Clinical Translatability of “Identified” Circulating miRNAs for Diagnosing Breast Cancer: Overview and Update
Abstract
1. Introduction
2. The Specificity of Identified Circulating miRNAs for Diagnosing Breast Cancer
3. Consistent Expression of Identified Circulating miRNA in Blood and Tumor Tissues
4. Clinical Trials Including Breast Cancer-Associated miRNAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Reliable Biomarkers to Identify New and Recurrent Cancer. Eur. J. Breast Health 2017, 13, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, P.; Incoronato, M. Usefulness of Traditional Serum Biomarkers for Management of Breast Cancer Patients. Biomed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maric, P.; Ozretic, P.; Levanat, S.; Oreskovic, S.; Antunac, K.; Beketic-Oreskovic, L. Tumor Markers in Breast Cancer—Evaluation of their Clinical Usefulness. Coll. Antropol. 2011, 35, 241–247. [Google Scholar] [PubMed]
- Duffy, M.J.; McDermott, E.W.; Crown, J. Blood-based biomarkers in breast cancer: From proteins to circulating tumor cells to circulating tumor DNA. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2018, 40. [Google Scholar] [CrossRef] [PubMed]
- Sestak, I.; Cuzick, J. Markers for the identification of late breast cancer recurrence. Breast Cancer Res. 2015, 17, 10. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Atasoy, U.; Sauter, E.R. Circulating microRNAs in breast cancer and healthy subjects. BMC Res. Notes 2009, 2, 89–89. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Nishida, N.; Calin, G.A.; Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Jarry, J.; Schadendorf, D.; Greenwood, C.; Spatz, A.; van Kempen, L.C. The validity of circulating microRNAs in oncology: Five years of challenges and contradictions. Mol. Oncol. 2014, 8, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W. Circulating MicroRNA Biomarker Studies: Pitfalls and Potential Solutions. Clin. Chem. 2015, 61, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Lam, S.; Nagahara, M.; Hoon, D.S.B. Circulating microRNA Biomarkers as Liquid Biopsy for Cancer Patients: Pros and Cons of Current Assays. J. Clin. Med. 2015, 4, 1890–1907. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, J.; Gao, Y.; Cui, C.; Zhang, S.; Li, J.; Zhou, Y.; Cui, Q. HMDD v3.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 2019, 47, D1013–D1017. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. ICD-11 Version 2018. Available online: https://icd.who.int/browse11/l-m/en (accessed on March 2019).
- Qiu, C.; Chen, G.; Cui, Q. Towards the understanding of microRNA and environmental factor interactions and their relationships to human diseases. Sci. Rep. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Kelly, R.; Newell, J.; Kerin, M.J. Systemic miRNA-195 Differentiates Breast Cancer from Other Malignancies and Is a Potential Biomarker for Detecting Noninvasive and Early Stage Disease. Oncologist 2010, 15, 673–682. [Google Scholar] [CrossRef]
- Roth, C.; Rack, B.; Mueller, V.; Janni, W.; Pantel, K.; Schwarzenbach, H. Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 2010, 12, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, J.; Medico, L.; Wang, D.; Ambrosone, C.B.; Liu, S. A Pilot Study of Circulating miRNAs as Potential Biomarkers of Early Stage Breast Cancer. PLoS ONE 2010, 5, e13735. [Google Scholar] [CrossRef]
