A Retrospective Evaluation of Risk of Peripartum Cardiac Dysfunction in Survivors of Childhood, Adolescent and Young Adult Malignancies
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
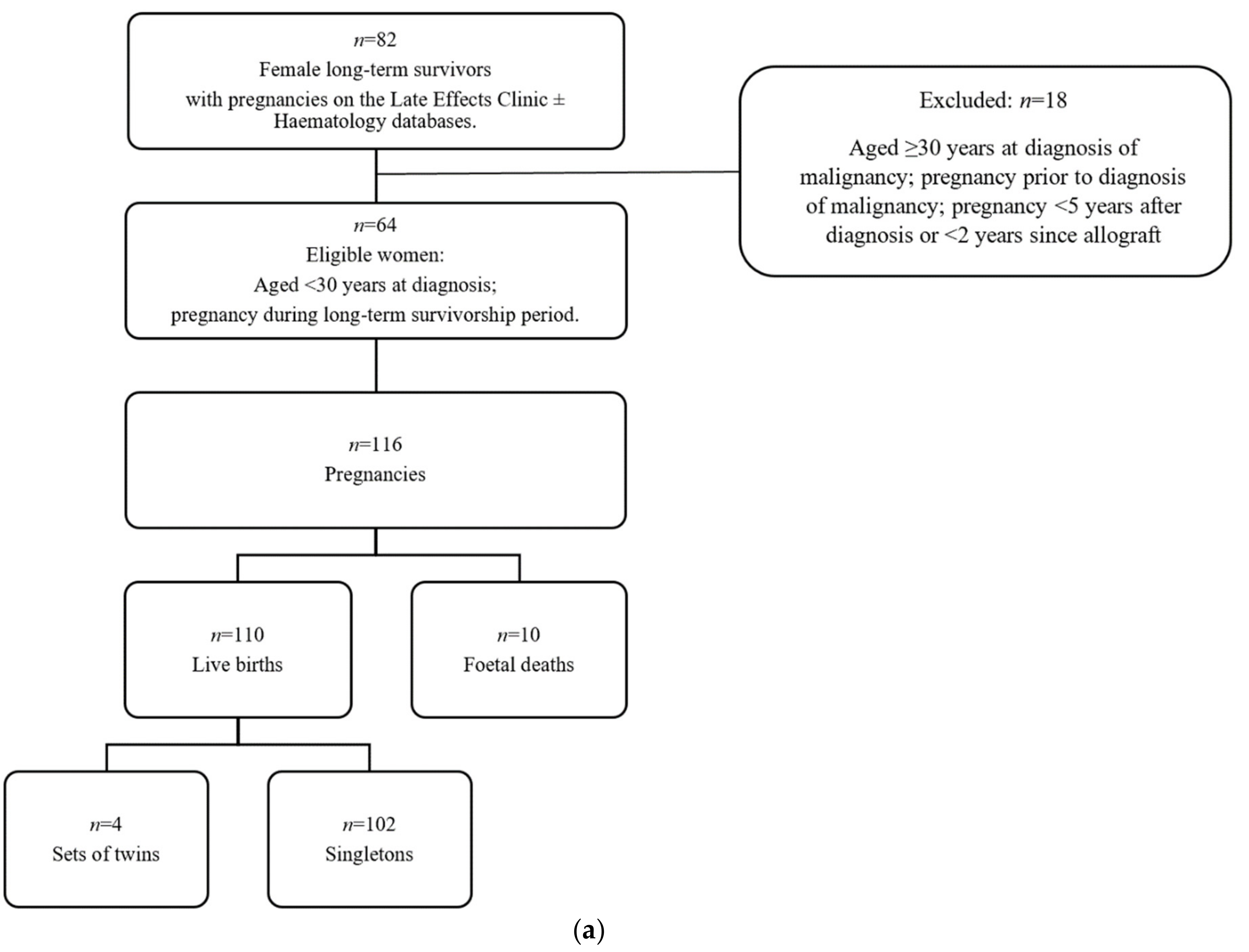
2.2. Characteristics of Pregnancies and Live Births
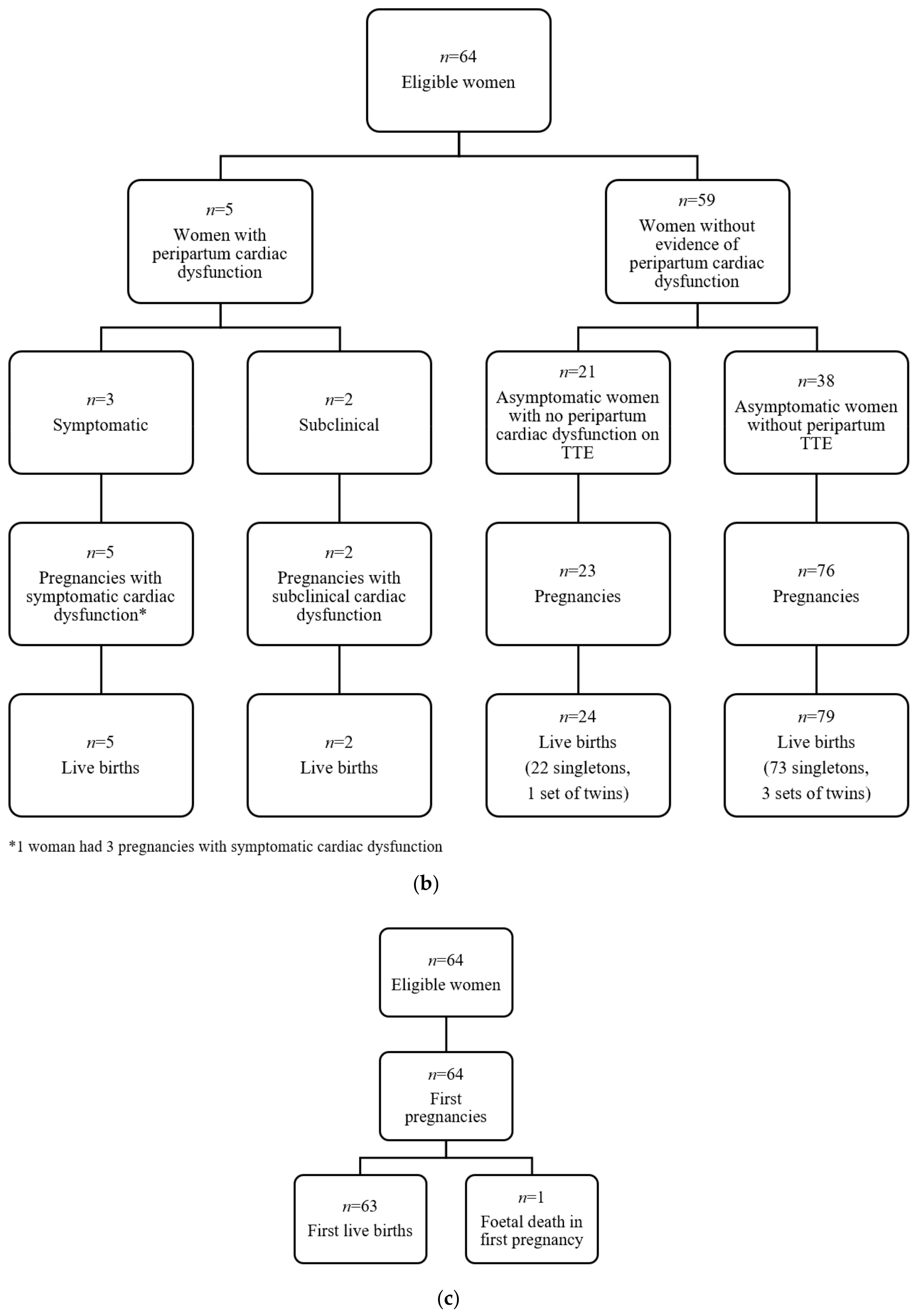
2.3. Characteristics of Cardiac Events
2.4. Symptomatic Cardiac Events
2.5. Subclinical Cardiac Events
