Phase I Dose Escalation Study with Expansion Cohort of the Addition of Nab-Paclitaxel to Capecitabine and Oxaliplatin (CapOx) as First-Line Treatment of Metastatic Esophagogastric Adenocarcinoma (ACTION Study)
Abstract
1. Introduction
2. Results
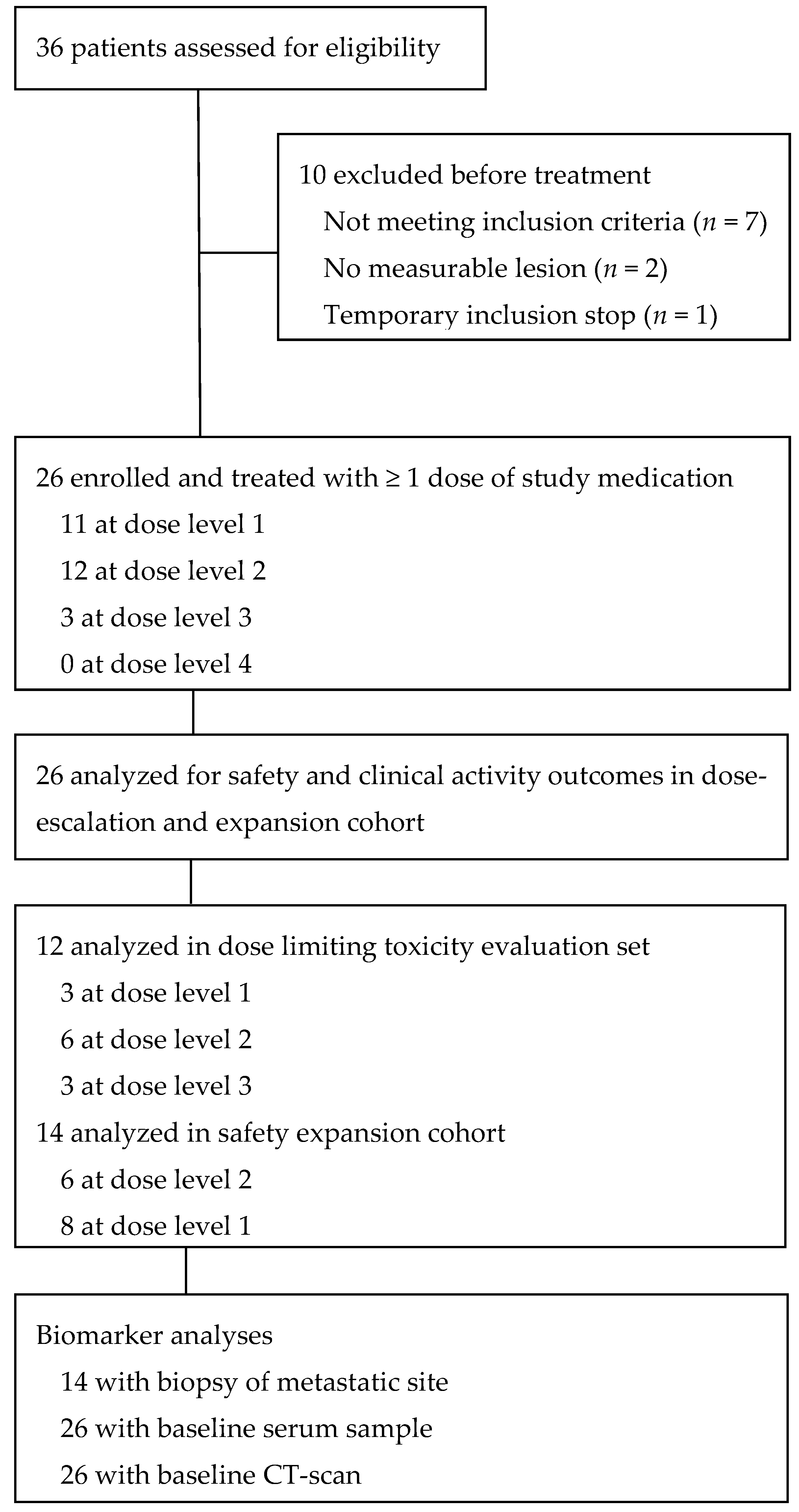
2.1. Patients
2.2. Safety and Tolerability
2.2.1. Dose Escalation, DLTs, and MTD
2.2.2. Treatment-Related Adverse Events
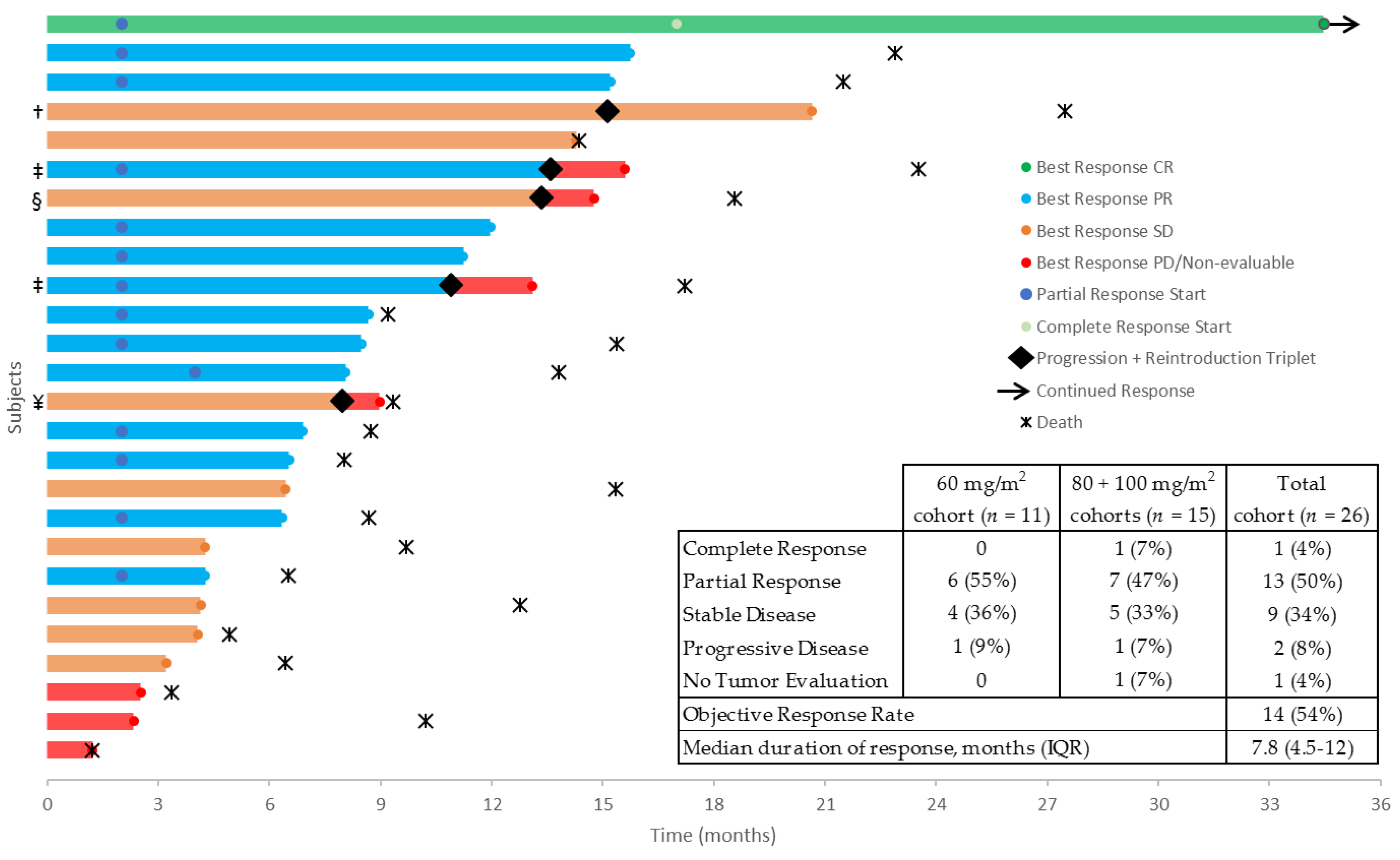
2.3. Drug Exposure and Clinical Activity
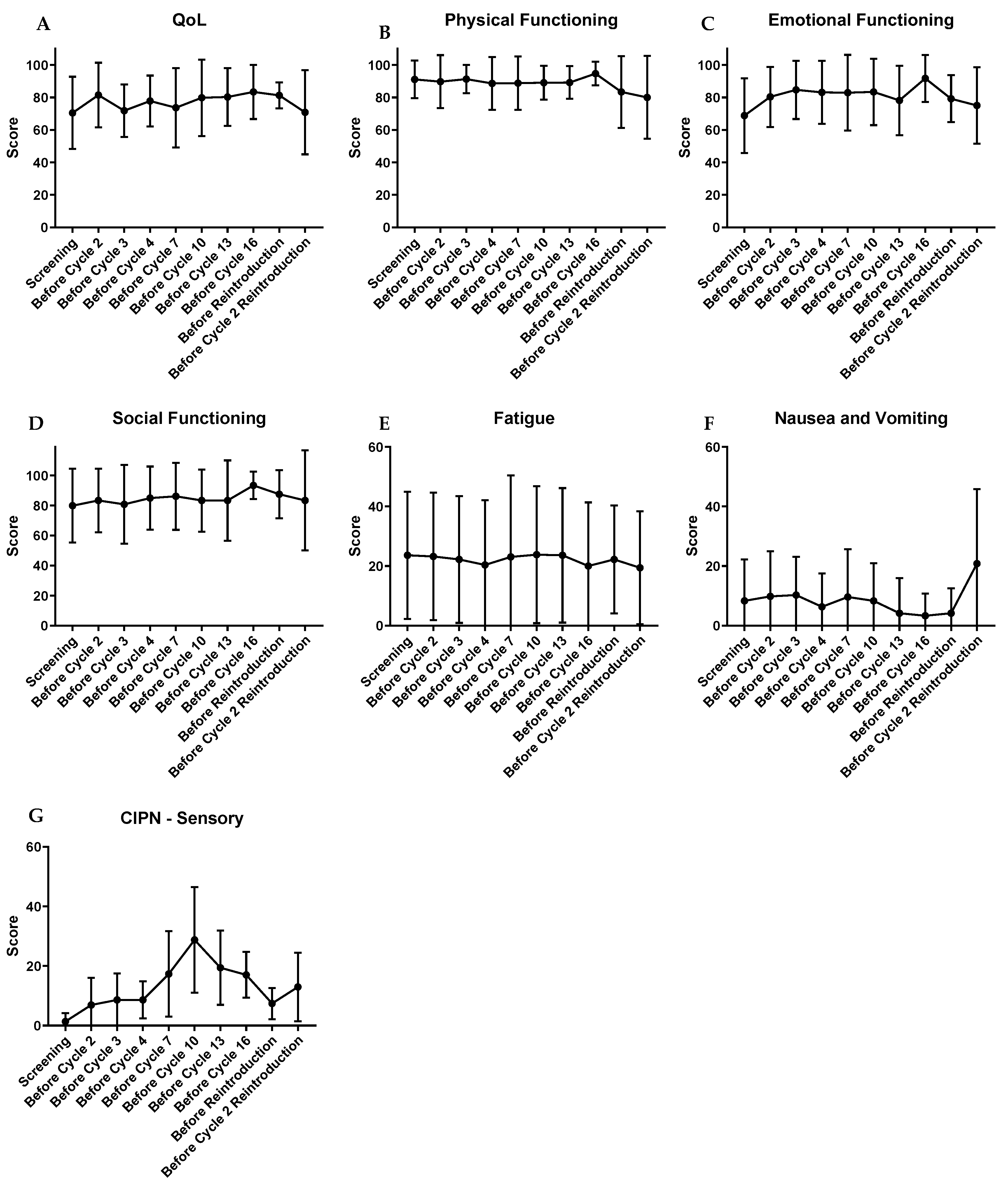
2.4. Patient-Reported Health-Related Quality of Life and Neurotoxicity
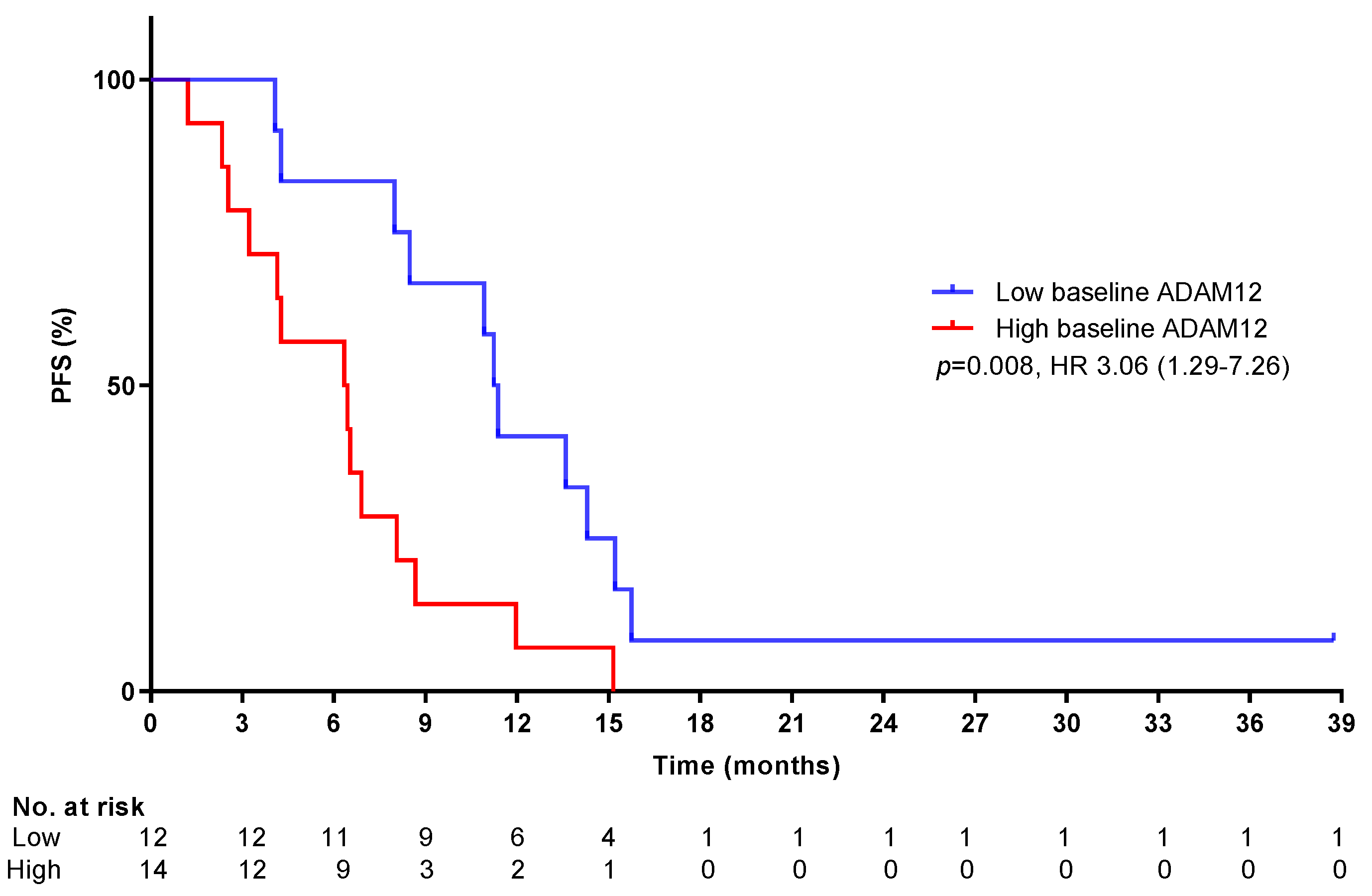
2.5. Biomarkers
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design
4.3. Study Assessments
4.3.1. Toxicity
4.3.2. Self-Reported Health-Related Quality of Life and Neurotoxicity
4.3.3. Biomarkers
4.3.4. Tumor Volume Assessment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R.; et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ter Veer, E.; Haj Mohammad, N.; van Valkenhoef, G.; Ngai, L.L.; Mali, R.M.A.; Anderegg, M.C.; van Oijen, M.G.H.; van Laarhoven, H.W.M. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: A network meta-analysis. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Takashima, A.; Fujitani, K.; Koeda, K.; Hara, H.; Nakayama, N.; Hironaka, S.; Nishikawa, K.; Makari, Y.; Amagai, K.; et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): An open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 277–287. [Google Scholar] [CrossRef]
- Hawkes, E.; Okines, A.F.; Papamichael, D.; Rao, S.; Ashley, S.; Charalambous, H.; Koukouma, A.; Chau, I.; Cunningham, D. Docetaxel and irinotecan as second-line therapy for advanced oesophagogastric cancer. Eur. J. Cancer 2011, 47, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Underwood, T.J.; Hayden, A.L.; Derouet, M.; Garcia, E.; Noble, F.; White, M.J.; Thirdborough, S.; Mead, A.; Clemons, N.; Mellone, M.; et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J. Pathol. 2015, 235, 466–477. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.B.; El-Ashry, D.; Turley, E.A. Hyaluronan, cancer-associated fibroblasts and the tumor microenvironment in malignant progression. Front. Cell Dev. Biol. 