Prostate Cancer Development Is Not Affected by Statin Use in Patients with Elevated PSA Levels
Abstract
1. Introduction
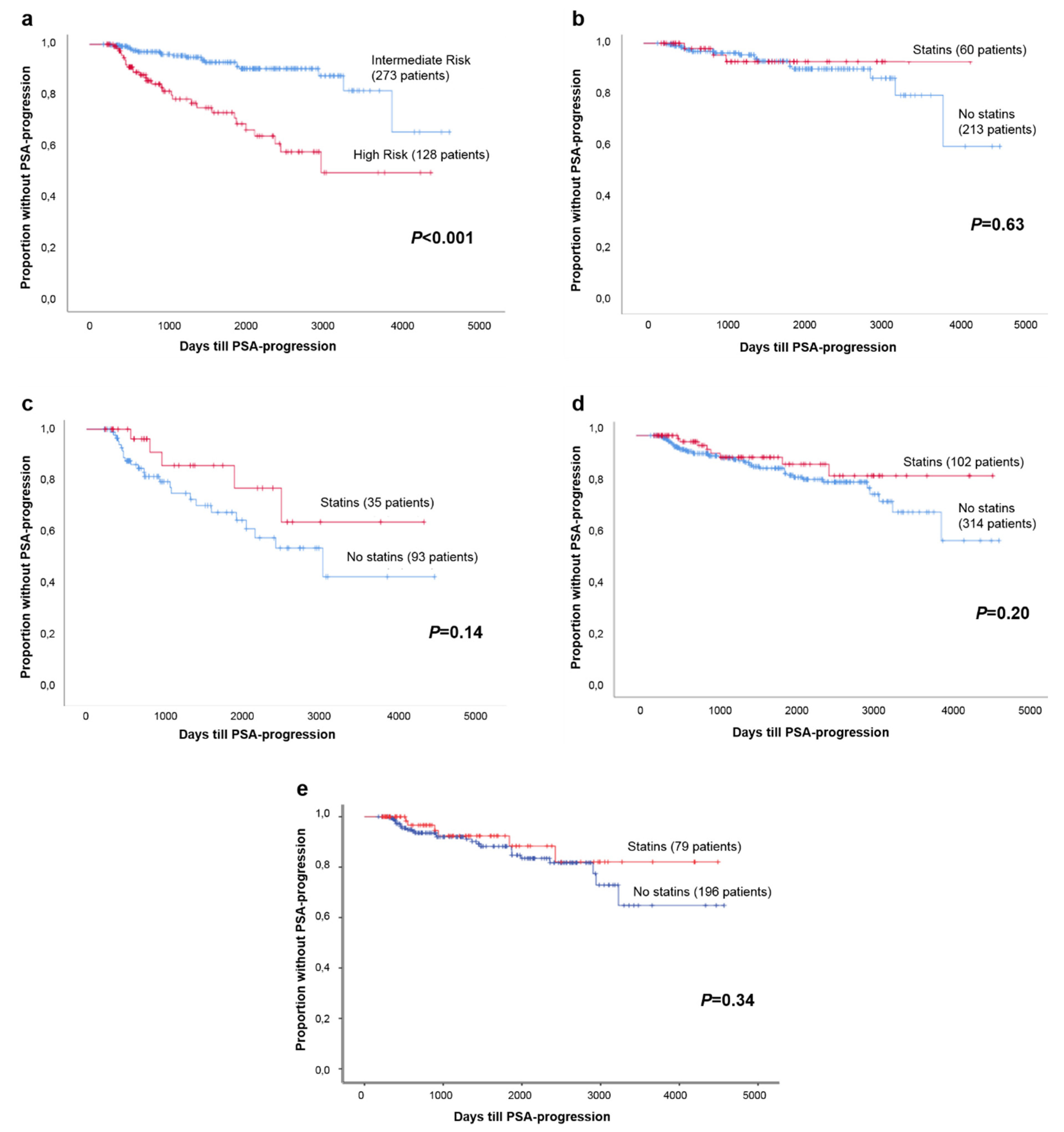
2. Results
3. Discussion
4. Material and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyle, P.; Ferlay, J. Cancer incidence and mortality in Europe, 2004. Ann. Oncol. 2005, 16, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Andriole, G.L.; Thompson, I.M., Jr.; Freedland, S.J. Smoking and Prostate Cancer: A Systematic Review. Eur. Urol. Focus 2015, 1, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Huncharek, M.; Haddock, K.S.; Reid, R.; Kupelnick, B. Smoking as a risk factor for prostate cancer: A meta-analysis of 24 prospective cohort studies. Am. J. Public Health 2010, 100, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Vandersluis, A.D.; Guy, D.E.; Klotz, L.H.; Fleshner, N.E.; Kiss, A.; Parker, C.; Venkateswaran, V. The role of lifestyle characteristics on prostate cancer progression in two active surveillance cohorts. Prostate Cancer Prostatic Dis. 2016, 19, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Thorat, M.A.; Andriole, G.; Brawley, O.W.; Brown, P.H.; Culig, Z.; Eeles, R.A.; Ford, L.G.; Hamdy, F.C.; Holmberg, L.; et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014, 15, e484–e492. [Google Scholar] [CrossRef]
- Hoque, A.; Chen, H.; Xu, X.C. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 2008, 17, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: A randomised placebo-controlled trial [ISRCTN48489393]. BMC Med. 2005, 3, 6. [Google Scholar] [CrossRef]
- Strandberg, T.E.; Pyorala, K.; Cook, T.J.; Wilhelmsen, L.; Faergeman, O.; Thorgeirsson, G.; Pedersen, T.R.; Kjekshus, J.; Group, S. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004, 364, 771–777. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Sitaras, N.M. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int. J. Cancer 2008, 123, 899–904. [Google Scholar] [CrossRef]
- Murtola, T.J.; Tammela, T.L.; Lahtela, J.; Auvinen, A. Cholesterol-lowering drugs and prostate cancer risk: A population-based case-control study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2226–2232. [Google Scholar] [CrossRef]