2.6. Risk Factors for Cardiac Dysfunction and Symptomatic Cardiac Dysfunction Per First Live-Birth
2.7. Incidence of PPCM Per Live Birth and Comparison with the General Population
2.8. Maternal Outcomes Beyond the Postpartum Period
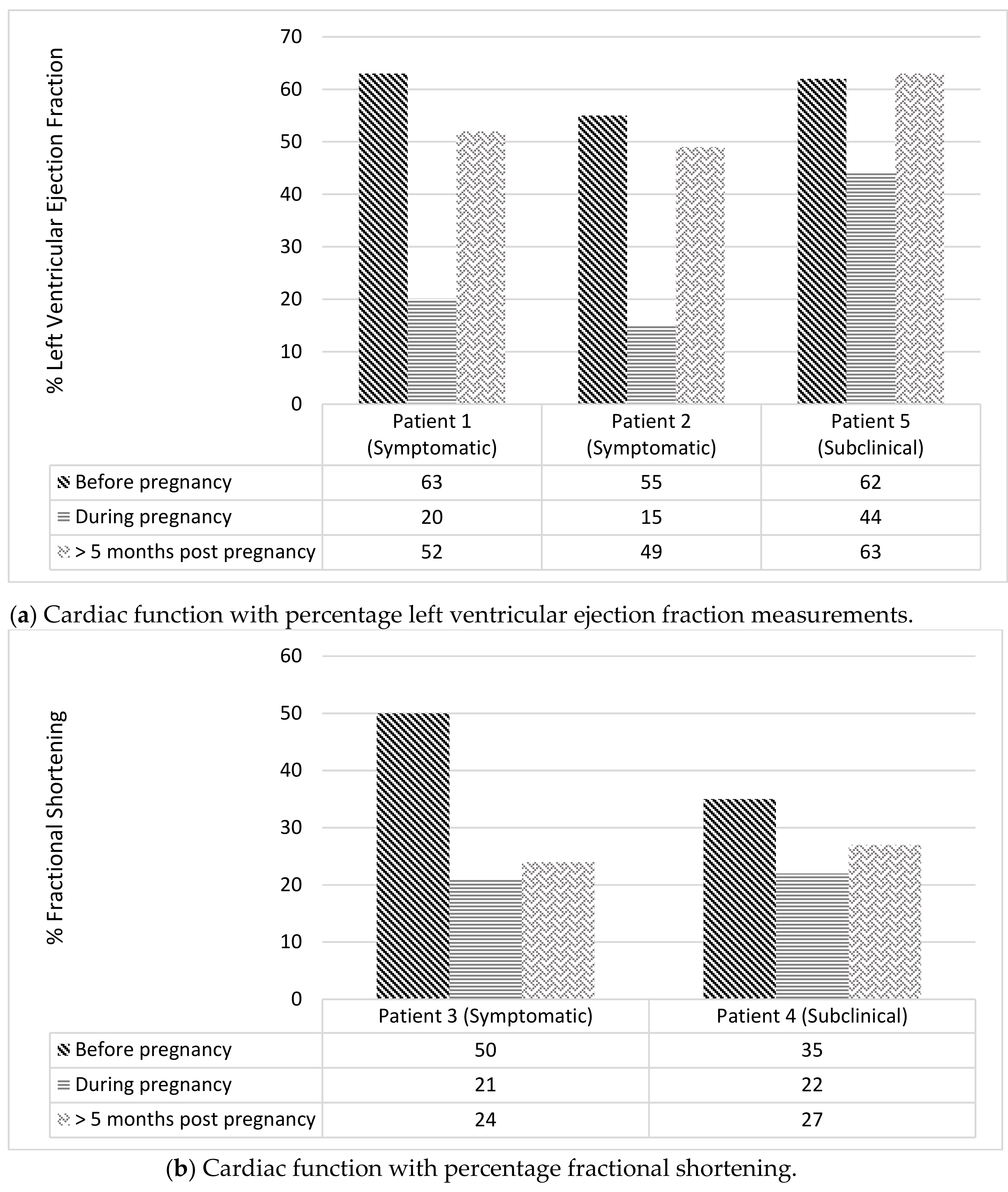
2.8.1. Outcomes for Women with Cardiac Events (n = 5)
2.8.2. Outcomes for Women with No Cardiac Events (n = 59)
3. Materials and Methods
3.1. Patient Selection and Study Design
3.2. Definitions of Cardiac Dysfunction
- (a)
- Clinical features: Measured by the New York Heart Association (NYHA) Functional Classification [15] with demonstration of specific clinical signs of cardiac decompensation: pulmonary oedema (radiographic or clinical), auscultation of S3, description of orthopnoea or paroxysmal nocturnal dyspnoea and/or response to diuretic therapy.