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, M.F.; van Laarhoven, H.W. The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: A systematic review and critical appraisal. Cancer Metastasis Rev. 2015, 34, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.R.; Brugge, J.S. A stiff blow from the stroma: Collagen crosslinking drives tumor progression. Cancer Cell 2009, 16, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Courrech Staal, E.F.; Wouters, M.W.; van Sandick, J.W.; Takkenberg, M.M.; Smit, V.T.; Junggeburt, J.M.; Spitzer-Naaykens, J.M.; Karsten, T.; Hartgrink, H.H.; Mesker, W.E.; et al. The stromal part of adenocarcinomas of the oesophagus: Does it conceal targets for therapy? Eur. J. Cancer 2010, 46, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Grabsch, H.; Ivanova, T.; Tan, I.B.; Murray, J.; Ooi, C.H.; Wright, A.I.; West, N.P.; Hutchins, G.G.; Wu, J.; et al. Comprehensive genomic meta-analysis identifies intra-tumoural stroma as a predictor of survival in patients with gastric cancer. Gut 2013, 62, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Sethi, T.; Rintoul, R.C.; Moore, S.M.; MacKinnon, A.C.; Salter, D.; Choo, C.; Chilvers, E.R.; Dransfield, I.; Donnelly, S.C.; Strieter, R.; et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999, 5, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, E.A.; van der Zalm, A.P.; Steins, A.; Creemers, A.; Hermsen, S.; Rentenaar, R.; Klein, M.; Waasdorp, C.; Hooijer, G.K.J.; Meijer, S.L.; et al. Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc. Natl. Acad. Sci. USA 2019, 116, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Redfern, A.; Agarwal, V.; Thompson, E.W. Hypoxia as a signal for prison breakout in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 250–263. [Google Scholar] [CrossRef]
- Hockel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Qiao, M.; Xu, Y.; Guan, W.; Wang, L. Cancer associated fibroblasts tailored tumor microenvironment of therapy resistance in gastrointestinal cancers. J. Cell Physiol. 2018, 233, 6359–6369. [Google Scholar] [CrossRef]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Ajani, J.A.; Fodor, M.B.; Tjulandin, S.A.; Moiseyenko, V.M.; Chao, Y.; Cabral Filho, S.; Majlis, A.; Assadourian, S.; Van Cutsem, E. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J. Clin. Oncol. 2005, 23, 5660–5667. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Pauligk, C.; Homann, N.; Hartmann, J.T.; Moehler, M.; Probst, S.; Rethwisch, V.; Stoehlmacher-Williams, J.; Prasnikar, N.; Hollerbach, S.; et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: A randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur. J. Cancer 2013, 49, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Blum Murphy, M.A.; Qiao, W.; Mewada, N.; Wadhwa, R.; Elimova, E.; Takashi, T.; Ho, L.; Phan, A.; Baker, J.; Ajani, J. A phase I/II study of docetaxel, oxaliplatin, and fluorouracil (D-FOX) chemotherapy in patients with untreated locally unresectable or metastatic adenocarcinoma of the stomach and gastroesophageal junction. Am. J. Clin. Oncol. 2018, 41, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.D.; Fazio, N.; Stupp, R.; Falk, S.; Bernhard, J.; Saletti, P.; Koberle, D.; Borner, M.M.; Rufibach, K.; Maibach, R.; et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: A randomized phase II trial of the Swiss Group for Clinical Cancer Research. J. Clin. Oncol. 2007, 25, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Janjigian, Y.Y.; Stoller, R.; Shibata, S.; Kemeny, M.; Krishnamurthi, S.; Su, Y.B.; Ocean, A.; Capanu, M.; Mehrotra, B.; et al. Randomized multicenter phase II study of modified Docetaxel, Cisplatin, and Fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: A study of the US gastric cancer consortium. J. Clin. Oncol. 2015, 33, 3874–3879. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Boni, C.; Tabernero, J.; Massuti, B.; Middleton, G.; Dane, F.; Reichardt, P.; Pimentel, F.L.; Cohn, A.; Follana, P.; et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: A randomized phase II study. Ann. Oncol. 2015, 26, 149–156. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E.; et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J. Clin. Oncol. 2006, 24, 4991–4997. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Li, J.; Bai, Y.; Liu, T.; Jiao, S.; Dai, G.; Xu, J.; Liu, Y.; Fan, N.; et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer 2016, 19, 234–244. [Google Scholar] [CrossRef]
- Li, C.P.; Chen, J.S.; Chen, L.T.; Yen, C.J.; Lee, K.D.; Su, W.P.; Lin, P.C.; Lu, C.H.; Tsai, H.J.; Chao, Y. A phase II study of weekly docetaxel and cisplatin plus oral tegafur/uracil and leucovorin as first-line chemotherapy in patients with locally advanced or metastatic gastric cancer. Br. J. Cancer 2010, 103, 1343–1348. [Google Scholar] [CrossRef]