- Singal, R.; Khurana, V.; Caldito, G.; Fort, C. Statins and prostate cancer risk: A large case control study in veterans. J. Clin. Oncol. 2005, 23, 107s. [Google Scholar] [CrossRef]
- Platz, E.A.; Leitzmann, M.F.; Visvanathan, K.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. Statin drugs and risk of advanced prostate cancer. J. Natl. Cancer Inst. 2006, 98, 1819–1825. [Google Scholar] [CrossRef]
- Kaye, J.A.; Jick, H. Statin use and cancer risk in the General Practice Research Database. Br. J. Cancer 2004, 90, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Howard, L.E.; Cooperberg, M.R.; Kane, C.J.; Aronson, W.J.; Terris, M.K.; Amling, C.L.; Freedland, S.J. Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2014, 114, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Mohler, J.; Bahnson, R.R.; Boston, B.; Busby, J.E.; D’Amico, A.; Eastham, J.A.; Enke, C.A.; George, D.; Horwitz, E.M.; Huben, R.P.; et al. NCCN clinical practice guidelines in oncology: Prostate cancer. J. Natl. Compr. Cancer Netw. 2010, 8, 162–200. [Google Scholar] [CrossRef]
- Scosyrev, E.; Tobis, S.; Donsky, H.; Wu, G.; Joseph, J.; Rashid, H.; Messing, E. Statin use and the risk of biochemical recurrence of prostate cancer after definitive local therapy: A meta-analysis of eight cohort studies. BJU Int. 2013, 111, E71–E77. [Google Scholar] [CrossRef]
- Raval, A.D.; Thakker, D.; Negi, H.; Vyas, A.; Salkini, M.W. Association between statins and clinical outcomes among men with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2016, 19, 222. [Google Scholar] [CrossRef]
- Krane, L.S.; Kaul, S.A.; Stricker, H.J.; Peabody, J.O.; Menon, M.; Agarwal, P.K. Men presenting for radical prostatectomy on preoperative statin therapy have reduced serum prostate specific antigen. J. Urol. 2010, 183, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Schild, S.E.; Schild, M.H.; Wong, W.; Vora, S.; Herman, M.G.; Fatyga, M. Statins and Metformin Use Is Associated with Lower PSA Levels in Prostate Cancer Patients Presenting for Radiation Therapy. J. Cancer Ther. 2017, 8, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.J.; Goldberg, K.C.; Platz, E.A.; Freedland, S.J. The influence of statin medications on prostate-specific antigen levels. J. Natl. Cancer Inst. 2008, 100, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- CBS. Centraal Bureau van de Statistiek. In Population Based Information [Dutch]; CBS: The Hague, The Netherlands, 2017. [Google Scholar]
