The Helicobacter pylori Urease Virulence Factor Is Required for the Induction of Hypoxia-Induced Factor-1α in Gastric Cells
Abstract
1. Introduction
2. Results
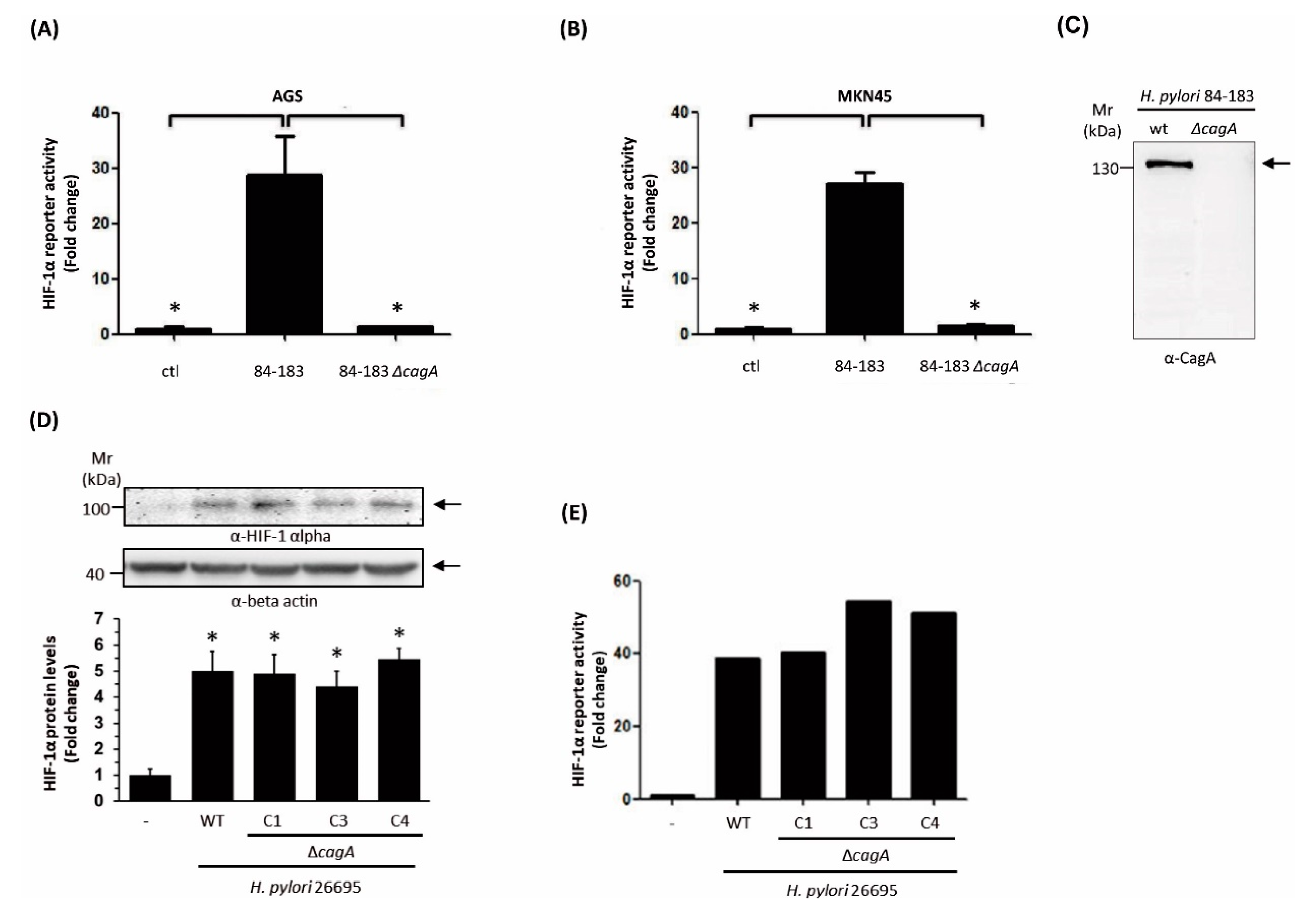
2.1. Characterization of the Ability of Helicobacter pylori Mutant Strains to Induce HIF-1α in Gastric Cells
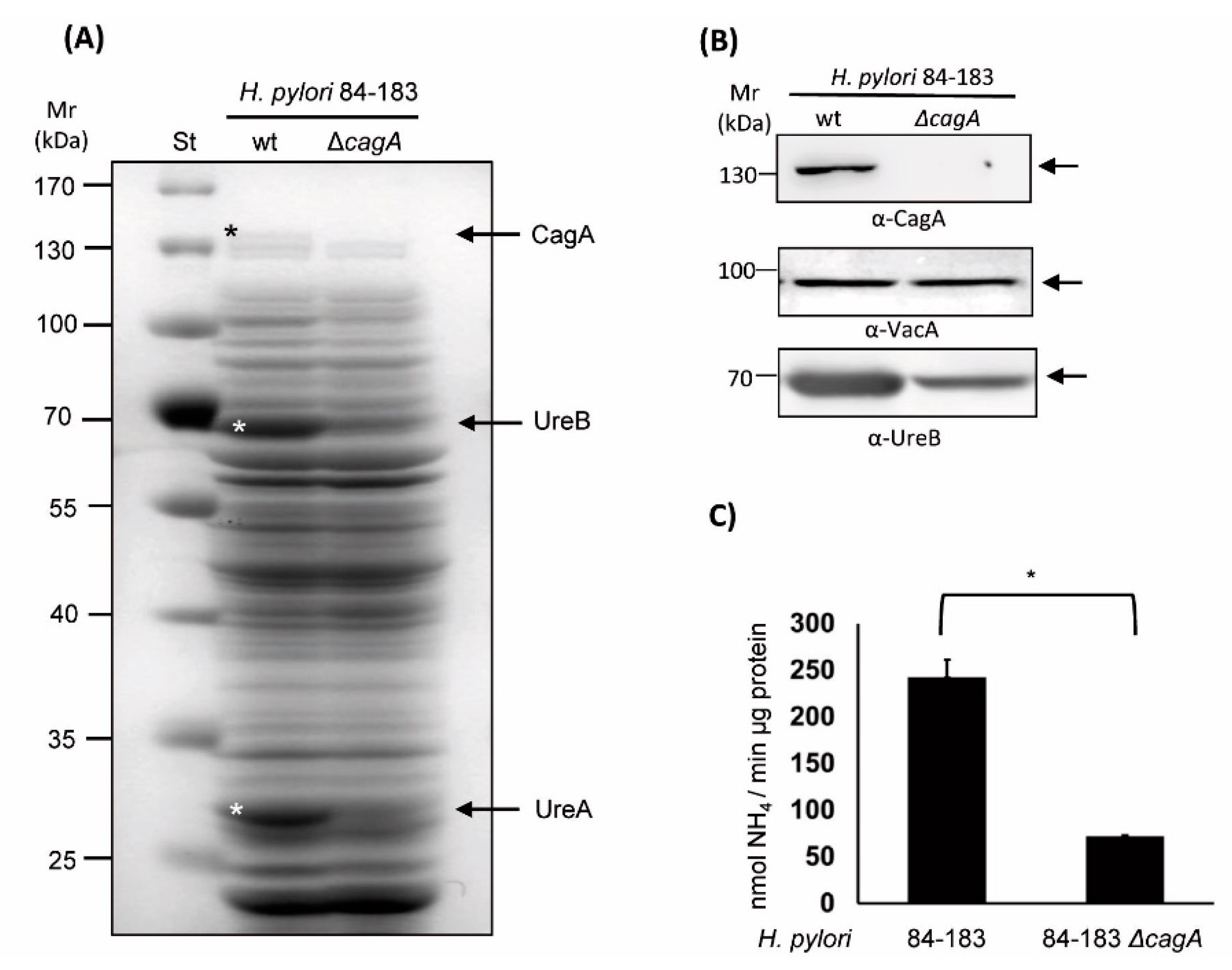
