Wnt/β-Catenin Signaling in Liver Cancers
Abstract
1. Introduction
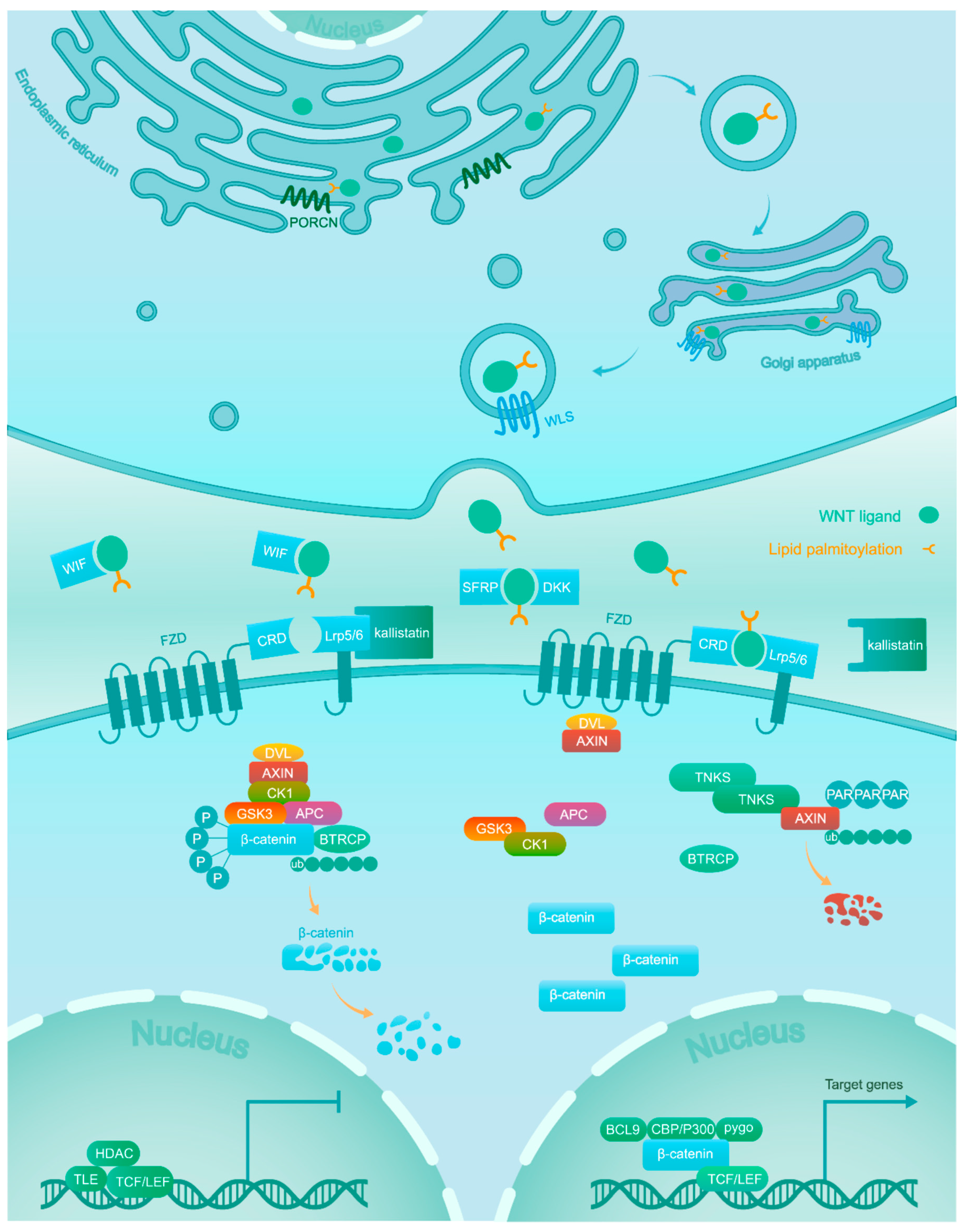
2. Wnt/β-Catenin Signaling
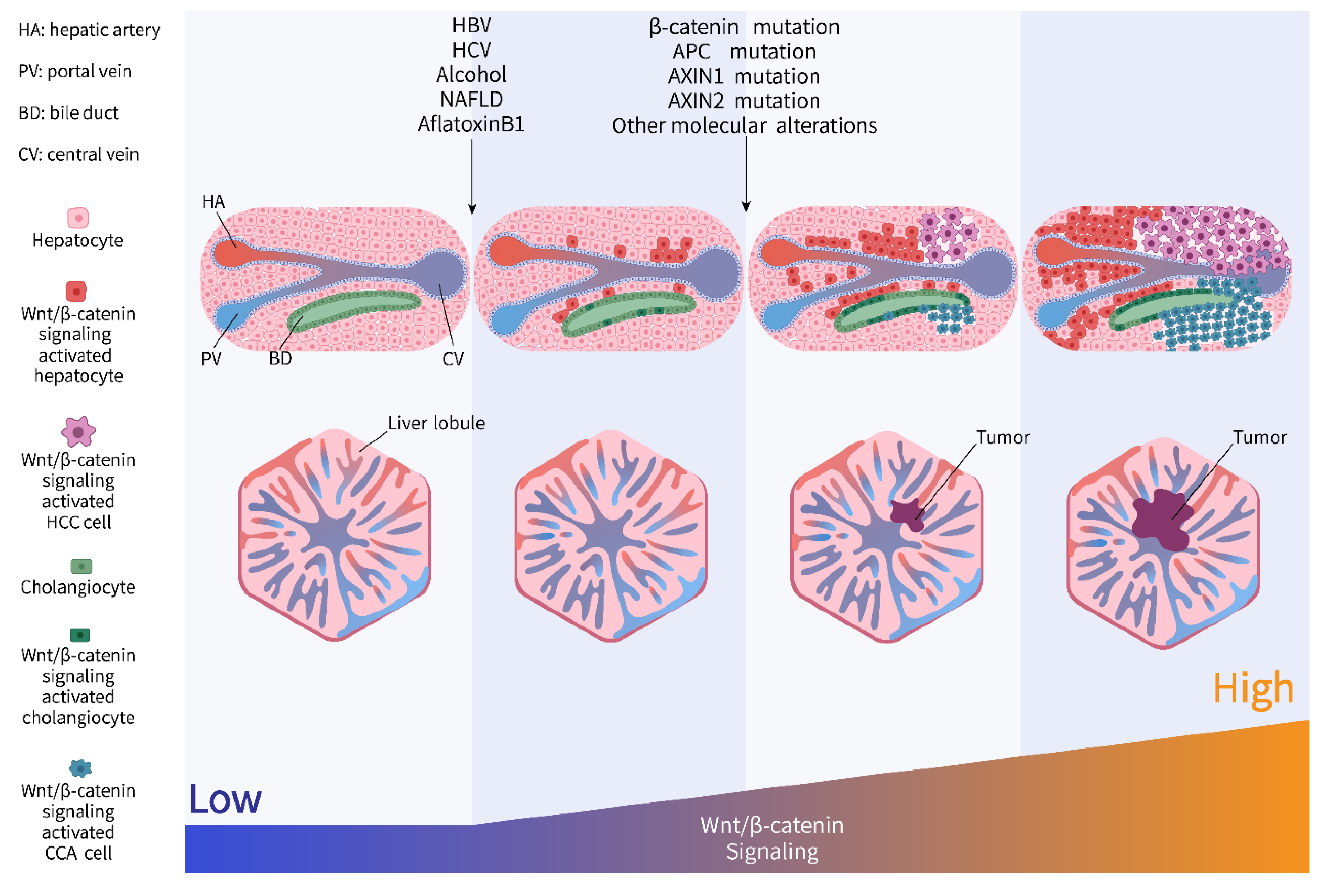
3. Precancerous Lesion
3.1. Hepatitis Viruses
3.1.1. HBV
3.1.2. HCV
3.2. Alcohol Abuse
3.3. Non-Alcoholic Fatty Liver Disease (NAFLD)
3.4. Aflatoxin-B1 Exposure
4. Liver Cancers
4.1. Hepatocellular Carcinoma
4.2. Cholangiocarcinoma
4.3. Hepatoblastoma
5. Targeting Wnt/β-Catenin Signaling in Liver Cancers
6. Future Perspectives
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| CCA | Cholangiocarcinoma |
| HB | Hepatoblastoma |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| FAP | Familial adenomatous polyposis |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Obesity-induced non-alcoholic steatohepatitis |
| ER | Endoplasmic reticulum |
| PORCN | Wnt acyl-transferase porcupine |
| SFRP | Secreted frizzled-related protein |
| WIF | Wnt inhibitory factor |
| LRP | Low-density lipoprotein receptor-related protein |
| APC | Adenomatous polyposis coli |
| β-TRCP | β-transducin repeat containing protein |
| CRD | Cysteine rich domain |
| FZD | Frizzled |
| DVL | Disheveled |
| PAR | Poly-ADP-ribosylation |
| TNKS | Tankyrase |
| TCF/LEF | T-cell factor/lymphoid enhancer-binding factor |
| TLE | Transducin-like enhancer of split |
| HDAC | Histone deacetylase |
| HBx | Hepatitis B viral X protein |
| HBsAg | Hepatitis B surface antigen |
| LINE | Long interspersed nuclear element |
| SHP-2 | Src homology region 2 domain-containing phosphatase-2 |
| EGFR | Epidermal growth factor receptor |
| FGF | Fibroblast growth factor |
| DEN | Diethylnitrosamine |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| ACLP | Aortic carboxypeptidase-like protein |
| CRC | Colorectal cancer |
References
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Darbari, A.; Sabin, K.M.; Shapiro, C.N.; Schwarz, K.B. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003, 38, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Aretz, S.; Koch, A.; Uhlhaas, S.; Friedl, W.; Propping, P.; von Schweinitz, D.; Pietsch, T. Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr. Blood Cancer 2006, 47, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Liu, B.R.; Du, Y.J.; Chen, J.; Cheng, Y.Q.; Xu, W.; Wang, X.H. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol. Lett. 2014, 7, 1175–1178. [Google Scholar] [CrossRef]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2012, 13, 11–26. [Google Scholar] [CrossRef]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Fuhler, G.M.; Smits, R.; Peppelenbosch, M.P. Action and function of Wnt/beta-catenin signaling in the progression from chronic hepatitis C to hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 419–431. [Google Scholar] [CrossRef]
- Peifer, M.; Polakis, P. Wnt signaling in oncogenesis and embryogenesis—A look outside the nucleus. Science 2000, 287, 1606–1609. [Google Scholar] [CrossRef]
- Dahmani, R.; Just, P.A.; Perret, C. The Wnt/beta-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 709–713. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; McBride, J.D.; Zhou, K.; Lee, K.; Zhou, Y.; Liu, Z.; Ma, J.X. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes 2013, 62, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Concordet, J.P.; Lassot, I.; Albert, I.; del los Santos, R.; Durand, H.; Perret, C.; Rubinfeld, B.; Margottin, F.; Benarous, R.; et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 1999, 9, 207–210. [Google Scholar] [CrossRef]