- (b)
- Pharmacological treatments: Requiring anti-failure therapy or diuretic therapy.
- (c)
- Functional quantification: Abnormal systolic function defined as FS < 28% or LVEF < 50%, as evidenced on TTE or cardiac MRI.
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 4.0; Children’s Oncology Group: Monrovia, CA, USA, 2013; Available online: http//:www.survivorshipguidelines.org (accessed on 5 July 2019).
- Groarke, J.D.; Nohria, A. Anthracycline Cardiotoxicity A New Paradigm for an Old Classic. Circulation 2015, 131, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.J.; Jorden, M. Pathology of Radiation and Anthracycline Cardiotoxicity. Pediatr. Blood Cancer 2005, 44, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.C.M.; Van Der Pal, H.J.H.; Offringa, M.; Van Dalen, E.C.; Voûte, P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann. Oncol. 2002, 13, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Colan, S.D.; Gelber, R.D.; Perez-Atayde, A.; Sallan, S.E.; Sanders, S.P. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 1991, 324, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female Sex and Higher Drug Dose as Risk Factors for Late Cardiotoxic Effects of Doxorubicin Therapy for Childhood Cancer. N. Engl. J. Med. 1995, 332, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Section on Hematology/Oncology Children’s Oncology Group. Long-term follow up care for Pediatric cancer survivors. Pediatrics 2008, 123, 906–915. [Google Scholar]
- Katz, A.; Maoz, C.; Thaler, M.; Grossman, E.; Rosenthal, T. Peripartum heart failure occurring in a patient previously treated with doxorubicin. Am. J. Med. Sci. 1997, 314, 399–400. [Google Scholar] [PubMed]
- Scheggi, V.; Mori, F. A 29-year-old pregnant woman with a history of anthracycline-induced clinical heart failure. Health 2011, 3, 37–38. [Google Scholar] [CrossRef][Green Version]
- Abboud, J.; Murad, Y.; Chen-Scarabelli, C.; Saravolatz, L.; Scarabelli, T.M. Peripartum cardiomyopathy: A comprehensive review. Int. J. Cardiol. 2007, 118, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lewey, J.; Haythe, J. Cardiomyopathy in pregnancy. Semin. Perinatol. 2014, 38, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Cancer in Adolescents and Young Adults in Australia; CAN 59; AIHW: Canberra, Australia, 2011.