- Cookson, V.J.; Bentley, M.A.; Hogan, B.V.; Horgan, K.; Hayward, B.E.; Hazelwood, L.D.; Hughes, T.A. Circulating microRNA profiles reflect the presence of breast tumours but not the profiles of microRNAs within the tumours. Cell. Oncol. 2012, 35, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.-J.; Santarpia, L.; Kim, J.; Esteva, F.J.; Moretti, E.; Buzdar, A.U.; Di Leo, A.; Le, X.-F.; Bast, R.C., Jr.; Park, S.-T.; et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer 2012, 118, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Van Schooneveld, E.; Wouters, M.C.A.; Van der Auwera, I.; Peeters, D.J.; Wildiers, H.; Van Dam, P.A.; Vergote, I.; Vermeulen, P.B.; Dirix, L.Y.; Van Laere, S.J. Expression profiling of cancerous and normal breast tissues identifies microRNAs that are differentially expressed in serum from patients with (metastatic) breast cancer and healthy volunteers. Breast Cancer Res. 2012, 14, R34. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Milde-Langosch, K.; Steinbach, B.; Mueller, V.; Pantel, K. Diagnostic potential of PTEN-targeting miR-214 in the blood of breast cancer patients. Breast Cancer Res. Treat. 2012, 134, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Schrauder, M.G.; Strick, R.; Schulz-Wendtland, R.; Strissel, P.L.; Kahmann, L.; Loehberg, C.R.; Lux, M.P.; Jud, S.M.; Hartmann, A.; Hein, A.; et al. Circulating Micro-RNAs as Potential Blood-Based Markers for Early Stage Breast Cancer Detection. PLoS ONE 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, C.; Lu, Z.; Guo, L.; Ge, Q. Analysis of serum genome-wide microRNAs for breast cancer detection. Clin. Chim. Acta 2012, 413, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Cuk, K.; Zucknick, M.; Heil, J.; Madhavan, D.; Schott, S.; Turchinovich, A.; Arlt, D.; Rath, M.; Sohn, C.; Benner, A.; et al. Circulating microRNAs in plasma as early detection markers for breast cancer. Int. J. Cancer 2013, 132, 1602–1612. [Google Scholar] [CrossRef]
- Eichelser, C.; Flesch-Janys, D.; Chang-Claude, J.; Pantel, K.; Schwarzenbach, H. Deregulated Serum Concentrations of Circulating Cell-Free MicroRNAs miR-17, miR-34a, miR-155, and miR-373 in Human Breast Cancer Development and Progression. Clin. Chem. 2013, 59, 1489–1496. [Google Scholar] [CrossRef]
- Mar-Aguilar, F.; Mendoza-Ramirez, J.A.; Malagon-Santiago, I.; Espino-Silva, P.K.; Santuario-Facio, S.K.; Ruiz-Flores, P.; Rodriguez-Padilla, C.; Resendez-Perez, D. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis. Markers 2013, 34, 163–169. [Google Scholar] [CrossRef]
- Cuk, K.; Zucknick, M.; Madhavan, D.; Schott, S.; Golatta, M.; Heil, J.; Marme, F.; Turchinovich, A.; Sinn, P.; Sohn, C.; et al. Plasma MicroRNA Panel for Minimally Invasive Detection of Breast Cancer. PLoS ONE 2013, 8, e76729. [Google Scholar] [CrossRef]
- Chan, M.; Liaw, C.S.; Ji, S.M.; Tan, H.H.; Wong, C.Y.; Thike, A.A.; Tan, P.H.; Ho, G.H.; Lee, A.S.-G. Identification of Circulating MicroRNA Signatures for Breast Cancer Detection. Clin. Cancer Res. 2013, 19, 4477–4487. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.K.O.; Li, R.; Shin, V.Y.; Jin, H.C.; Leung, C.P.H.; Ma, E.S.K.; Pang, R.; Chua, D.; Chu, K.-M.; Law, W.L.; et al. Circulating microRNAs as Specific Biomarkers for Breast Cancer Detection. PLoS ONE 2013, 8, e53141. [Google Scholar] [CrossRef] [PubMed]
- Sochor, M.; Basova, P.; Pesta, M.; Dusilkova, N.; Bartos, J.; Burda, P.; Pospisil, V.; Stopka, T. Oncogenic MicroRNAs: miR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC Cancer 2014, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- Kodahl, A.R.; Lyng, M.B.; Binder, H.; Cold, S.; Gravgaard, K.; Knoop, A.S.; Ditzel, H.J. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: A case control study. Mol. Oncol. 2014, 8, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Zearo, S.; Kim, E.; Zhu, Y.; Zhao, J.T.; Sidhu, S.B.; Robinson, B.G.; Soon, P.S.H. MicroRNA-484 is more highly expressed in serum of early breast cancer patients compared to healthy volunteers. BMC Cancer 2014, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M.; Miller, N.; Wall, D.; Martyn, L.M.; Ball, G.; Sweeney, K.J.; Kerin, M.J. Identification and Validation of Oncologic miRNA Biomarkers for Luminal A-like Breast Cancer. PLoS ONE 2014, 9, e87032. [Google Scholar] [CrossRef]