- Zhong, Y.; Katavolos, P.; Nguyen, T.; Lau, T.; Boggs, J.; Sambrone, A.; Kan, D.; Merchant, M.; Harstad, E.; Diaz, D.; et al. Tankyrase Inhibition Causes Reversible Intestinal Toxicity in Mice with a Therapeutic Index <1. Toxicol. Pathol. 2016, 44, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Pollock, K.; Guettler, S. Regulation of Wnt/beta-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding. Br. J. Pharm. 2017, 174, 4611–4636. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Mok, M.T.; Yang, P.; Cheng, A.S. Epigenetic Activation of Wnt/beta-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis. Cancers (Basel) 2016, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Jamieson, C.; Johnson, M.; Molloy, M.P.; Henderson, B.R. Specific armadillo repeat sequences facilitate beta-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J. Biol. Chem. 2012, 287, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wei, W.; Chua, M.-S.; So, S. WNT/β-catenin pathway activation in hepatocellular carcinoma: A clinical perspective. Gastrointest. Cancer Targets Ther. 2014, 4, 49–63. [Google Scholar] [CrossRef]
- Lau, C.C.; Sun, T.; Ching, A.K.; He, M.; Li, J.W.; Wong, A.M.; Co, N.N.; Chan, A.W.; Li, P.S.; Lung, R.W.; et al. Viral-human chimeric transcript predisposes risk to liver cancer development and progression. Cancer Cell 2014, 25, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Tiollais, P.; Pourcel, C.; Dejean, A. The hepatitis B virus. Nature 1985, 317, 489–495. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, L.; Shan, X.; Shan, X.; Tang, J.; Zhou, F.; Chen, Q.; Quan, H.; Nie, D.; Zhang, W.; et al. Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. Int. J. Cancer 2014, 135, 635–646. [Google Scholar] [CrossRef]
- Hsieh, A.; Kim, H.S.; Lim, S.O.; Yu, D.Y.; Jung, G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/beta-catenin signaling. Cancer Lett. 2011, 300, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.Y.; Kim, C.M.; Park, Y.M.; Ryu, W.S. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology 2004, 39, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gao, Y.Q.; Feng, H.; Lee, Y.Y.; Li, M.S.; Tian, Y.; Go, M.Y.; Yu, D.Y.; Cheung, Y.S.; Lai, P.B.; et al. Cell cycle-related kinase mediates viral-host signalling to promote hepatitis B virus-associated hepatocarcinogenesis. Gut 2014, 63, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.; Rana, M.A.; Husnain, T.; Ijaz, B. Modulation of Wnt signaling pathway by hepatitis B virus. Arch. Virol. 2017, 162, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhao, C.; Ren, J.; Ma, Z.M.; Xie, Y.H.; Wen, Y.M. Gene-expression profiles of a hepatitis B small surface antigen-secreting cell line reveal upregulation of lymphoid enhancer-binding factor 1. J. Gen. Virol. 2007, 88, 2966–2976. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Ma, Z.M.; Zhao, C.; Wan, D.F.; Wen, Y.M. Role of hepatitis B surface antigen in the development of hepatocellular carcinoma: Regulation of lymphoid enhancer-binding factor 1. J. Exp. Clin. Cancer Res. 2009, 28, 58. [Google Scholar] [CrossRef]
- Niknafs, Y.S.; Chinnaiyan, A.M. RNA identity crisis: Hepatitis B walks the LINE. Cancer Cell 2014, 25, 259–260. [Google Scholar] [CrossRef][Green Version]
- Budzinska, M.A.; Shackel, N.A.; Urban, S.; Tu, T. Cellular Genomic Sites of Hepatitis B Virus DNA Integration. Genes (Basel) 2018, 9, 365. [Google Scholar] [CrossRef]
- Cao, Q.; Imbeaud, S.; Datta, S.; Zucman-Rossi, J. Authors’ response: Virus-host interactions in HBV-related hepatocellular carcinoma: More to be revealed? Gut 2015, 64, 853–854. [Google Scholar] [CrossRef]
- Lin, M.V.; King, L.Y.; Chung, R.T. Hepatitis C virus-associated cancer. Annu. Rev. Pathol. 2015, 10, 345–370. [Google Scholar] [CrossRef]
- McLauchlan, J. Properties of the hepatitis C virus core protein: A structural protein that modulates cellular processes. J. Viral. Hepat. 2000, 7, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.B.; Ray, R. Hepatitis C virus core protein: Intriguing properties and functional relevance. FEMS Microbiol. Lett. 2001, 202, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, T.; Zhou, Y.; Kawai, S.; Eguchi, H.; Wands, J.R.; Li, J. Hepatitis C virus core protein stimulates hepatocyte growth: Correlation with upregulation of wnt-1 expression. Hepatology 2005, 41, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, X.; Tang, J.; Cao, Y.; Hu, P.; Zhou, F.; Shan, X.; Cai, X.; Chen, Q.; Ling, N. Enhancement of canonical Wnt/β-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS ONE 2011, 6, e27496. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Qureshi, S.A.; Hashmi, Z.Y.; Raza, A.; Ahmad, J.; Rahman, M.; Iqbal, M. Promoter hypermethylation of Wnt pathway inhibitors in hepatitis C virus-induced multistep hepatocarcinogenesis. Virol. J. 2014, 11, 117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quan, H.; Zhou, F.; Nie, D.; Chen, Q.; Cai, X.; Shan, X.; Zhou, Z.; Chen, K.; Huang, A.; Li, S. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial—mesenchymal transition. Oncogene 2014, 33, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Ripoli, M.; Barbano, R.; Balsamo, T.; Piccoli, C.; Brunetti, V.; Coco, M.; Mazzoccoli, G.; Vinciguerra, M.; Pazienza, V. Hypermethylated levels of E-cadherin promoter in Huh-7 cells expressing the HCV core protein. Virus Res. 2011, 160, 74–81. [Google Scholar] [CrossRef]
- Sawey, E.T.; Chanrion, M.; Cai, C.; Wu, G.; Zhang, J.; Zender, L.; Zhao, A.; Busuttil, R.W.; Yee, H.; Stein, L. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 2011, 19, 347–358. [Google Scholar] [CrossRef]
- Park, C.Y.; Choi, S.H.; Kang, S.M.; Kang, J.I.; Ahn, B.Y.; Kim, H.; Jung, G.; Choi, K.Y.; Hwang, S.B. Nonstructural 5A protein activates beta-catenin signaling cascades: Implication of hepatitis C virus-induced liver pathogenesis. J. Hepatol. 2009, 51, 853–864. [Google Scholar] [CrossRef]
- Street, A.; Macdonald, A.; Crowder, K.; Harris, M. The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J. Biol. Chem. 2004, 279, 12232–12241. [Google Scholar] [CrossRef]
- Macdonald, A.; Harris, M. Hepatitis C virus NS5A: Tales of a promiscuous protein. J. Gen. Virol. 2004, 85, 2485–2502. [Google Scholar] [CrossRef]
- Street, A.; Macdonald, A.; McCormick, C.; Harris, M. Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular β-catenin and stimulation of β-catenin-responsive transcription. J. Virol. 2005, 79, 5006–5016. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Ganju, R.K.; Groopman, J.E. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J. Biol. Chem. 2003, 278, 35755–35766. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Tsutsumi, R.; Kikuchi, I.; Obuse, C.; Saito, Y.; Seidi, A.; Karisch, R.; Fernandez, M.; Cho, T.; Ohnishi, N. SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor suppressor to an oncogenic driver. Mol. Cell 2011, 43, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, W.; Cheng, N.; Wang, K.; Li, B.; Jiang, X.; Sun, S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology 2012, 56, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Israsena, N.; Hu, M.; Fu, W.; Kan, L.; Kessler, J.A. The presence of FGF2 signaling determines whether β-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev. Biol. 2004, 268, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Igloi, Z.; Kazlauskas, A.; Saksela, K.; Macdonald, A.; Mankouri, J.; Harris, M. The hepatitis C virus NS5A protein blocks EGFR degradation via a proline motif dependent interaction. J. Gen. Virol. 2015, 96, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Holbro, T.; Hynes, N.E. Wnt1 and Wnt5a induce cyclin D1 expression through ErbB1 transactivation in HC11 mammary epithelial cells. EMBO Rep. 2003, 4, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Kwon, Y.-C.; Liu, S.; Hagedorn, C.H.; Ray, R.B.; Ray, R. Interferon-α inducible protein 6 impairs EGFR activation by CD81 and inhibits hepatitis C virus infection. Sci. Rep. 2015, 5, 9012. [Google Scholar] [CrossRef]