- Hecht, J.R.; Bang, Y.J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—A randomized phase III trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Jeung, H.C.; Rha, S.Y.; Kim, H.S.; Jung, I.; Nam, B.H.; Lee, K.H.; Chung, H.C. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur. J. Cancer 2012, 48, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Kripp, M.; Al-Batran, S.E.; Rosowski, J.; Pauligk, C.; Homann, N.; Hartmann, J.T.; Moehler, M.; Hofheinz, R.D. Quality of life of older adult patients receiving docetaxel-based chemotherapy triplets for esophagogastric adenocarcinoma: A randomized study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Gastric Cancer 2014, 17, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Bang, Y.J.; Gotovkin, E.A.; Hamamoto, Y.; Kang, Y.K.; Moiseyenko, V.M.; Ohtsu, A.; Van Cutsem, E.; Al-Sakaff, N.; Urspruch, A.; et al. Quality of life in the trastuzumab for gastric cancer trial. Oncologist 2014, 19, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Abramov, M.; Bondarenko, I.; Shparyk, Y.; Gorbunova, V.; Hontsa, A.; Otchenash, N.; Alsina, M.; Lazarev, S.; Feliu, J.; et al. A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann. Oncol. 2017, 28, 2142–2148. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Dank, M.; Zaluski, J.; Barone, C.; Valvere, V.; Yalcin, S.; Peschel, C.; Wenczl, M.; Goker, E.; Cisar, L.; Wang, K.; et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann. Oncol. 2008, 19, 1450–1457. [Google Scholar] [CrossRef]
- Moehler, M.; Kanzler, S.; Geissler, M.; Raedle, J.; Ebert, M.P.; Daum, S.; Flieger, D.; Seufferlein, T.; Galle, P.R.; Hoehler, T.; et al. A randomized multicenter phase II study comparing capecitabine with irinotecan or cisplatin in metastatic adenocarcinoma of the stomach or esophagogastric junction. Ann. Oncol. 2010, 21, 71–77. [Google Scholar] [CrossRef]
- Shah, M.A.; Bang, Y.J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; McCaffery, I.; Shames, D.S.; et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: The METGastric randomized clinical trial. JAMA Oncol. 2017, 3, 620–627. [Google Scholar] [CrossRef]