- Eichelser, C.; Stueckrath, I.; Mueller, V.; Milde-Langosch, K.; Wikman, H.; Pantel, K.; Schwarzenbach, H. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget 2014, 5, 9650–9663. [Google Scholar] [CrossRef]
- Stuekrath, I.; Rack, B.; Janni, W.; Jaeger, B.; Pantel, K.; Schwarzenbach, H. Aberrant plasma levels of circulating miR-16, miR-107, miR-130a and miR-146a are associated with lymph node metastasis and receptor status of breast cancer patients. Oncotarget 2015, 6, 13387–13401. [Google Scholar] [CrossRef]
- Matamala, N.; Teresa Vargas, M.; Gonzalez-Campora, R.; Minambres, R.; Ignacio Arias, J.; Menendez, P.; Andres-Leon, E.; Gomez-Lopez, G.; Yanowsky, K.; Calvete-Candenas, J.; et al. Tumor MicroRNA Expression Profiling Identifies Circulating MicroRNAs for Early Breast Cancer Detection. Clin. Chem. 2015, 61, 1098–1106. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Jin, X.; Wang, Z.; Wu, Y.; Zhao, D.; Chen, G.; Li, D.; Wang, X.; Cao, H.; et al. A circulating miRNA signature as a diagnostic biomarker for non-invasive early detection of breast cancer. Breast Cancer Res. Treat. 2015, 154, 423–434. [Google Scholar] [CrossRef]
- Shimomura, A.; Shiino, S.; Kawauchi, J.; Takizawa, S.; Sakamoto, H.; Matsuzaki, J.; Ono, M.; Takeshita, F.; Niida, S.; Shimizu, C.; et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016, 107, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Zavridou, M.; Sourvinou, I.; Yousef, G.; Kounelis, S.; Malamos, N.; Georgoulias, V.; Lianidou, E. Direct Comparison of Metastasis-Related miRNAs Expression Levels in Circulating Tumor Cells, Corresponding Plasma, and Primary Tumors of Breast Cancer Patients. Clin. Chem. 2016, 62, 1002–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, K.; Wang, Y.-W.; Wang, Y.-Y.; Song, Y.; Zhu, J.; Si, P.-C.; Ma, R. Identification of microRNA biomarkers in the blood of breast cancer patients based on microRNA profiling. Gene 2017, 619, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Swellam, M.; El Magdoub, H.M.; Hassan, N.M.; Hefny, M.M.; Sobeih, M.E. Potential diagnostic role of circulating MiRNAs in breast cancer: Implications on clinicopathological characters. Clin. Biochem. 2018, 56, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-K.; Luo, Q.; Peng, H.; Li, J.; Zhao, M.; Wang, J.; Gu, Y.-Y.; Li, Y.; Yuan, P.; Zhao, G.-H.; et al. A Panel of Serum Noncoding RNAs for the Diagnosis and Monitoring of Response to Therapy in Patients with Breast Cancer. Med. Sci. Monit. 2018, 24, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Mao, Y.; Sun, Q.; Liu, F.; Lin, J.-S.; Liu, Y.; Cui, J.; Jiang, Y. Branched rolling circle amplification method for measuring serum circulating microRNA levels for early breast cancer detection. Cancer Sci. 2018, 109, 2897–2906. [Google Scholar] [CrossRef]
- Papadaki, C.; Stratigos, M.; Markakis, G.; Spiliotaki, M.; Mastrostamatis, G.; Nikolaou, C.; Mavroudis, D.; Agelaki, S. Circulating microRNAs in the early prediction of disease recurrence in primary breast cancer. Breast Cancer Res. 2018, 20, 72. [Google Scholar] [CrossRef]
