer, and gastric cancer. Infect. Immun. 2004, 72, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Wessler, S.; Hartig, R.; Backert, S. Helicobacter pylori activates protein kinase C delta to control Raf in MAP kinase signalling: Role in AGS epithelial cell scattering and elongation. Cell Motil. Cytoskelet. 2009, 66, 874–892. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Zabler, D.; Schmidt, D.; Hartig, R.; Brandt, S.; Backert, S. Importance of EGF receptor, HER2/Neu and Erk1/2 kinase signalling for host cell elongation and scattering induced by the Helicobacter pylori CagA protein: Antagonistic effects of the vacuolating cytotoxin VacA. Cell. Microbiol. 2009, 11, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Posselt, G.; Backert, S.; Wessler, S. The functional interplay of Helicobacter pylori factors with gastric epithelial cells induces a multi-step process in pathogenesis. Cell Commun. Signal. 2013, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Wessler, S.; Backert, S. Abl family of tyrosine kinases and microbial pathogenesis. Int. Rev. Cell Mol. Biol. 2011, 286, 271–300. [Google Scholar] [PubMed]
- Backert, S.; Feller, S.M.; Wessler, S. Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem. Sci. 2008, 33, 80–90. [Google Scholar] [CrossRef]
- Hunter, T. Tyrosine phosphorylation: Thirty years and counting. Curr. Opin. Cell Biol. 2009, 21, 140–146. [Google Scholar] [CrossRef]
- Shi, L.; Ji, B.; Kolar-Znika, L.; Boskovic, A.; Jadeau, F.; Combet, C.; Grangeasse, C.; Franjevic, D.; Talla, E.; Mijakovic, I. Evolution of bacterial protein-tyrosine kinases and their relaxed specificity toward substrates. Genome Biol. Evol. 2014, 6, 800–817. [Google Scholar] [CrossRef]
- Alm, R.A.; Trust, T.J. Analysis of the genetic diversity of Helicobacter pylori: The tale of two genomes. J. Mol. Med. (Berl.) 1999, 77, 834–846. [Google Scholar] [CrossRef]
- Saju, P.; Murata-Kamiya, N.; Hayashi, T.; Senda, Y.; Nagase, L.; Noda, S.; Matsusaka, K.; Funata, S.; Kunita, A.; Urabe, M.; et al. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nat. Microbiol. 2016, 1, 16026. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Selbach, M. Tyrosine-phosphorylated bacterial effector proteins: The enemies within. Trends Microbiol. 2005, 13, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Paul, F.E.; Brandt, S.; Guye, P.; Daumke, O.; Backert, S.; Dehio, C.; Mann, M. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe 2009, 5, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sason, H.; Milgrom, M.; Weiss, A.M.; Melamed-Book, N.; Balla, T.; Grinstein, S.; Backert, S.; Rosenshine, I.; Aroeti, B. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol. Biol. Cell 2009, 20, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Clyne, M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2011, 16, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pachathundikandi, S.; Brandt, S.; Madassery, J.; Backert, S. Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-α. PLoS ONE 2011, 6, e19614. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, T.; Hofbaur, S.; Tegtmeyer, N.; Huber, S.; Sewald, N.; Wessler, S.; Backert, S.; Rieder, G. Helicobacter pylori CagL dependent induction of gastrin expression via a novel αvβ5-integrin-integrin linked kinase signalling complex. Gut 2012, 61, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Conradi, J.; Tegtmeyer, N.; Woźna, M.; Wissbrock, M.; Michalek, C.; Gagell, C.; Cover, T.L.; Frank, R.; Sewald, N.; Backert, S. An RGD helper sequence in CagL of Helicobacter pylori assists in interactions with integrins and injection of CagA. Front. Cell. Infect. Microbiol. 2012, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.; Tegtmeyer, N.; Rohde, M.; Rowland, M.; Oyarzabal, O.A.; Backert, S. Live Helicobacter pylori in the root canal of endodontic-infected deciduous teeth. J. Gastroenterol. 2012, 47, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Rivas Traverso, F.; Rohde, M.; Oyarzabal, O.A.; Lehn, N.; Schneider-Brachert, W.; Ferrero, R.L.; Fox, J.G.; Berg, D.E.; Backert, S. Electron microscopic, genetic and protein expression analyses of Helicobacter acinonychis strains from a Bengal tiger. PLoS ONE 2013, 8, e71220. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Dörfel, P.; Lurz, R.; Börner, T. Rolling-circle replication of mitochondrial DNA in the higher plant Chenopodium album (L.). Mol Cell Biol. 1996, 16, 6285–6294. [Google Scholar] [CrossRef]