- Diao, J.; Pantua, H.; Ngu, H.; Komuves, L.; Diehl, L.; Schaefer, G.; Kapadia, S.B. Hepatitis C virus induces epidermal growth factor receptor activation via CD81 binding for viral internalization and entry. J. Virol. 2012, 86, 10935–10949. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Review Cross-talk of WNT and FGF Signaling Pathways at GSK3β to Regulate β-Catenin and SNAIL Signaling Cascades. Cancer Biol. Ther. 2006, 5, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Van, N.D.; Falk, C.S.; Vondran, F.W.; Helfritz, F.; Wedemeyer, H.; Manns, M.P.; Ciesek, S.; von Hahn, T. Modulation of HCV reinfection after orthotopic liver transplantation by fibroblast growth factor-2 and other non-interferon mediators. Gut 2015, 65, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Yu, T.; de la Monte, S.M.; Wands, J.R.; Derdak, Z.; Kim, M. Restoration of Wnt/beta-catenin signaling attenuates alcoholic liver disease progression in a rat model. J. Hepatol. 2015, 63, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Q.; de la Monte, S.M.; Tong, M.; Huang, C.K.; Kim, M. Chronic Ethanol-Induced Impairment of Wnt/beta-Catenin Signaling is Attenuated by PPAR-delta Agonist. Alcohol. Clin. Exp. Res. 2015, 39, 969–979. [Google Scholar] [CrossRef]
- Behari, J.; Sylvester, K.G. Role of the Wnt/beta-Catenin Pathway in the Pathogenesis of Alcoholic Liver Disease. Curr. Mol. Pharm. 2017, 10, 186–194. [Google Scholar] [CrossRef]
- Liu, S.; Yeh, T.H.; Singh, V.P.; Shiva, S.; Krauland, L.; Li, H.; Zhang, P.; Kharbanda, K.; Ritov, V.; Monga, S.P.; et al. Beta-catenin is essential for ethanol metabolism and protection against alcohol-mediated liver steatosis in mice. Hepatology 2012, 55, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Lehwald, N.; Tao, G.Z.; Jang, K.Y.; Papandreou, I.; Liu, B.; Liu, B.; Pysz, M.A.; Willmann, J.K.; Knoefel, W.T.; Denko, N.C.; et al. Beta-Catenin regulates hepatic mitochondrial function and energy balance in mice. Gastroenterology 2012, 143, 754–764. [Google Scholar] [CrossRef]
- Mercer, K.E.; Hennings, L.; Ronis, M.J. Alcohol consumption, Wnt/beta-catenin signaling, and hepatocarcinogenesis. Adv. Exp. Med. Biol. 2015, 815, 185–195. [Google Scholar] [CrossRef]
- Lai, K.K.Y.; Kweon, S.M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef]
- Bhala, N.; Angulo, P.; van der Poorten, D.; Lee, E.; Hui, J.M.; Saracco, G.; Adams, L.A.; Charatcharoenwitthaya, P.; Topping, J.H.; Bugianesi, E.; et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: An international collaborative study. Hepatology 2011, 54, 1208–1216. [Google Scholar] [CrossRef]
- Takada, I.; Kouzmenko, A.P.; Kato, S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Go, G.W.; Srivastava, R.; Hernandez-Ono, A.; Gang, G.; Smith, S.B.; Booth, C.J.; Ginsberg, H.N.; Mani, A. The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue. Cell Metab. 2014, 19, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Go, G.W. Low-Density Lipoprotein Receptor-Related Protein 6 (LRP6) Is a Novel Nutritional Therapeutic Target for Hyperlipidemia, Non-Alcoholic Fatty Liver Disease, and Atherosclerosis. Nutrients 2015, 7, 4453–4464. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, K.; Srivastava, R.; Dong, C.; Go, G.W.; Li, N.; Iwakiri, Y.; Mani, A. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 2015, 29, 3436–3445. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Tomita, K.; Suzuki, T.; Furuhashi, H.; Irie, R.; Nishikawa, M.; Yamamoto, J.; Hibi, T.; Miura, S.; Minamino, T.; et al. Aortic carboxypeptidase-like protein, a WNT ligand, exacerbates nonalcoholic steatohepatitis. J. Clin. Invest. 2018, 128, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Debebe, A.; Medina, V.; Chen, C.Y.; Mahajan, I.M.; Jia, C.; Fu, D.; He, L.; Zeng, N.; Stiles, B.W.; Chen, C.L.; et al. Wnt/beta-catenin activation and macrophage induction during liver cancer development following steatosis. Oncogene 2017, 36, 6020–6029. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wong, V.W.; Wong, G.L.; Yang, W.; Sun, H.; Shen, J.; Tong, J.H.; Go, M.Y.; Cheung, Y.S.; Lai, P.B.; et al. Histone Deacetylase HDAC8 Promotes Insulin Resistance and beta-Catenin Activation in NAFLD-Associated Hepatocellular Carcinoma. Cancer Res. 2015, 75, 4803–4816. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Schwank, J.; Staib, F.; Wang, X.W.; Harris, C.C. TP53 mutations and hepatocellular carcinoma: Insights into the etiology and pathogenesis of liver cancer. Oncogene 2007, 26, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Feng, Y.; Wu, T.; Srinivas, S.; Yang, W.; Fan, J.; Yang, C.; Wang, S. Aflatoxin B1 negatively regulates Wnt/beta-catenin signaling pathway through activating miR-33a. PLoS ONE 2013, 8, e73004. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, J.; Huang, K.; Luo, Y.; Zhang, B.; Xu, W. miR-34a screened by miRNA profiling negatively regulates Wnt/beta-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Sci. Rep. 2015, 5, 16732. [Google Scholar] [CrossRef] [PubMed]
- Devereux, T.R.; Stern, M.C.; Flake, G.P.; Yu, M.C.; Zhang, Z.Q.; London, S.J.; Taylor, J.A. CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Mol. Carcinog. 2001, 31, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Nhieu, J.T.; Renard, C.A.; Wei, Y.; Cherqui, D.; Zafrani, E.S.; Buendia, M.A. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am. J. Pathol. 1999, 155, 703–710. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Alsinet, C.; Savic, R.; Cabellos, L.; Toffanin, S.; Hoshida, Y.; Villanueva, A.; Minguez, B.; Newell, P.; Tsai, H.W.; et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin. Cancer Res. 2012, 18, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Fan, S.T.; Ng, I.O. beta-Catenin mutation and overexpression in hepatocellular carcinoma: Clinicopathologic and prognostic significance. Cancer 2001, 92, 136–145. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chao, C.C. Identification of the beta-catenin/JNK/prothymosin-alpha axis as a novel target of sorafenib in hepatocellular carcinoma cells. Oncotarget 2015, 6, 38999–39017. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Puig, P.; Zucman-Rossi, J. Genetics of hepatocellular tumors. Oncogene 2006, 25, 3778–3786. [Google Scholar] [CrossRef]
- Fujimoto, A.; Totoki, Y.; Abe, T.; Boroevich, K.A.; Hosoda, F.; Nguyen, H.H.; Aoki, M.; Hosono, N.; Kubo, M.; Miya, F.; et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat. Genet. 2012, 44, 760–764. [Google Scholar] [CrossRef]
- Monga, S.P. beta-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef]
- Bruix, J.; Han, K.H.; Gores, G.; Llovet, J.M.; Mazzaferro, V. Liver cancer: Approaching a personalized care. J. Hepatol. 2015, 62, S144–S156. [Google Scholar] [CrossRef] [PubMed]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-beta-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lisby, A.; Ma, C.; Lo, N.; Ehmer, U.; Hayer, K.E.; Furth, E.E.; Viatour, P. Promotion of growth factor signaling as a critical function of beta-catenin during HCC progression. Nat. Commun. 2019, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- Fodde, R.; Smits, R.; Clevers, H. APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer 2001, 1, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Pez, F.; Lopez, A.; Kim, M.; Wands, J.R.; Caron de Fromentel, C.; Merle, P. Wnt signaling and hepatocarcinogenesis: Molecular targets for the development of innovative anticancer drugs. J. Hepatol. 2013, 59, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Lin, L.; Yang, S.; Bhuvaneshwar, K.; Wang, H.; Gusev, Y.; Lee, M.H.; Kallakury, B.; Shivapurkar, N.; Cahn, K.; et al. betaII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology 2015, 61, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Ng, K.Y.; Tong, M.; Lau, E.Y.; Lee, T.K.; Chan, K.W.; Yuan, Y.F.; Cheung, T.T.; Cheung, S.T.; Wang, X.Q.; et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/beta-catenin activation in liver cancer stem cells. Hepatology 2016, 64, 2062–2076. [Google Scholar] [CrossRef]
- Jain, S.; Chang, T.T.; Hamilton, J.P.; Lin, S.Y.; Lin, Y.J.; Evans, A.A.; Selaru, F.M.; Lin, P.W.; Chen, S.H.; Block, T.M.; et al. Methylation of the CpG sites only on the sense strand of the APC gene is specific for hepatocellular carcinoma. PLoS ONE 2011, 6, e26799. [Google Scholar] [CrossRef]