- Papadaki, C.; Stoupis, G.; Tsalikis, L.; Monastirioti, A.; Papadaki, M.; Maliotis, N.; Stratigos, M.; Mastrostamatis, G.; Mavroudis, D.; Agelaki, S. Circulating miRNAs as a marker of metastatic disease and prognostic factor in metastatic breast cancer. Oncotarget 2019, 10, 966–981. [Google Scholar] [CrossRef]
- Masuda, T.; Shinden, Y.; Noda, M.; Ueo, H.; Hu, Q.; Yoshikawa, Y.; Tsuruda, Y.; Kuroda, Y.; Ito, S.; Eguchi, H.; et al. Circulating Pre-microRNA-488 in Peripheral Blood Is a Potential Biomarker for Predicting Recurrence in Breast Cancer. Anticancer Res. 2018, 38, 4515–4523. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Zhu, J.; Cheng, W.; Zhu, W.; Shu, Y.; Liu, P. MiR-4728-3p could act as a marker of HER2 status. Cancer Biomark. 2015, 15, 807–814. [Google Scholar] [CrossRef]
- Su, C.M.; Wang, M.Y.; Hong, C.C.; Chen, H.A.; Su, Y.H.; Wu, C.H.; Huang, M.T.; Chang, Y.W.; Jiang, S.S.; Sung, S.Y.; et al. miR-520h is crucial for DAPK2 regulation and breast cancer progression. Oncogene 2016, 35, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Cava, C.; Bertoli, G.; Ripamonti, M.; Mauri, G.; Zoppis, I.; Della Rosa, P.A.; Gilardi, M.C.; Castiglioni, I. Integration of mRNA Expression Profile, Copy Number Alterations, and microRNA Expression Levels in Breast Cancer to Improve Grade Definition. PLoS ONE 2014, 9, e97681. [Google Scholar] [CrossRef] [PubMed]
- Murria Estal, R.; Palanca Suela, S.; de Juan Jimenez, I.; Egoavil Rojas, C.; Garcia-Casado, Z.; Juan Fita, M.J.; Sanchez Heras, A.B.; Segura Huerta, A.; Chirivella Gonzalez, I.; Sanchez-Izquierdo, D.; et al. MicroRNA signatures in hereditary breast cancer. Breast Cancer Res. Treat. 2013, 142, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.H.; Wang, F.; Chapin, W.; Huang, R.S. Identification of MicroRNAs as Breast Cancer Prognosis Markers through the Cancer Genome Atlas. PLoS ONE 2016, 11, e0168284. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, Z.; Guo, J.; Ding, X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol. Oncol. 2010, 119, 586–593. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Z.; Ning, H.; Zhang, K.; Pan, D.; Ding, K.; Huang, W.; Kang, X.-L.; Wang, Y.; Chen, X. MicroRNA-21 in peripheral blood mononuclear cells as a novel biomarker in the diagnosis and prognosis of prostate cancer. Cancer Biomark. 2016, 17, 223–230. [Google Scholar] [CrossRef]
- Liu, J.; Mao, Q.; Liu, Y.; Hao, X.; Zhang, S.; Zhang, J. Analysis of miR-205 and miR-155 expression in the blood of breast cancer patients. Chin. J. Cancer Res. 2013, 25, 46–54. [Google Scholar]
- Li, X.-X.; Gao, S.-Y.; Wang, P.-Y.; Zhou, X.; Li, Y.-J.; Yu, Y.; Yan, Y.-F.; Zhang, H.-H.; Lv, C.-J.; Zhou, H.-H.; et al. Reduced expression levels of let-7c in human breast cancer patients. Oncol. Lett. 2015, 9, 1207–1212. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Gong, H.-T.; Li, B.-F.; Lv, C.-L.; Wang, H.-T.; Zhou, H.-H.; Li, X.-X.; Xie, S.-Y.; Jiang, B.-F. Higher expression of circulating miR-182 as a novel biomarker for breast cancer. Oncol. Lett. 2013, 6, 1681–1686. [Google Scholar] [CrossRef]
- Thakur, S.; Grover, R.K.; Gupta, S.; Yadav, A.K.; Das, B.C. Identification of Specific miRNA Signature in Paired Sera and Tissue Samples of Indian Women with Triple Negative Breast Cancer. PLoS ONE 2016, 11, e0158946. [Google Scholar] [CrossRef]
- Cecene, G.; Ak, S.; Eskiler, G.G.; Demirdogen, E.; Erturk, E.; Gokgoz, S.; Polatkan, V.; Egeli, U.; Tunca, B.; Tezcan, G.; et al. Circulating miR-195 as a Therapeutic Biomarker in Turkish Breast Cancer Patients. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 4241–4246. [Google Scholar] [PubMed]