2.2. The Isogenic cagA mutant H. pylori 84-183 Strain Displayed Reduced Urease Protein Levels and Activity
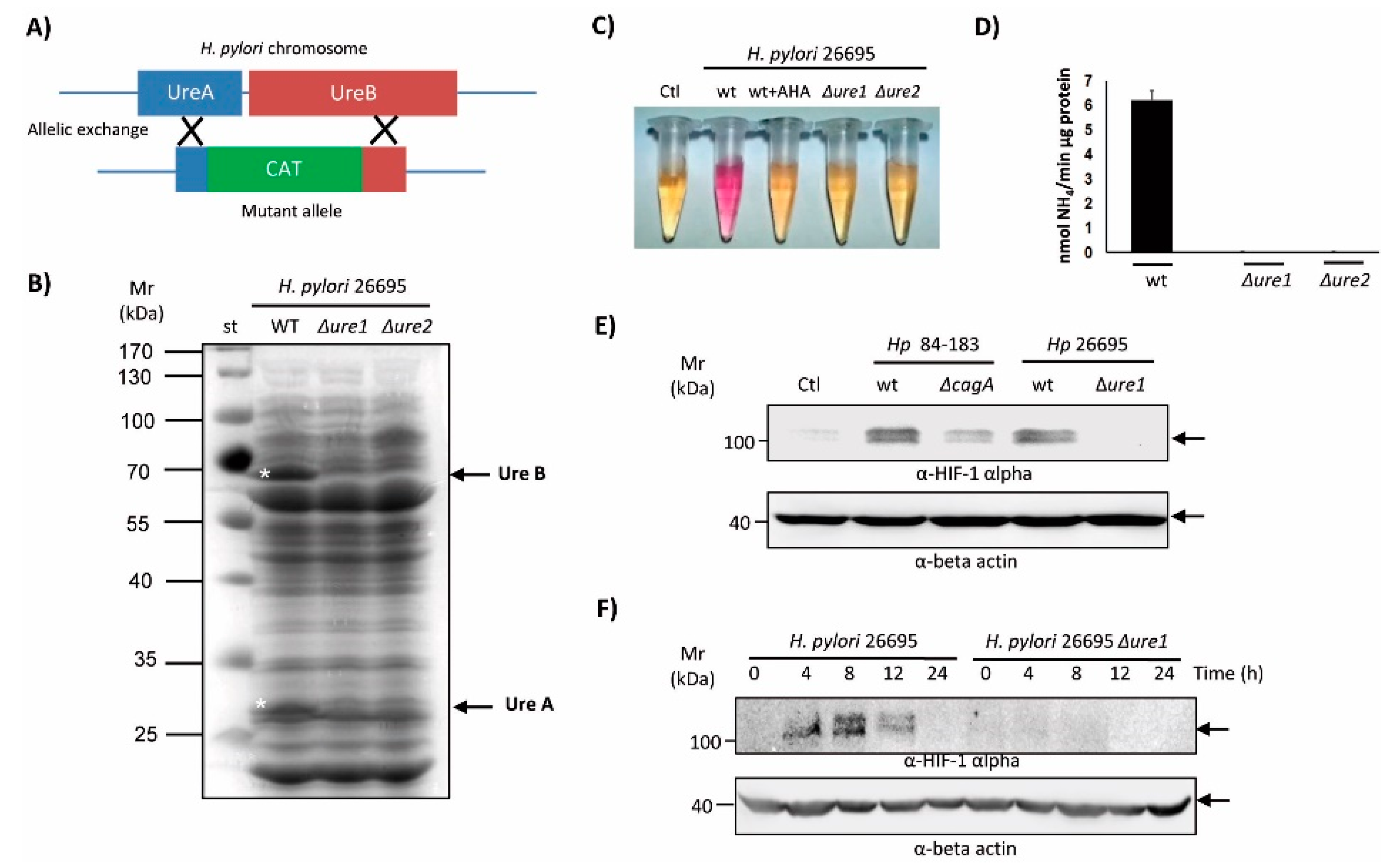