- Backert, S.; Lurz, R.; Oyarzabal, O.A.; Börner, T. High content, size and distribution of single-stranded DNA in the mitochondria of Chenopodium album (L.). Plant. Mol. Biol. 1997, 33, 1037–1050. [Google Scholar] [CrossRef]
- Brisslert, M.; Enarsson, K.; Lundin, S.; Karlsson, A.; Kusters, J.G.; Svennerholm, A.M.; Backert, S.; Quiding-Järbrink, M. Helicobacter pylori induce neutrophil transendothelial migration: Role of the bacterial HP-NAP. FEMS Microbiol. Lett. 2005, 249, 95–103. [Google Scholar] [CrossRef]
- Brandt, S.; Kwok, T.; Hartig, R.; König, W.; Backert, S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 2005, 102, 9300–9305. [Google Scholar] [CrossRef] [PubMed]
- Moese, S.; Selbach, M.; Zimny-Arndt, U.; Jungblut, P.R.; Meyer, T.F.; Backert, S. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: Processing or breakage? Proteomics 2001, 1, 618–629. [Google Scholar] [CrossRef]
- Hoy, B.; Geppert, T.; Boehm, M.; Reisen, F.; Plattner, P.; Gadermaier, G.; Sewald, N.; Ferreira, F.; Briza, P.; Schneider, G. Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. J. Biol. Chem. 2012, 287, 10115–10120. [Google Scholar] [CrossRef] [PubMed]
- Moese, S.; Selbach, M.; Kwok, T.; Brinkmann, V.; König, W.; Meyer, T.F.; Backert, S. Helicobacter pylori induces AGS cell motility and elongation via independent signaling pathways. Infect. Immun. 2004, 72, 3646–3649. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Wittelsberger, R.; Hartig, R.; Wessler, S.; Martinez-Quiles, N.; Backert, S. Serine phosphorylation of cortactin controls focal adhesion kinase activity and cell scattering induced by Helicobacter pylori. Cell Host Microbe 2011, 9, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Hoy, B.; Löwer, M.; Weydig, C.; Carra, G.; Tegtmeyer, N.; Geppert, T.; Schröder, P.; Sewald, N.; Backert, S.; Schneider, G.; et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010, 11, 798–804. [Google Scholar] [PubMed]
- Moese, S.; Selbach, M.; Brinkmann, V.; Karlas, A.; Haimovich, B.; Backert, S.; Meyer, T.F. The Helicobacter pylori CagA protein disrupts matrix adhesion of gastric epithelial cells by dephosphorylation of vinculin. Cell. Microbiol. 2007, 9, 1148–1161. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Hartig, R.; Delahay, R.M.; Rohde, M.; Brandt, S.; Conradi, J.; Takahashi, S.; Smolka, A.J.; Sewald, N.; Backert, S. A small fibronectin-mimicking protein from bacteria induces cell spreading and focal adhesion formation. J. Biol. Chem. 2010, 285, 23515–23526. [Google Scholar] [CrossRef]
- Traverso, F.R.; Bohr, U.R.; Oyarzabal, O.A.; Rohde, M.; Clarici, A.; Wex, T.; Kuester, D.; Malfertheiner, P.; Fox, J.G.; Backert, S. Morphologic, genetic, and biochemical characterization of Helicobacter magdeburgensis, a novel species isolated from the intestine of laboratory mice. Helicobacter 2010, 15, 403–415. [Google Scholar] [CrossRef]
| Day | Step |
|---|---|
| 0 | Peptide synthesis and LPH coupling |
| 0 | Collection of pre-immune serum |
| 0 | First immunization of 2 rabbits |
| 7 | First boost |
| 14 | Second boost |
| 28 | Third boost |
| 42 | Bleeding, fourth boost |
| 56 | Fifth boost |
| 70 | Sixth boost |
| 77 | Bleeding |
| 105 | Final bleeding, serum IgG purification |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachathundikandi, S.K.; Gutiérrez-Escobar, A.J.; Tegtmeyer, N. Tailor-Made Detection of Individual Phosphorylated and Non-Phosphorylated EPIYA-Motifs of Helicobacter pylori Oncoprotein CagA. Cancers 2019, 11, 1163. https://doi.org/10.3390/cancers11081163
Pachathundikandi SK, Gutiérrez-Escobar AJ, Tegtmeyer N. Tailor-Made Detection of Individual Phosphorylated and Non-Phosphorylated EPIYA-Motifs of Helicobacter pylori Oncoprotein CagA. Cancers. 2019; 11(8):1163. https://doi.org/10.3390/cancers11081163
Chicago/Turabian StylePachathundikandi, Suneesh Kumar, Andrés Julián Gutiérrez-Escobar, and Nicole Tegtmeyer. 2019. "Tailor-Made Detection of Individual Phosphorylated and Non-Phosphorylated EPIYA-Motifs of Helicobacter pylori Oncoprotein CagA" Cancers 11, no. 8: 1163. https://doi.org/10.3390/cancers11081163
APA StylePachathundikandi, S. K., Gutiérrez-Escobar, A. J., & Tegtmeyer, N. (2019). Tailor-Made Detection of Individual Phosphorylated and Non-Phosphorylated EPIYA-Motifs of Helicobacter pylori Oncoprotein CagA. Cancers, 11(8), 1163. https://doi.org/10.3390/cancers11081163