- Zorginstituut Nederland. GIP (Genees—En hulpmiddelen Informatie Project) databank. In Medication Use per Age [Dutch]; Zorginstituut Nederland: Diemen, The Netherlands, 2017. [Google Scholar]
- Caro-Maldonado, A.; Camacho, L.; Zabala-Letona, A.; Torrano, V.; Fernandez-Ruiz, S.; Zamacola-Bascaran, K.; Arreal, L.; Valcarcel-Jimenez, L.; Martin-Martin, N.; Flores, J.M.; et al. Low-dose statin treatment increases prostate cancer aggressiveness. Oncotarget 2018, 9, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhang, G.; Wang, X.; Xu, X. Body mass index and incidence of nonaggressive and aggressive prostate cancer: A dose-response meta-analysis of cohort studies. Oncotarget 2017, 8, 97584–97592. [Google Scholar] [CrossRef] [PubMed]
- Presti, J.C., Jr. Obesity and prostate cancer. Curr. Opin. Urol. 2005, 15, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.L.; Bell, R. Androgen physiology. Semin. Reprod. Med. 2006, 24, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Qin, X.J.; Zhang, H.L.; Xiao, W.J.; Zhu, Y.; Gu, C.Y.; Dai, B.; Shi, G.H.; Ye, D.W. Serum lipid profiles: Novel biomarkers predicting advanced prostate cancer in patients receiving radical prostatectomy. Asian J. Androl. 2015, 17, 239–244. [Google Scholar] [CrossRef]
- Hull, G.W.; Rabbani, F.; Abbas, F.; Wheeler, T.M.; Kattan, M.W.; Scardino, P.T. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J. Urol. 2002, 167, 528–534. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J., Jr.; Dotan, Z.A.; Fearn, P.A.; Kattan, M.W. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J. Natl. Cancer Inst. 2006, 98, 715–717. [Google Scholar] [CrossRef]
- Tourinho-Barbosa, R.; Srougi, V.; Nunes-Silva, I.; Baghdadi, M.; Rembeyo, G.; Eiffel, S.S.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X.; et al. Biochemical recurrence after radical prostatectomy: What does it mean? Int. Braz. J. Urol. 2018, 44, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kupelian, P.; Katcher, J.; Levin, H.; Zippe, C.; Klein, E. Correlation of clinical and pathologic factors with rising prostate-specific antigen profiles after radical prostatectomy alone for clinically localized prostate cancer. Urology 1996, 48, 249–260. [Google Scholar] [CrossRef]