- Dai, H.; Gallagher, D.; Schmitt, S.; Pessetto, Z.Y.; Fan, F.; Godwin, A.K.; Tawfik, O. Rote of miR-139 as a surrogate marker for tumor aggression in breast cancer. Hum. Pathol. 2017, 61, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liang, J.; Xu, J.; Li, X.; Xing, S.; Li, H.; Liu, W.; Liu, D.; Xu, J.; Huang, L.; et al. Identification and Validation of Circulating MicroRNA Signatures for Breast Cancer Early Detection Based on Large Scale Tissue-Derived Data. J. Breast Cancer 2018, 21, 363. [Google Scholar] [CrossRef] [PubMed]
- Qattan, A.; Intabli, H.; Alkhayal, W.; Eltabache, C.; Tweigieri, T.; Bin Amer, S. Robust expression of tumor suppressor miRNA’s let-7 and miR-195 detected in plasma of Saudi female breast cancer patients. BMC Cancer 2017, 17, 799. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Liang, J.-L.; Kuo, Y.-L.; Lee, H.-H.; Calkins, M.J.; Chang, H.-T.; Lin, F.-C.; Chen, Y.-C.; Hsu, T.-I.; Hsiao, M.; et al. miR-105/93-3p promotes chemoresistance and circulating miR-105/93-3p acts as a diagnostic biomarker for triple negative breast cancer. Breast Cancer Res. 2017, 19, 133. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Y.; Xia, T.; Zhou, X.; Huang, Z.; Zhang, H.; Zhu, W.; Ding, Q.; Wang, S. Circulating microRNAs from the miR-106a-363 cluster on chromosome X as novel diagnostic biomarkers for breast cancer. Breast Cancer Res. Treat. 2018, 170, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating microRNAs as Novel Minimally Invasive Biomarkers for Breast Cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef]
- Abdulhussain, M.M.; Hasan, N.A.; Hussain, A.G. Interrelation of the Circulating and Tissue MicroRNA-21 with Tissue PDCD4 Expression and the Invasiveness of Iraqi Female Breast Tumors. Indian J. Clin. Biochem. 2019, 34, 26–38. [Google Scholar] [CrossRef]
- Si, H.Y.; Sun, X.M.; Chen, Y.J.; Cao, Y.; Chen, S.M.; Wang, H.C.; Hu, C.J. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 223–229. [Google Scholar] [CrossRef]
- Watson, A.K.; Witwer, K.W. Do Platform-Specific Factors Explain MicroRNA Profiling Disparities? Clin. Chem. 2012, 58, 472–474. [Google Scholar] [CrossRef]
- Kirschner, M.B.; van Zandwijk, N.; Reid, G. Cell-free microRNAs: Potential biomarkers in need of standardized reporting. Front. Genet. 2013, 4, 56–56. [Google Scholar] [CrossRef]
- Rice, J.; Roberts, H.; Burton, J.; Pan, J.; States, V.; Rai, S.N.; Galandiuk, S. Assay Reproducibility in Clinical Studies of Plasma miRNA. PLoS ONE 2015, 10, e0121948. [Google Scholar] [CrossRef]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef]
- Leidner, R.S.; Li, L.; Thompson, C.L. Dampening Enthusiasm for Circulating MicroRNA in Breast Cancer. PLoS ONE 2013, 8, e57841. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ramasubramanian, B.; Kanji, S.; Chakraborty, A.R.; Hague, S.J.; Chakravarti, A. Circulating microRNAs in cancer: Hope or hype? Cancer Lett. 2016, 381, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.; Bonilla, P.; Ravishankar, Y.G.; Contag, A.; Gopal, N.; LaCour, S.; Lee, T.; Niemz, A. Technology in MicroRNA Profiling: Circulating MicroRNAs as Noninvasive Cancer Biomarkers in Breast Cancer. JALA 2015, 20, 574–588. [Google Scholar] [CrossRef]
- Fang, R.; Zhu, Y.; Hu, L.; Khadka, V.S.; Ai, J.; Zou, H.; Ju, D.; Jiang, B.; Deng, Y.; Hu, X. Plasma MicroRNA Pair Panels as Novel Biomarkers for Detection of Early Stage Breast Cancer. Front. Physiol. 2019, 9, 1879. [Google Scholar] [CrossRef] [PubMed]