- Loilome, W.; Bungkanjana, P.; Techasen, A.; Namwat, N.; Yongvanit, P.; Puapairoj, A.; Khuntikeo, N.; Riggins, G.J. Activated macrophages promote Wnt/beta-catenin signaling in cholangiocarcinoma cells. Tumour Biol. 2014, 35, 5357–5367. [Google Scholar] [CrossRef]
- Boulter, L.; Guest, R.V.; Kendall, T.J.; Wilson, D.H.; Wojtacha, D.; Robson, A.J.; Ridgway, R.A.; Samuel, K.; Van Rooijen, N.; Barry, S.T.; et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J. Clin. Invest. 2015, 125, 1269–1285. [Google Scholar] [CrossRef]
- Goeppert, B.; Konermann, C.; Schmidt, C.R.; Bogatyrova, O.; Geiselhart, L.; Ernst, C.; Gu, L.; Becker, N.; Zucknick, M.; Mehrabi, A.; et al. Global alterations of DNA methylation in cholangiocarcinoma target the Wnt signaling pathway. Hepatology 2014, 59, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wan, M.; Xu, Y.; Li, Z.; Leng, K.; Kang, P.; Cui, Y.; Jiang, X. Long noncoding RNA PCAT1 regulates extrahepatic cholangiocarcinoma progression via the Wnt/beta-catenin-signaling pathway. Biomed Pharm. 2017, 94, 55–62. [Google Scholar] [CrossRef]
- Huang, G.L.; Luo, Q.; Rui, G.; Zhang, W.; Zhang, Q.Y.; Chen, Q.X.; Shen, D.Y. Oncogenic activity of retinoic acid receptor gamma is exhibited through activation of the Akt/NF-kappaB and Wnt/beta-catenin pathways in cholangiocarcinoma. Mol. Cell. Biol. 2013, 33, 3416–3425. [Google Scholar] [CrossRef] [PubMed]
- Merino-Azpitarte, M.; Lozano, E.; Perugorria, M.J.; Esparza-Baquer, A.; Erice, O.; Santos-Laso, A.; O’Rourke, C.J.; Andersen, J.B.; Jimenez-Aguero, R.; Lacasta, A.; et al. SOX17 regulates cholangiocyte differentiation and acts as a tumor suppressor in cholangiocarcinoma. J. Hepatol. 2017, 67, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K.; Wang, J.; Wu, X.; Liu, X.; Li, B.; Zhu, Y.; Yu, Y.; Cheng, Q.; Hu, Z.; et al. Underexpression of LKB1 tumor suppressor is associated with enhanced Wnt signaling and malignant characteristics of human intrahepatic cholangiocarcinoma. Oncotarget 2015, 6, 18905–18920. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.O.; Monga, S.P. Wnt/beta-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Dubbink, H.J.; Hollink, I.; Avenca Valente, C.; Wang, W.; Liu, P.; Doukas, M.; van Noesel, M.M.; Dinjens, W.N.M.; Wagner, A.; Smits, R. A novel tissue-based ss-catenin gene and immunohistochemical analysis to exclude familial adenomatous polyposis among children with hepatoblastoma tumors. Pediatr. Blood Cancer 2018, 65, e26991. [Google Scholar] [CrossRef]
- Adebayo Michael, A.O.; Ko, S.; Tao, J.; Moghe, A.; Yang, H.; Xu, M.; Russell, J.O.; Pradhan-Sundd, T.; Liu, S.; Singh, S.; et al. Inhibiting Glutamine-Dependent mTORC1 Activation Ameliorates Liver Cancers Driven by beta-Catenin Mutations. Cell Metab. 2019, 29, 1135–1150. [Google Scholar] [CrossRef]
- Wei, W.; Chua, M.S.; Grepper, S.; So, S.K. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol. Cancer 2011, 10, 16. [Google Scholar] [CrossRef]
- Jimeno, A.; Gordon, M.; Chugh, R.; Messersmith, W.; Mendelson, D.; Dupont, J.; Stagg, R.; Kapoun, A.M.; Xu, L.; Uttamsingh, S.; et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 7490–7497. [Google Scholar] [CrossRef]
- Dose Escalation Study of OMP-54F28 in Combination with Sorafenib in Patients With Hepatocellular Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02069145 (accessed on 24 February 2014).
- A Dose Escalation Study of OMP-54F28 in Subjects with Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01608867 (accessed on 31 May 2019).
- Dose Escalation Study of OMP-54F28 in Combination With Paclitaxel and Carboplatin in Patients With Recurrent Platinum-Sensitive Ovarian Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02092363 (accessed on 20 March 2014).
- Dose Escalation Study of OMP-54F28 in Combination With Nab-Paclitaxel and Gemcitabine in Patients with Previously Untreated Stage IV Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02050178 (accessed on 30 January 2014).