- Tan, P.; Wei, S.; Yang, L.; Tang, Z.; Cao, D.; Liu, L.; Lei, J.; Fan, Y.; Gao, L.; Wei, Q. The effect of statins on prostate cancer recurrence and mortality after definitive therapy: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 29106. [Google Scholar] [CrossRef] [PubMed]
- Ishak-Howard, M.B.; Okoth, L.A.; Cooney, K.A. Statin use and the risk of recurrence after radical prostatectomy in a cohort of men with inherited and/or early-onset forms of prostate cancer. Urology 2014, 83, 1356–1361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A.; Committee, E.G. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v69–v77. [Google Scholar] [CrossRef]
- Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother. Rep. 1966, 50, 125–128. [Google Scholar] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. American Joint Committee on Cancer Staging Manual; Springer: New York, NY, USA, 2010. [Google Scholar]
- Nahm, F.S. Nonparametric statistical tests for the continuous data: The basic concept and the practical use. Korean J. Anesthesiol. 2016, 69, 8–14. [Google Scholar] [CrossRef]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric-Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
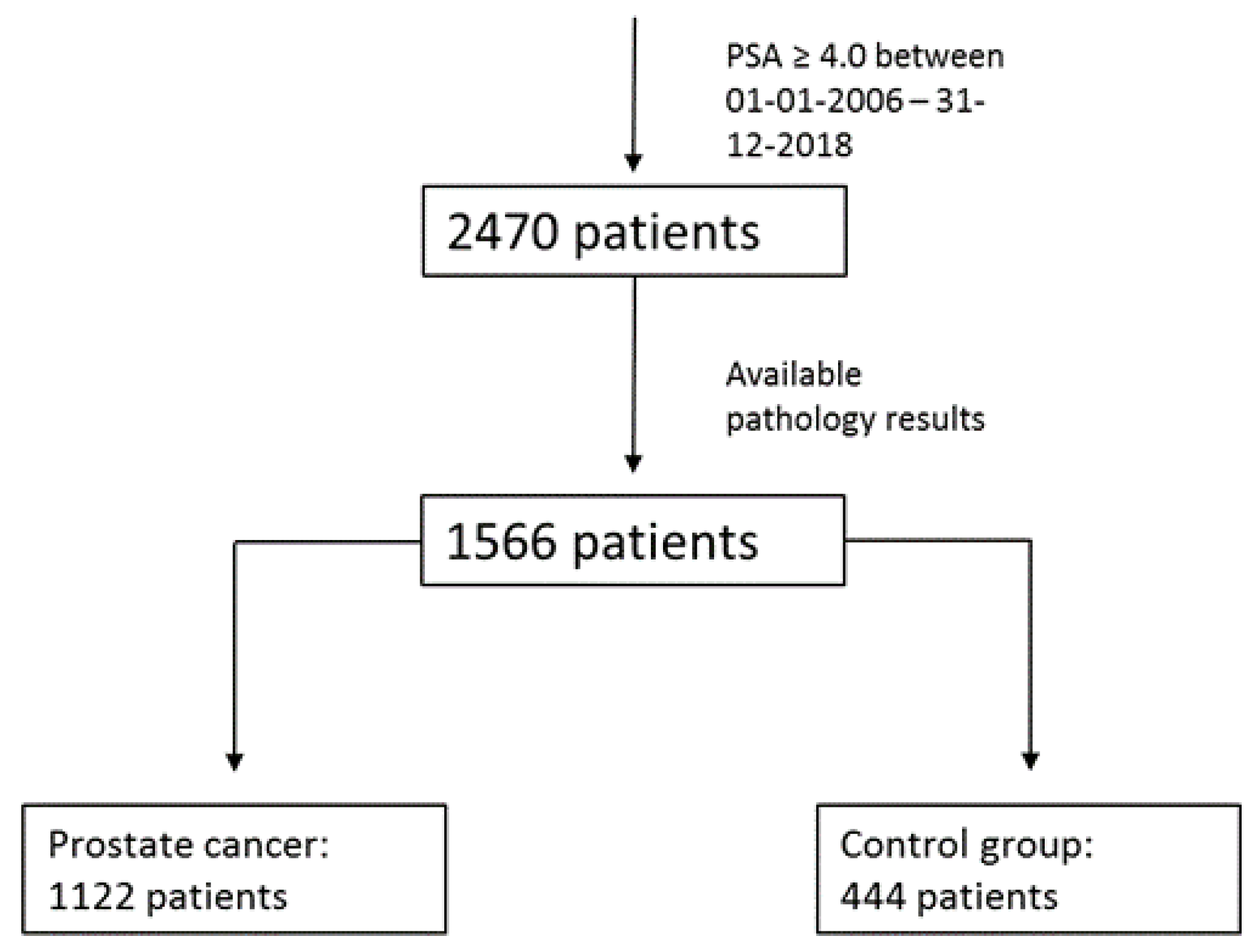
| Parameters | Prostate Cancer (n = 1122) | Control Group (n = 444) | p |
|---|---|---|---|
| Age, yrs; median (IQR) | 66 (61–70) | 64 (59–70) | 0.24 |
| Body Mass Index, kg/m2; mean (SD) | 26.5 (3.6) | 25.7 (4.3) | 0.01 |
| First PSA measurement; median (IQR) | 9.9 (6.7–16.3) | 6.4 (4.8–10.3) | 0.93 |
| Statin use, n (%) | 252 (22.5) | 83 (18.7) | 0.10 |
| Type of statins, n (%) | |||
| Simvastatin | 160 (63.5) | 45 (54.2) | 0.13 |
| Atorvastatin | 44 (17.5) | 22 (26.5) | 0.07 |
| Rosuvastatin | 22 (8.7) | 7 (8.4) | 0.93 |
| Pravastatin | 26 (10.3) | 9 (10.8) | 0.89 |
| Gleason score, n (%) | |||
| 6 | 219 (19.5) | ||
| 7 | 661 (58.9) | ||
| ≥8 | 242 (21.6) | ||
| Pathological T-stage, n (%) | |||
| T2b or lower | 104 (9.3) | ||
| T2c or higher | 533 (47.5) | ||
| Missing | 485 (43.2) | ||
| ESMO-classification, n (%) | |||
| Low Risk | 103 (9.2) | ||
| Intermediate Risk | 588 (52.4) | ||
| High Risk | 431 (38.4) | ||
| Surgery, n (%) | |||
| Received prostate surgery | 728 (64.9) | ||
| No prostate surgery | 394 (35.1) | ||