- Ter Veer, E.; Haj Mohammad, N.; van Valkenhoef, G.; Ngai, L.L.; Mali, R.M.; van Oijen, M.G.; van Laarhoven, H.W. Second- and third-line systemic therapy in patients with advanced esophagogastric cancer: A systematic review of the literature. Cancer Metastasis Rev. 2016, 35, 439–456. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Boku, N.; Mizusawa, J.; Iwasa, S.; Kadowaki, S.; Nakayama, N.; Azuma, M.; Sakamoto, T.; Shitara, K.; Okuno, T.; et al. Phase III study comparing triplet chemotherapy with S-1 and cisplatin plus docetaxel versus doublet chemotherapy with S-1 and cisplatin for advanced gastric cancer (JCOG1013). In Proceedings of the ASCO 2018, Chicago, IL, USA, 1–5 June 2018. [Google Scholar]
- Kawamoto, Y.; Komatsu, Y.; Yuki, S.; Sawada, K.; Muranaka, T.; Harada, K.; Nakatsumi, H.; Fukushima, H.; Ishiguro, A.; Dazai, M.; et al. Study protocol of HGCSG1404 SNOW study: A phase I/II trial of combined chemotherapy of S-1, nab-paclitaxel and oxaliplatin administered biweekly to patients with advanced gastric cancer. BMC Cancer 2017, 17, 837. [Google Scholar] [CrossRef] [PubMed]
- Zaanan, A.; Samalin, E.; Aparicio, T.; Bouche, O.; Laurent-Puig, P.; Manfredi, S.; Michel, P.; Monterymard, C.; Moreau, M.; Rougier, P.; et al. Phase III randomized trial comparing 5-fluorouracil and oxaliplatin with or without docetaxel in first-line advanced gastric cancer chemotherapy (GASTFOX study). Dig. Liver Dis. 2018, 50, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, V.L.; Damhofer, H.; Waasdorp, C.; van Rijssen, L.B.; Dijk, F.; Wilmink, J.W.; Besselink, M.G.; Busch, O.R.; Medema, J.P.; Shiansong Li, J.; et al. ADAM12 is a circulating marker for stromal activation in pancreatic cancer and predicts response to chemotherapy. Oncogenesis 2019. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; LaPlant, B.R.; Gross, G.G.; Bane, C.L.; Palmieri, F.M.; North Central Cancer Treatment, G. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531). Ann. Oncol. 2009, 20, 449–453. [Google Scholar] [CrossRef]
- Frustaci, S.; Buonadonna, A.; Turchet, E.; Corona, G.; Tabaro, G.; Miolo, G.; Torrisi, E.; Lo Re, G.; Tumolo, S.; Toffoli, G. Phase I trial of docetaxel, oxaliplatin, and capecitabine (DOC) in untreated gastric cancer patients. Int. J. Clin. Oncol. 2013, 18, 510–516. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M.; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur. J. Cancer 2005, 41, 1690–1696. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization For Research And Treatment Of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Postma, T.J.; Aaronson, N.K.; Heimans, J.J.; Muller, M.J.; Hildebrand, J.G.; Delattre, J.Y.; Hoang-Xuan, K.; Lanteri-Minet, M.; Grant, R.; Huddart, R.; et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef]