- Swellam, M.; Zahran, R.F.K.; Abo El-Sadat Taha, H.; El-Khazragy, N.; Abdel-Malak, C. Role of some circulating MiRNAs on breast cancer diagnosis. Arch. Physiol. Biochem. 2018, 1–9. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Wang, M.; Li, Z.; Zhao, Z.; Wang, R.X.; Wu, R.; Yuan, Z.; Cui, R.; Jiao, K.; et al. MicroRNA-155, induced by FOXP3 through transcriptional repression of BRCA1, is associated with tumor initiation in human breast cancer. Oncotarget 2017, 8, 41451–41464. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Zhan, M.; Mann, D.L.; Stass, S.A.; Jiang, F. Differential miRNA expressions in peripheral blood mononuclear cells for diagnosis of lung cancer. Lab. Investig. 2015, 95, 1197–1206. [Google Scholar] [CrossRef]
- Su, J.; Leng, Q.; Lin, Y.; Ma, J.; Jiang, F.; Lee, C.-J.; Fang, H.; Jiang, F. Integrating Circulating Immunological and Sputum Biomarkers for the Early Detection of Lung Cancer. Biomark. Cancer 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Wu, H.-C.; Terry, M.B.; Santella, R.M. microRNA Expression in Prospectively Collected Blood as a Potential Biomarker of Breast Cancer Risk in the BCFR. Anticancer Res. 2015, 35, 3969–3977. [Google Scholar] [PubMed]
- Baine, M.J.; Chakraborty, S.; Smith, L.M.; Mallya, K.; Sasson, A.R.; Brand, R.E.; Batra, S.K. Transcriptional Profiling of Peripheral Blood Mononuclear Cells in Pancreatic Cancer Patients Identifies Novel Genes with Potential Diagnostic Utility. PLoS ONE 2011, 6, e17014. [Google Scholar] [CrossRef] [PubMed]
- Incoronato, M.; Aiello, M.; Infante, T.; Cavaliere, C.; Grimaldi, A.M.; Mirabelli, P.; Monti, S.; Salvatore, M. Radiogenomic Analysis of Oncological Data: A Technical Survey. Int. J. Mol. Sci. 2017, 18, E805. [Google Scholar] [CrossRef] [PubMed]
- Pezuk, J.A.; Araujo Miller, T.L.; Barbosa Bevilacqua, J.L.; Simoes Dornellas de Barros, A.C.; Martins de Andrade, F.E.; de Andrade e Macedo, L.F.; Aguilar, V.; Menardo Claro, A.N.; Camargo, A.A.; Favoretto Galante, P.A.; et al. Measuring plasma levels of three microRNAs can improve the accuracy for identification of malignant breast lesions in women with BI-RADS 4 mammography. Oncotarget 2017, 8, 83940–83948. [Google Scholar] [CrossRef] [PubMed]
- Lyng, M.B.; Kodahl, A.R.; Binder, H.; Ditzel, H.J. Prospective validation of a blood-based 9-miRNA profile for early detection of breast cancer in a cohort of women examined by clinical mammography. Mol. Oncol. 2016, 10, 1621–1626. [Google Scholar] [CrossRef]
- Zaleski, M.; Kobilay, M.; Schroeder, L.; Debald, M.; Semaan, A.; Hettwer, K.; Uhlig, S.; Kuhn, W.; Hartmann, G.; Holdenrieder, S. Improved sensitivity for detection of breast cancer by combination of miR-34a and tumor markers CA 15-3 or CEA. Oncotarget 2018, 9, 22523–22536. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Shen, H.; Deng, R.; Xue, K. Combination of miR-21 with Circulating Tumor Cells Markers Improve Diagnostic Specificity of Metastatic Breast Cancer. Cell Biochem. Biophys. 2015, 73, 87–91. [Google Scholar] [CrossRef]
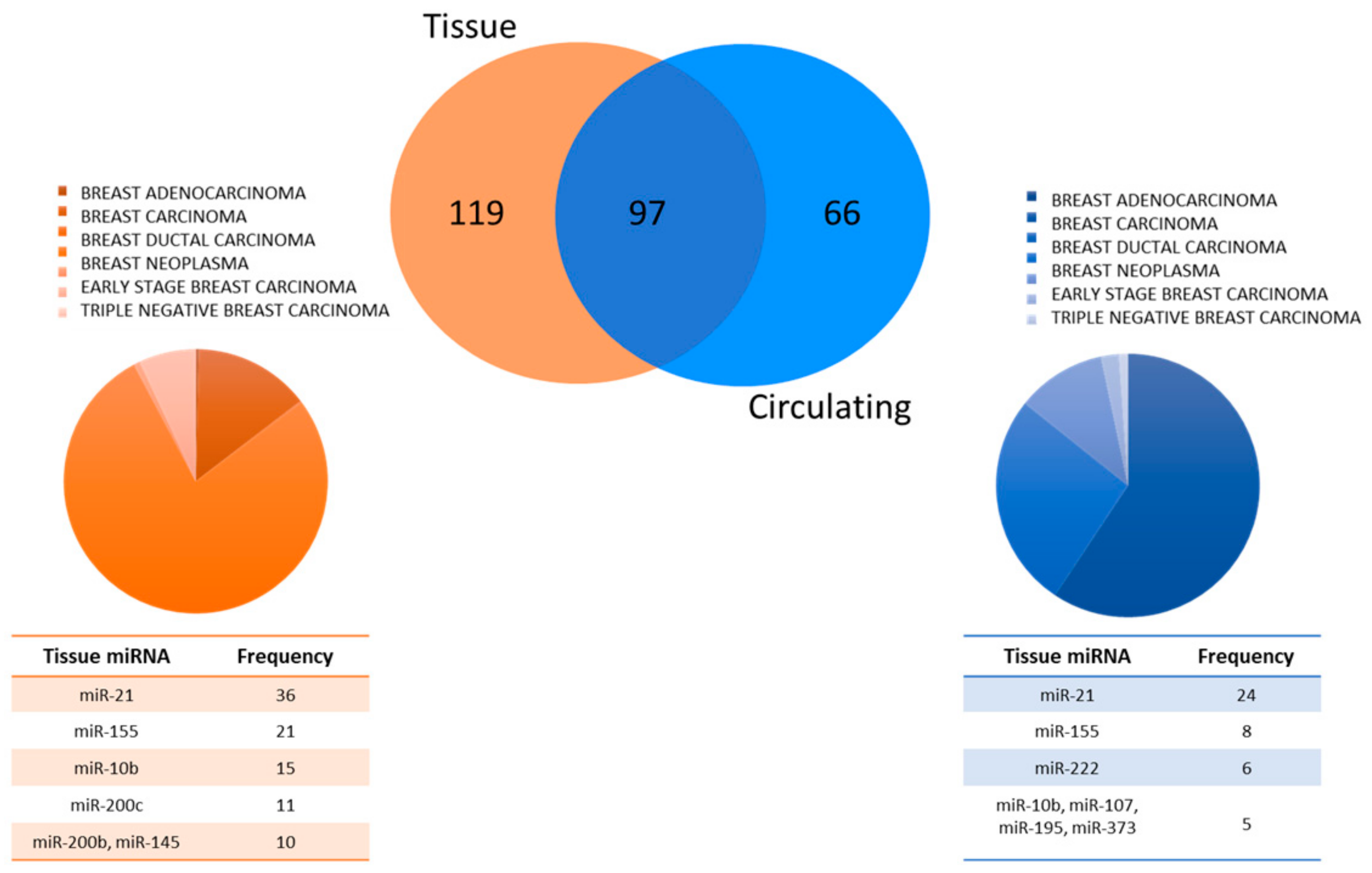
| Total Number of Panels | Recurring Circulating miRNAs | Circulating miRNA Frequency in Panels |
|---|---|---|
| 31 | miR-10b | 3 |
| miR-16 | 5 | |
| miR-21 | 8 | |
| miR-24 | 3 | |
| miR-29a | 4 | |
| miR-107 | 3 | |
| miR-145 | 3 | |
| miR-155 | 7 | |
| miR-195 | 3 | |
| miR-200c | 3 | |
| miR-222 | 4 | |
| miR-373 | 3 |
| Sample | Bloodstream Expression | Tissue Expression | Concordance | Patients (N) | Method | Reference |
|---|---|---|---|---|---|---|
| Serum | ↑ miR-21, miR-106a and miR-155; ↓ miR-126, miR-199a and miR-335 | ↑ miR-21, miR-106a and miR-155; ↓ miR-126, miR-199a and miR-335 | Yes | 68 BC tissues and adjacent non-tumour tissues and matching serum samples and 40 normal control sera | RT-qPCR | [56] |