- Arafat, K.; Iratni, R.; Takahashi, T.; Parekh, K.; Al Dhaheri, Y.; Adrian, T.E.; Attoub, S. Inhibitory Effects of Salinomycin on Cell Survival, Colony Growth, Migration, and Invasion of Human Non-Small Cell Lung Cancer A549 and LNM35: Involvement of NAG-1. PLoS ONE 2013, 8, e66931. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/beta-catenin and mTORC1 signaling in breast and prostate cancer cells. J. Cell. Biochem. 2014, 115, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Fan, S.; Ma, W.; Fan, P.; Wang, B.; Zhang, J.; Wang, H.; Tang, B.; Zhang, Q.; Yu, X.; et al. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014, 5, e1039. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Zhao, Z.Q.; Li, J.C.; Liang, Y.; Yin, J.Q.; Zou, C.Y.; Xie, X.B.; Zeng, Y.X.; Shen, J.N.; Kang, T.; et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett. 2011, 311, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dai, W.; Wang, Y.; Shen, M.; Chen, K.; Cheng, P.; Zhang, Y.; Wang, C.; Li, J.; Zheng, Y.; et al. The synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma. PLoS ONE 2014, 9, e97414. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, L.; Dai, W.Q.; Xu, Y.P.; Wu, D.; Lin, C.L.; Wu, S.M.; Cheng, P.; Zhang, Y.; Shen, M.; et al. Salinomycin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells in vitro and in vivo. PLoS ONE 2012, 7, e50638. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chua, M.S.; Grepper, S.; So, S.K. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol. Cancer 2009, 8, 76. [Google Scholar] [CrossRef]
- He, B.; Reguart, N.; You, L.; Mazieres, J.; Xu, Z.; Lee, A.Y.; Mikami, I.; McCormick, F.; Jablons, D.M. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 2005, 24, 3054–3058. [Google Scholar] [CrossRef]
- He, B.; You, L.; Uematsu, K.; Xu, Z.; Lee, A.Y.; Matsangou, M.; McCormick, F.; Jablons, D.M. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Mikami, I.; You, L.; He, B.; Xu, Z.; Batra, S.; Lee, A.Y.; Mazieres, J.; Reguart, N.; Uematsu, K.; Koizumi, K.; et al. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC Cancer 2005, 5, 53. [Google Scholar] [CrossRef]
- Rhee, C.S.; Sen, M.; Lu, D.; Wu, C.; Leoni, L.; Rubin, J.; Corr, M.; Carson, D.A. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene 2002, 21, 6598–6605. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Dong, A.; Fernandez-Ruiz, V.; Shan, J.; Kawa, M.; Martinez-Anso, E.; Prieto, J.; Qian, C. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res. 2009, 69, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- A Study of DKN-01 in Multiple Myeloma or Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01457417 (accessed on 24 October 2011).
- A Study of DKN-01 and Lenalidomide/Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. Available online: https://clinicaltrials.gov/ct2/show/NCT01711671 (accessed on 22 October 2012).
- A Study of DKN-01 in Combination With Paclitaxel or Pembrolizumab (P102). Available online: https://clinicaltrials.gov/ct2/show/NCT02013154 (accessed on 17 December 2013).
- Study of DKN-01 and Gemcitabine/Cisplatin in Patients with Carcinoma to Primary to the Intra- or Extra-Hepatic Biliary System or Gallbladder. Available online: https://clinicaltrials.gov/ct2/show/NCT02375880 (accessed on 3 March 2015).
- A Study of DKN-01 as a Monotherapy or in Combination With Paclitaxel in Patients with Recurrent Epithelial Endometrial or Epithelial Ovarian Cancer (P204). Available online: https://clinicaltrials.gov/ct2/show/NCT03395080 (accessed on 10 January 2018).
- Bendell, J.C.; Murphy, J.E.; Mahalingam, D.; Halmos, B.; Sirard, C.A.; Landau, S.B.; Ryan, D.P. A Phase 1 study of DKN-01, an anti-DKK1 antibody, in combination with paclitaxel (pac) in patients with DKK1 relapsed or refractory esophageal cancer (EC) or gastro-esophageal junction tumors (GEJ). J. Clin. Oncol. 2016, 34, 111. [Google Scholar] [CrossRef]
- DKN-01 Inhibition in Advanced Liver Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03645980 (accessed on 24 August 2018).
- Combination of Targeted and Immunotherapy for Advanced Biliary Tract and Esophagogastric Gastric Cancer (DYNAMIC). Available online: https://clinicaltrials.gov/ct2/show/NCT03818997 (accessed on 28 January 2019).
- CGX1321 in Subjects With Advanced Solid Tumors and CGX1321 with Pembrolizumab in Subjects With Advanced GI Tumors (Keynote 596). Available online: https://clinicaltrials.gov/ct2/show/NCT02675946 (accessed on 5 February 2016).
- Phase 1 Dose Escalation Study of CGX1321 in Subjects with Advanced Gastrointestinal Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03507998 (accessed on 25 April 2018).
- Li, C.; Cao, J.; Zhang, N.; Tu, M.; Xu, F.; Wei, S.; Chen, X.; Xu, Y. Identification of RSPO2 Fusion Mutations and Target Therapy Using a Porcupine Inhibitor. Sci. Rep. 2018, 8, 14244. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, L.; Liu, P.; Jairam, K.; Yin, Y.; Chen, K.; Sprengers, D.; Peppelenbosch, M.P.; Pan, Q.; Smits, R. Blocking Wnt Secretion Reduces Growth of Hepatocellular Carcinoma Cell Lines Mostly Independent of beta-Catenin Signaling. Neoplasia 2016, 18, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, X.; Jia, T.; Wei, W.; Chua, M.S.; So, S. Tankyrase inhibitors attenuate WNT/beta-catenin signaling and inhibit growth of hepatocellular carcinoma cells. Oncotarget 2015, 6, 25390–25401. [Google Scholar] [CrossRef] [PubMed]
- Gwak, J.; Lee, J.H.; Chung, Y.H.; Song, G.Y.; Oh, S. Small molecule-based promotion of PKCalpha-mediated beta-catenin degradation suppresses the proliferation of CRT-positive cancer cells. PLoS ONE 2012, 7, e46697. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Apte, U.; Cieply, B.; Singh, S.; Monga, S.P. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia 2007, 9, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef]
- Safety and Efficacy Study of PRI-724 in Subjects with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01302405 (accessed on 24 February 2011).
- Safety and Efficacy Study of PRI-724 in Subjects with Advanced Myeloid Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT01606579 (accessed on 25 May 2012).
- Safety and Efficacy Study of PRI-724 Plus Gemcitabine in Subjects with Advanced or Metastatic Pancreatic Adenocarcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01764477 (accessed on 9 January 2013).