- Pavlakis, N.; Sjoquist, K.M.; Martin, A.J.; Tsobanis, E.; Yip, S.; Kang, Y.K.; Bang, Y.J.; Alcindor, T.; O’Callaghan, C.J.; Burnell, M.J.; et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): A multinational placebo-controlled phase II trial. J. Clin. Oncol. 2016, 34, 2728–2735. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | n (%)/Median (Range) |
|---|---|
| Median Age at Start Study | 63 (45–75) |
| Sex | |
| Male | 23 (89%) |
| Female | 3 (11%) |
| ECOG performance status | |
| 0 | 20 (77%) |
| 1 | 6 (23%) |
| Primary tumor location | |
| Esophagus | 9 (35%) |
| Gastro-esophageal junction | 13 (50%) |
| Stomach | 4 (15%) |
| Disease status | |
| Synchronous metastases | 16 (62%) |
| Metachronous metastases | 10 (39%) |
| Number of organs involved | |
| 1 | 10 (39%) |
| 2 | 10 (39%) |
| 3 | 3 (11%) |
| 4 | 3 (11%) |
| Organs involved | |
| Lymph nodes | 18 (69%) |
| Liver | 12 (46%) |
| Lung | 6 (23%) |
| Bone | 3 (12%) |
| Peritoneum | 3 (12%) |
| Locoregional recurrence | 2 (7%) |
| Other * | 5 (19%) |
| Prior (neo)adjuvant treatment | |
| Previous radiotherapy | 9 (35%) |
| Previous chemotherapy ** | 10 (39%) |
| Previous gastrectomy | 1 (4%) |
| Previous esophagectomy | 5 (19%) |
| Median time since last (neo-)adjuvant chemotherapy in months (range) | 9.8 (1.5–31.7) |
| Adverse Event | 60 mg/m2 (n = 11) | 80–100 mg/m2 (n = 15) | ||
|---|---|---|---|---|
| Grade 1/2 | Grade 3/4/5 | Grade 1/2 | Grade 3/4/5 | |
| Hematological | n (%) | n (%) | n (%) | n (%) |
| Thrombocytopenia | 0 | 1 (9%) | 1 (6%) | 1 (6%) |
| Leukocytopenia | 0 | 0 | 0 | 1 (6%) |
| Non-febrile neutropenia | 0 | 2 (18%) | 1 (6%) | 3 (20%) |
| Non-Hematological | n (%) | n (%) | n (%) | n (%) |
| Peripheral sensory neuropathy | 9 (82%) | 0 | 15 (100%) | 0 |
| Fatigue | 8 (73%) | 0 | 12 (80%) | 0 |
| Nausea | 7 (64%) | 1 (9) | 10 (66%) | 1 (6%) |
| Diarrhea | 8 (73%) | 1 (9) | 9 (60%) | 5 (33%) |
| Dysgeusia | 6 (55%) | 0 | 9 (60%) | 0 |
| Alopecia | 3 (27%) | 0 | 8 (53%) | 0 |
| Vomiting | 4 (36%) | 1 (9) | 8 (53%) | 2 (13%) |
| Anorexia | 6 (55%) | 1 (9) | 7 (47%) | 0 |
| Oral mucositis | 1 (9%) | 0 | 4 (26%) | 1 (6%) |