- Allen, A. The cardiotoxicity of chemotherapeutic drugs. Semin. Oncol. 1992, 19, 529–542. [Google Scholar] [PubMed]
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed.; Little, Brown & Co.: Boston, MA, USA, 1994; pp. 253–256.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Campbell, B.; Wheeler, G.; Seymour, J.; Ritchie, D.; Goroncy, N. The Late Effects Clinic in action: For survivors of childhood malignancy. Acta Oncol. 2007, 46, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.R.; Mulrooney, D.A.; Hudson, M.M.; Ness, K.K.; Green, D.M.; Howard, S.C.; Krasin, M.; Metzger, M.L. Pregnancy-associated cardiomyopathy in survivors of childhood cancer. J. Cancer Surviv. 2016, 10, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.; Davidi, O.; Goshen, Y.; Hod, M.; Yaniv, I.; Hirsch, R. Pregnancy outcome in women treated with doxorubicin for childhood cancer. Am. J. Obstet. Gynecol. 2003, 189, 835–837. [Google Scholar] [CrossRef]
- Van Dalen, E.C.; Van Der Pal, H.J.H.; Van Den Bos, C.; Kok, W.E.M.; Carona, H.N.; Kremer, L.C.M. Clinical heart failure during pregnancy and delivery in a cohort of female childhood cancer survivors treated with anthracyclines. Eur. J. Cancer 2006, 42, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lipshultz, S.E. Pathophysiology of Anthracycline and Radiation-Associated Cardiomyopathies: Implications for Screening and Prevention. Pediatr. Blood Cancer 2005, 44, 600–606. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | n = 64 |
|---|---|
| Age at diagnosis of malignancy (years) | |
| Median, (Range) | 18 (2–29) |
| Malignancy Type (n, %) | |
| Leukaemia | 8 (12.5) |
| (a) ALL* | 4 (6) |
| (b) AML* | 3 (5) |
| (c) Dual phenotypes | 1 (2) |
| Lymphoma | 42 (66) |
| (a) NHL* | 10 (16) |
| (b) HL* | 32 (50) |
| Osteosarcoma | 1 (1.5) |
| Ewing sarcoma | 5 (8) |
| Hepatoblastoma | 1 (1.5) |
| Wilm’s Tumour | 4 (6) |
| Other solid tumour | 3 (5) |
| Anti-malignancy therapies (n, %) | |
| Anthracycline chemotherapy | 55 (86) |
| Non-anthracycline based treatment | 9 (14) |
| Chest radiotherapy | 37 (58) |
| (a) Chest radiotherapy only | 5 (8) |
| (b) Anthracyclines & chest radiotherapy | 28 (44) |
| (c) Chest radiotherapy & non-anthracycline chemotherapy | 4 (6) |
| Cumulative anthracycline dose (mg/m2) | n = 55 |
| Median, (Range) | 270 (150–600) |
| ≤300 mg/m2 (n, %) | 41 (74) |
| >300 mg/m2 (n, %) | 13 (24) |
| Unknown | 1 (2) |
| Total chest radiotherapy dose (Gy) | n = 37 |
| Median, [Range] | 36 (12–50) |
| Pregnancies and live-births (n) | |
| Total number of reported pregnancies | 116 |
| Live births | 110 |
| Singleton births | 102 |
| Twin births | 4 |
| Foetal deaths (n) | |
| Total | 10 |
| (a) Miscarriage | 7 |
| (b) Elective termination | 2 |
| (c) Ectopic pregnancy | 1 |
| Time from malignancy diagnosis to first pregnancy (years) | |
| Median, (Range) | 11 (5–37) |
| Unknown | 1 |
| Maternal age at onset of first pregnancy (years) | |
| Median, (Range) | 31 (19–42) |
| Unknown | 1 |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age at cancer diagnosis (years) | 9 | 4 | 13 | 12 | 14 |
| Cancer type | Ewing sarcoma | Hepatoblastoma | Ewing sarcoma | Ewing sarcoma | Hodgkin lymphoma |
| Anthracycline dose mg/m2 | 480 | 360 | 280 | 440 | 301.5 |
| Number of pregnancies | 1 | 1 | 3 | 2 | 2 |
| Number of live births | 1 | 1 | 3 | 2 | 1 |