- An Open Label, Single Arm, Dose Escalation Phase 1 Trial of PRI-724 in Patients with HCV-induced Cirrhosis. Available online: https://clinicaltrials.gov/ct2/show/NCT02195440 (accessed on 21 July 2014).
- Kimura, K.; Ikoma, A.; Shibakawa, M.; Shimoda, S.; Harada, K.; Saio, M.; Imamura, J.; Osawa, Y.; Kimura, M.; Nishikawa, K.; et al. Safety, Tolerability, and Preliminary Efficacy of the Anti-Fibrotic Small Molecule PRI-724, a CBP/beta-Catenin Inhibitor, in Patients with Hepatitis C Virus-related Cirrhosis: A Single-Center, Open-Label, Dose Escalation Phase 1 Trial. EBioMed. 2017, 23, 79–87. [Google Scholar] [CrossRef]
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, R.T.; Teo, J.L.; Kim, H.Y.; Moon, S.H.; et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 12682–12687. [Google Scholar] [CrossRef] [PubMed]
- Handeli, S.; Simon, J.A. A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma and PPARdelta activities. Mol. Cancer 2008, 7, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chua, M.S.; Grepper, S.; So, S. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int. J. Cancer 2010, 126, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Lepourcelet, M.; Chen, Y.N.; France, D.S.; Wang, H.; Crews, P.; Petersen, F.; Bruseo, C.; Wood, A.W.; Shivdasani, R.A. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 2004, 5, 91–102. [Google Scholar] [CrossRef]
- Mologni, L.; Brussolo, S.; Ceccon, M.; Gambacorti-Passerini, C. Synergistic effects of combined Wnt/KRAS inhibition in colorectal cancer cells. PLoS ONE 2012, 7, e51449. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Dar, M.J.; Monga, S.P. Pegylated interferon alpha targets Wnt signaling by inducing nuclear export of beta-catenin. J. Hepatol. 2011, 54, 506–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhanasekaran, R.; Nault, J.C.; Roberts, L.R.; Zucman-Rossi, J. Genomic Medicine and Implications for Hepatocellular Carcinoma Prevention and Therapy. Gastroenterology 2019, 156, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouze, E.; Blanc, J.F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Boyault, S.; Rickman, D.S.; de Reynies, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Herault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007, 45, 42–52. [Google Scholar] [CrossRef]
| Etiological Factors | Roles to Regulate Wnt/β-Catenin Signaling | References |
|---|---|---|
| HBV | ||
| HBx |
| [20] |
| [21,22,23] | |
| HBsAg |
| [24,25,26] |
| other |
| [18,27] |
| HCV | ||
| core protein |
| [33,34] |
| [35,36] | |
| [37] | |
| NS5A |
| [39] |
| [40,41,42] | |
| E2 |
| [43,44] |
| others |
| [45] |
| [46,47] | |
| Alcohol abuse |
| [53,54,55,56,57] |
| [58,59] | |
| NAFLD |
| [63,64,65] |
| [15,66,67,68] | |
| Aflatoxin-B1 |
| [71,72] |
| [73] |
| Targets | Compounds | Diseases | Stage | References |
|---|---|---|---|---|
| FZD7 | sFZD7 | HCC | Preclinical | [99] |
| FZD8 | OMP-54F28 | HCC, ovarian cancer, pancreas cancer | Phase 1 | [100,101,102,103,104] |
| LRP5/6 | Salinomycin | breast, prostate, lung, gastric, osteosarcoma, HCC | Preclinical | [105,106,107,108,109,110] |
| Wnt1 | Anti-Wnt1 | HCC, CRC, lung cancer, sarcoma, breast cancer, head-neck squamous cell carcinoma | Preclinical | [111,112,113,114,115] |
| Wnt ligands | WIF-Fc/ SFRP-Fc | HCC | Preclinical | [116] |
| DKK1 | DKN-01 | HCC, CCA, biliary tract cancer, gallbladder cancer, and other cancers | Phase 1/2 | [117,118,119,120,121,122,123,124] |
| PORCN | CGX1321 | HCC, CCA, and other cancers | Phase 1 | [125,126,127] |
| IWP12 | HCC and CRC | Preclinical | [128] | |
| Tankyrase | XAV939/WXL-8 | HCC | Preclinical | [129] |
| β-catenin phosphorylation | CGK062 | CRC, HCC, prostate cancer | Preclinical | [130] |
| β-catenin | β-catenin siRNA | HCC | Preclinical | [131] |
| BBI608 | Glioblastoma, CRC, HCC, gastric cancer, pancreas cancer, lung cancer | Phase 1/2 | [132] | |
| β-catenin/CBP | PRI-724 | Pancreatic adenocarcinoma, leukemia, CRC, HCV-induced cirrhosis, solid tumor | Phase 1/2 | [133,134,135,136,137,138] |
| β-catenin/TCF | PKF115-548 PKF222-815 CGP049090 FH535 | HCC, CRC, lung cancer | Preclinical | [139,140,141,142] |
| β-catenin nuclear export | Peg-IFN | HCC | Preclinical | [143] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Smits, R.; Hao, H.; He, C. Wnt/β-Catenin Signaling in Liver Cancers. Cancers 2019, 11, 926. https://doi.org/10.3390/cancers11070926
Wang W, Smits R, Hao H, He C. Wnt/β-Catenin Signaling in Liver Cancers. Cancers. 2019; 11(7):926. https://doi.org/10.3390/cancers11070926
Chicago/Turabian StyleWang, Wenhui, Ron Smits, Haiping Hao, and Chaoyong He. 2019. "Wnt/β-Catenin Signaling in Liver Cancers" Cancers 11, no. 7: 926. https://doi.org/10.3390/cancers11070926
APA StyleWang, W., Smits, R., Hao, H., & He, C. (2019). Wnt/β-Catenin Signaling in Liver Cancers. Cancers, 11(7), 926. https://doi.org/10.3390/cancers11070926