| Pain | 3 (27%) | 0 | 4 (26%) | 0 |
| Dehydration | 0 | 0 | 0 | 4 (26%) |
| Fever | 0 | 0 | 3 (20%) | 0 |
| Constipation | 3 (27%) | 0 | 2 (13%) | 0 |
| Cough | 0 | 0 | 2 (13%) | 0 |
| Dysphagia | 0 | 0 | 2 (13%) | 0 |
| Flu-like symptoms | 0 | 0 | 2 (13%) | 0 |
| Malaise | 2 (18%) | 0 | 2 (13%) | 0 |
| Non-cardiac chest pain | 0 | 0 | 2 (13%) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 1 (9%) | 0 | 2 (13%) | 0 |
| Maculopapular rash | 0 | 0 | 2 (13%) | 0 |
| Hypertension | 0 | 0 | 0 | 1 (6%) |
| Sepsis | 0 | 0 | 0 | 1 (6%) |
| Abdominal pain | 1 (9%) | 0 | 1 (6%) | 0 |
| Dizziness | 1 (9%) | 0 | 1 (6%) | 0 |
| Thromboembolic event | 1 (9%) | 0 | 1 (6%) | 0 |
| Drug Exposure | n (%)/Median (Range) |
|---|---|
| Median number of cycles ACTION (range) | 6 (2–6) |
| Patients completing 6 cycles | 20 (77%) |
| Reasons for premature study termination * | |
| Toxicity | 4 (15%) |
| Progressive disease | 2 (8%) |
| Patients continuing capecitabine monotherapy | 18 (69%) |
| Median number of cycles capecitabine monotherapy (range) | 7 (2–43) |
| Patients eligible for reintroduction | 5 (19%) |
| Median dose intensity oxaliplatin (range) ** | 40.2 (22.7–67.4) |
| Dose intensity oxaliplatin in % | 84.6% |
| Median dose intensity capecitabine (range) ** | 7424 (4530–9672) |
| Dose intensity capecitabine in % | 89.2% |
| Median dose intensity nab-paclitaxel (range) ** | 43.5 (25.7–82.9) |
| Dose intensity nab-paclitaxel in %, total cohort | 83.9% |
| 60 mg/m2 cohort | 37.5 (25.7–46.0) |
| Dose intensity in % | 87.8% |
| 80 mg/m2 cohort | 47.6 (27.7–82.9) |
| Dose intensity in % | 88.3% |
| 100 mg/m2 cohort | 47.3 (41.1–64.6) |
| Dose intensity in % | 78.9% |
| Baseline HRQoL (n = 24) | Mean (SD) | Median (IQR) |
|---|---|---|
| Global QoL/Global Health Score †‡ | 70.5 (22.2) | 75 (66.6–83.3) |
| Functioning scales †‡ | ||
| Physical functioning | 91.1 (11.6) | 93.3 (86.7–100) |
| Role functioning | 81.9 (23) | 91.7 (66.7–100) |
| Emotional functioning | 68.8 (23) | 66.7 (52.1–89.6) |
| Cognitive functioning | 93.1 (12.9) | 100 (83.3–100) |
| Social functioning | 79.9 (24.6) | 91.7 (66.7–100) |
| Symptom scales † | ||
| Fatigue | 23.6 (21.3) | 22.2 (2.8–33.3) |
| Nausea and vomiting | 8.3 (13.9) | 0 (0–16.7) |
| Pain | 18.8 (25.2) | 8.3 (0–33.3) |
| Dyspnea | 13.9 (19.5) | 0 (0–33.3) |