| Time interval between cancer diagnosis and first pregnancy (years) | 28 | 18 | 6 | 14 | 17 |
| Most recent pre-pregnancy transthoracic echocardiogram | Normal, LVEF 63% | Normal, LVEF 55% | Normal, FS 50% | Normal, FS 35% | Normal, LVEF 62% |
| Cardiac event | Symptomatic HF | Symptomatic HF | Symptomatic HF | Subclinical | Subclinical |
| Functional quantification of cardiac event | LVEF 20% | LVEF <15% | FS 21% | FS 22% | LVEF 44% |
| Predictor | Median | Number of Cardiac Events | Odds Ratio, (95% Confidence Interval (CI)) | p-Value |
|---|---|---|---|---|
| Cardiac Dysfunction (n = 20 †) * | ||||
| Age at diagnosis of malignancy | 14.5 years | 5 | 0.853, (0.682, 1.010) ** | 0.031 |
| Anthracycline dose (×10) | 300 mg/m² | 5 | 1.150, (1.010, 1.360) | 0.015 |
| Maternal age at pregnancy | 29.5 years | 5 | 0.972, (0.803, 1.170) ** | 0.960 |
| Time from cancer diagnosis to pregnancy | 10.5 years | 5 | 1.090, (0.941, 1.260) ** | 0.200 |
| Cancer Type | 0.140, (0.002, 1.900) | 0.130 | ||
| (a) Haematological malignancy (n = 11) | 1 | |||
| (b) Solid Tumour (n = 9) | 4 | |||
| Chest radiotherapy | 0.000, (0.000, 2.390) | 0.260 | ||
| (a) Chest radiotherapy (n = 6) | 0 | |||
| (b) No chest radiotherapy (n = 14) | 5 | |||
| Symptomatic cardiac dysfunction (n = 63) * | ||||
| Age at diagnosis of malignancy | 18 years | 3 | 0.846, (0.685, 0.994) ** | 0.062 |
| Anthracycline dose (×10) | 226.5 mg/m² | 3 | 1.070, (0.987, 1.160) | 0.083 |
| (missing n = 1) | ||||
| Maternal age at pregnancy | 31 years | 3 | 0.825, (0.622, 1.050) ** | 0.120 |
| (missing n = 1) | ||||
| Time from cancer diagnosis to pregnancy | 11 years | 3 | 1.080, (0.916, 1.280) ** | 0.260 |
| (missing n = 1) | ||||
| Cancer Type | 0.000, (0.000, 0.635) | 0.009 | ||
| (a) Haematological malignancy (n = 49) | 0 | |||
| (b) Solid Tumour (n = 14) | 3 | |||
| Chest radiotherapy | 0.000, (0.000, 1.760) | 0.074 | ||
| (a) Chest radiotherapy (n = 36) | 0 | |||
| (b) No chest radiotherapy (n = 27) | 3 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chait-Rubinek, L.; Mariani, J.A.; Goroncy, N.; Herschtal, A.; Wheeler, G.C.; Dwyer, M.K.; Seymour, J.F.; Campbell, B.A. A Retrospective Evaluation of Risk of Peripartum Cardiac Dysfunction in Survivors of Childhood, Adolescent and Young Adult Malignancies. Cancers 2019, 11, 1046. https://doi.org/10.3390/cancers11081046
Chait-Rubinek L, Mariani JA, Goroncy N, Herschtal A, Wheeler GC, Dwyer MK, Seymour JF, Campbell BA. A Retrospective Evaluation of Risk of Peripartum Cardiac Dysfunction in Survivors of Childhood, Adolescent and Young Adult Malignancies. Cancers. 2019; 11(8):1046. https://doi.org/10.3390/cancers11081046
Chicago/Turabian StyleChait-Rubinek, Lori, Justin A. Mariani, Natalie Goroncy, Alan Herschtal, Greg C. Wheeler, Mary K. Dwyer, John F. Seymour, and Belinda A. Campbell. 2019. "A Retrospective Evaluation of Risk of Peripartum Cardiac Dysfunction in Survivors of Childhood, Adolescent and Young Adult Malignancies" Cancers 11, no. 8: 1046. https://doi.org/10.3390/cancers11081046
APA StyleChait-Rubinek, L., Mariani, J. A., Goroncy, N., Herschtal, A., Wheeler, G. C., Dwyer, M. K., Seymour, J. F., & Campbell, B. A. (2019). A Retrospective Evaluation of Risk of Peripartum Cardiac Dysfunction in Survivors of Childhood, Adolescent and Young Adult Malignancies. Cancers, 11(8), 1046. https://doi.org/10.3390/cancers11081046