| PSA-progression after surgery, n (%) | |||
| No PSA-progression | 365 (87.7) | ||
| PSA-progression | 51 (12.3) | ||
| Follow-up in months; mean (SD) | 50.0 (33.3) |
| BMI | Statin User (n = 308) | Non-Statin User (n = 821) | p |
| BMI < 25 kg/m2, n (%) | 89 (28.9) | 322 (39.2) | 0.001 |
| BMI < 30 kg/m2, n (%) | 250 (81.1) | 705 (85.9) | 0.05 |
| ESMO-Classification, n (%) | BMI < 25 kg/m2, (n = 326) | BMI ≥ 25 kg/m2, (n = 618) | p |
| Low Risk | 18 (5.5) | 33 (5.3) | 0.91 |
| Intermediate Risk | 173 (53.1) | 348 (56.3) | 0.34 |
| High Risk | 135 (41.4) | 237 (38.3) | 0.36 |
| ESMO-Classification, n (%) | BMI < 30 kg/m2, (n = 796) | BMI ≥ 30 kg/m2, (n = 148) | p |
| Low Risk | 39 (4.9) | 12 (8.1) | 0.11 |
| Intermediate Risk | 447 (56.2) | 74 (50.0) | 0.17 |
| High Risk | 310 (38.9) | 62 (41.9) | 0.50 |
| ESMO-Classification, n (%) | Statin User (n = 252) | Non-Statin User (n = 870) | p |
| Low Risk | 22 (8.7) | 81 (9.3) | 0.78 |
| Intermediate Risk | 129 (51.2) | 459 (52.8) | 0.66 |
| High Risk | 101 (40.1) | 330 (37.9) | 0.54 |
| Age, Yrs; Median (IQR) | Prostate Cancer | Control Group | p |
|---|---|---|---|
| BMI < 30 kg/m2 | 66 (61–70) | 66 (60–72) | 0.20 |
| BMI ≥ 30 kg/m2 | 66 (61–70) | 68 (63–70) | 0.19 |
| Statin use | 68 (64–72) | 68 (62–72) | 0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meijer, D.; van Moorselaar, R.J.A.; Vis, A.N.; Bijnsdorp, I.V. Prostate Cancer Development Is Not Affected by Statin Use in Patients with Elevated PSA Levels. Cancers 2019, 11, 953. https://doi.org/10.3390/cancers11070953
Meijer D, van Moorselaar RJA, Vis AN, Bijnsdorp IV. Prostate Cancer Development Is Not Affected by Statin Use in Patients with Elevated PSA Levels. Cancers. 2019; 11(7):953. https://doi.org/10.3390/cancers11070953
Chicago/Turabian StyleMeijer, Dennie, R. Jeroen A. van Moorselaar, André N. Vis, and Irene V. Bijnsdorp. 2019. "Prostate Cancer Development Is Not Affected by Statin Use in Patients with Elevated PSA Levels" Cancers 11, no. 7: 953. https://doi.org/10.3390/cancers11070953
APA StyleMeijer, D., van Moorselaar, R. J. A., Vis, A. N., & Bijnsdorp, I. V. (2019). Prostate Cancer Development Is Not Affected by Statin Use in Patients with Elevated PSA Levels. Cancers, 11(7), 953. https://doi.org/10.3390/cancers11070953