| ↑ miR-222 | ↑ miR-222 | Yes | Screening step: 5 BC tissues and adjacent normal tissues and 13 BC serum and 10 healthy control serum. Validation step: 20 BC tissues and adjacent normal tissues and 50 BC serum and 50 healthy controls serum and 20 ovarian cancer serum and 20 benign breast cancer serum | SOLiD sequencing/RT-qPCR | [27] | |
| ↑ miR-15b, miR-16, miR-17, miR-25, miR-93, miR-107 and miR-185; ↓ miR-199a-5p | ↑ miR-15b, miR-16, miR-17, miR-25, miR-93, miR-107 and miR-185; ↓ miR-199a-5p | Yes | Markers discovery: 32 BC tissues and adjacent normal tissues and 32 BC serum and 22 healthy control serum. Validation stage: 132 BC serum and 101 healthy control serum. | LNA RT-PCR human miRNA panels (Exiqon)/RT-qPCR | [32] | |
| ↑ miR-21; ↓ miR-92a | ↑ miR-21; ↓ miR-92a | Yes | 48 BC tissue and 100 BC serum and 20 healthy control serum. | RT-qPCR | [57] | |
| ↓ miR-205; ↑ miR-155 | ↓ miR-205; ↑ miR-155 | Yes | 4 BC tissues and adjacent normal tissues and 20 BC serum and 10 healthy donors serum. | Microarray Chip/RT-qPCR | [58] | |
| ↓ let-7c | ↓ let-7c | Yes | 90 BC serum and 64 healthy control serum. | RT-qPCR | [59] | |
| ↑ miR-182 | ↑ miR-182 | Yes | 3 BC tissues and adjacent normal tissues; 46 BC serum and 58 healthy control serum. | RT-qPCR | [60] | |
| ↑ miR-21, miR-221, miR-210, let-7a; ↓ miR-195, miR-145 | ↑ miR-21, miR-221, miR-210 and Let-7a; ↓ miR-195, miR-145 | Yes | 85 BC tissues and adjacent normal tissues and 85 BC serum and 85 healthy donors serum; 15 benign breast tissues and adjacent normal tissues and 15 benign breast tissues serum and 15 healthy donors serum. | RT-qPCR | [61] | |
| ↑ miR-195 | ↓ miR-195 | No | 96 BC tissues and normal tissues; 48 pre-operative BC serum and 48 post-operative BC serum and 12 heathy control serum. | RT-qPCR | [62] | |
| ↑ miR-139 | ↑ miR-139 | Yes | 74 BC tissues and 18 paired serum and tissue samples of BC and 10 healthy donor serum. | RT-qPCR | [63] | |
| ↑ miR-21-5p and miR-21-3p;↓ miR-99a-5p | ↑ miR-21-5p and miR-21-3p; ↓miR-99a-5p | Yes | In silico (TGCA): 409 BC tissue and 87 healthy controls tissues. Samples 113 BC serum and 47 healthy controls serum. | RT-qPCR | [64] | |
| Plasma | ↓ miR-181a, miR-652, miR-29a and miR-223 | ↑ miR-181a and miR-652 | No | 11 BC tissues and adjacent normal tissue and 54 Luminal A-like breast cancer plasma and 56 healthy control plasma. | TaqMan human miRNA arrays/RT-qPCR | [37] |
| ↑ miR-505-5p, miR-125b-5p, miR-21-5p, miR-96-5p | ↑ miR-21-5p, miR-96-5p; ↓ miR-505-5p, miR-125b-5p | Partially | Markers discovery: 122 BC tumours and 11 healthy breast tissue. Discovery cohort: 83 BC plasma and 26 healthy control plasma. Validation cohort: 114 BC plasma and 116 healthy control plasma. | (LNA)-based miRNA microarrays | [40] | |
| ↑ miR-21, miR-146a, and miR-210 | ↑ miR-21 and miR-146a; ↓ miR-200c and miR-210 | Partially | 89 BC tissues; 30 not BC tissues; 55 BC plasma and 20 healthy donors plasma. | RT-qPCR | [43] | |
| ↑ miR-145 and miR-451 | ↑ miR-145 and miR-451 | Yes | Markers discovery: 5 BC tissue and adjacent normal tissue and 5 BC plasma and 5 normal control plasma. Marker validation: 170 BC plasma and 100 normal control plasma. Blind validation: 70 BC plasma and 50 normal control plasma. | TaqMan Array Human MicroRNA Panels A and B/RT-qPCR | [33] | |
| ↑ let-7 miRNA and miR-195 | ↓ let-7 miRNA and miR-195 | No | In silico (TGCA): 120 luminal samples and 38 Triple Negative samples and 87 control. Samples: 57 luminal BC plasma and 36 TNBC plasma and 34 healthy control. | miScript miRNA PCR Array /RT-qPCR | [65] | |