| Insomnia | 25 (31.5) | 0 (0–58.3) |
| Appetite loss | 22.2 (27.2) | 0 (0–33.3) |
| Constipation | 16.7 (22) | 0 (0–33.3) |
| Diarrhea | 22.2 (28.9) | 0 (0–33.3) |
| Financial difficulties | 6.9 (17) | 0 (0–0) |
| Neuropathy scales § | ||
| Sensory Neuropathy | 1.4 (2.9) | 0 (0–2.8) |
| Motor Neuropathy | 1 (2.4) | 0 (0–0) |
| Autonomic Neuropathy | 4.2 (8.9) | 0 (0–0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schokker, S.; van der Woude, S.O.; van Kleef, J.J.; van Zoen, D.J.; van Oijen, M.G.H.; Mearadji, B.; Beenen, L.F.M.; Stroes, C.I.; Waasdorp, C.; Jibodh, R.A.; et al. Phase I Dose Escalation Study with Expansion Cohort of the Addition of Nab-Paclitaxel to Capecitabine and Oxaliplatin (CapOx) as First-Line Treatment of Metastatic Esophagogastric Adenocarcinoma (ACTION Study). Cancers 2019, 11, 827. https://doi.org/10.3390/cancers11060827
Schokker S, van der Woude SO, van Kleef JJ, van Zoen DJ, van Oijen MGH, Mearadji B, Beenen LFM, Stroes CI, Waasdorp C, Jibodh RA, et al. Phase I Dose Escalation Study with Expansion Cohort of the Addition of Nab-Paclitaxel to Capecitabine and Oxaliplatin (CapOx) as First-Line Treatment of Metastatic Esophagogastric Adenocarcinoma (ACTION Study). Cancers. 2019; 11(6):827. https://doi.org/10.3390/cancers11060827
Chicago/Turabian StyleSchokker, Sandor, Stephanie O. van der Woude, Jessy Joy van Kleef, Daan J. van Zoen, Martijn G. H. van Oijen, Banafsche Mearadji, Ludo F. M. Beenen, Charlotte I. Stroes, Cynthia Waasdorp, R. Aarti Jibodh, and et al. 2019. "Phase I Dose Escalation Study with Expansion Cohort of the Addition of Nab-Paclitaxel to Capecitabine and Oxaliplatin (CapOx) as First-Line Treatment of Metastatic Esophagogastric Adenocarcinoma (ACTION Study)" Cancers 11, no. 6: 827. https://doi.org/10.3390/cancers11060827
APA StyleSchokker, S., van der Woude, S. O., van Kleef, J. J., van Zoen, D. J., van Oijen, M. G. H., Mearadji, B., Beenen, L. F. M., Stroes, C. I., Waasdorp, C., Jibodh, R. A., Creemers, A., Meijer, S. L., Hooijer, G. K. J., Punt, C. J. A., Bijlsma, M. F., & van Laarhoven, H. W. M. (2019). Phase I Dose Escalation Study with Expansion Cohort of the Addition of Nab-Paclitaxel to Capecitabine and Oxaliplatin (CapOx) as First-Line Treatment of Metastatic Esophagogastric Adenocarcinoma (ACTION Study). Cancers, 11(6), 827. https://doi.org/10.3390/cancers11060827