| ↑ miR-105 and miR-93-3p | ↑ miR-105 and miR-93-3p | Yes | In silico (Metabric): 204 TNBC and 1095 non-TNBC from Metabric database; 94 TNBC and 79 non-TNBC from GEO dataset. Samples: 13 TNBC normal and tumour tissue pairs and 12 non-TNBC N-T pairs and 130 plasma samples (12 healthy controls, 74 TNBC and 44 non-TNBC). | RT-qPCR | [66] | |
| Plasma and Serum | ↑ miR-106a-5p and miR-20b-5p | ↑ miR-106a-5p and miR-20b-5p | Yes | 200 BC plasma and 200 plasma controls and 204 BC serum and 202 serum control; 32 paired breast tissues. | RT-qPCR | [67] |
| Whole blood | ↑ miR-195 | ↑ miR-195 | Yes | 65 BC tissues and adjacent normal tissues and 83 BC whole blood and plasma and serum and 44 healthy control whole blood and plasma and serum. | RT-qPCR | [68] |
| A. Concordant | |||||
| miRNA | Total Studies (n°) | Circulating Expression | Tissue Expression | ||
| Up | Down | Up | Down | ||
| mir-155 | 16 | 4 | - | 12 | - |
| mir-373 | 6 | 3 | - | 3 | - |
| mir-20a-b | 6 | 4 | - | 2 | - |
| mir-181b | 5 | 1 | - | 4 | - |
| B. Partially Concordant | |||||
| miRNA | Total Studies (n°) | Circulating Expression | Tissue Expression | ||
| Up | Down | Up | Down | ||
| mir-21 | 38 | 10 | 1 | 25 | 2 |
| mir-10b | 12 | 2 | 1 | 5 | 4 |
| mir-200c | 10 | 1 | 1 | 3 | 5 |
| mir-145 | 11 | - | 3 | 1 | 7 |
| mir-222 | 8 | 3 | 2 | 3 | - |
| mir-200b | 6 | 1 | - | 2 | 3 |
| mir-195 | 5 | 1 | 2 | - | 2 |
| mir-210 | 6 | 1 | 1 | 3 | 1 |
| mir-182 | 8 | 2 | 2 | 2 | 2 |
| mir-19a | 5 | 1 | 1 | 1 | 2 |
| Identifier | Status | Study Type | Study Start Date | Title | Interventions |
|---|---|---|---|---|---|
| NCT01612871 | Completed | Interventional | 2012 | Circulating miRNAs as Biomarkers of Hormone Sensitivity in Breast Cancer | Drugs: -Tamoxifen -Letrozole -Anastrozole -Exemestane |
| NCT01722851 | Recruiting | Observational | 2011 | Circulating miRNAs. ICORG 10-11, V2 | -none |
| NCT03779022 | Recruiting | Observational | 2015 | miRNA and Relevant Biomarkers of BC Patients Undergoing Neoadjuvant Treatment | Genetic: -microRNA |
| NCT02618538 | Enrolling by invitation | Observational | 2015 | The ANDROMEDA Study. Predictive Value of Combined Criteria to Tailor Breast Cancer Screening | -none |
| NCT03255486 | Completed | Interventional | 2013 | Identification and Evaluation of Biomarkers of Resistance to Neoadjuvant Chemotherapy (IDEA SEIN) | Biological: -Blood sample |
| NCT03528473 | Recruiting | Interventional | 2019 | Adapted Physical Activity (APA) in a Breast Cancer Population | Behavioural: -Exercise |
| NCT03118882 | Completed | Interventional | 2010 | STI.VI. Study: How to Improve Lifestyles in Screening Contexts | Behavioural: -Diet -Physiological activity |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimaldi, A.M.; Incoronato, M. Clinical Translatability of “Identified” Circulating miRNAs for Diagnosing Breast Cancer: Overview and Update. Cancers 2019, 11, 901. https://doi.org/10.3390/cancers11070901
Grimaldi AM, Incoronato M. Clinical Translatability of “Identified” Circulating miRNAs for Diagnosing Breast Cancer: Overview and Update. Cancers. 2019; 11(7):901. https://doi.org/10.3390/cancers11070901
Chicago/Turabian StyleGrimaldi, Anna Maria, and Mariarosaria Incoronato. 2019. "Clinical Translatability of “Identified” Circulating miRNAs for Diagnosing Breast Cancer: Overview and Update" Cancers 11, no. 7: 901. https://doi.org/10.3390/cancers11070901
APA StyleGrimaldi, A. M., & Incoronato, M. (2019). Clinical Translatability of “Identified” Circulating miRNAs for Diagnosing Breast Cancer: Overview and Update. Cancers, 11(7), 901. https://doi.org/10.3390/cancers11070901