2.3. An Isogenic Urease Mutant of the H. pylori 26695 Strain Reduced the Ability to Induce HIF-1α Protein Expression in AGS Cells
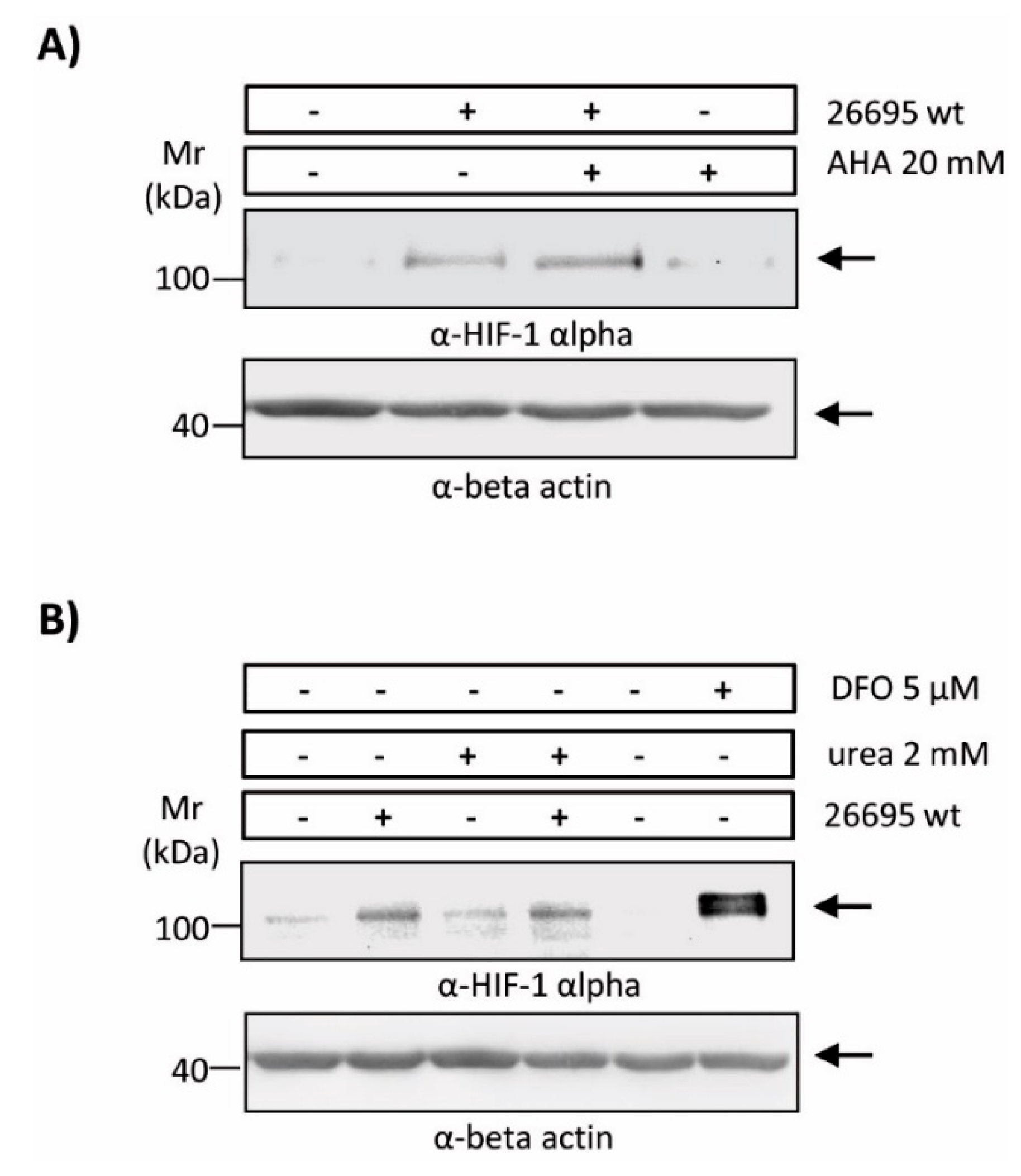
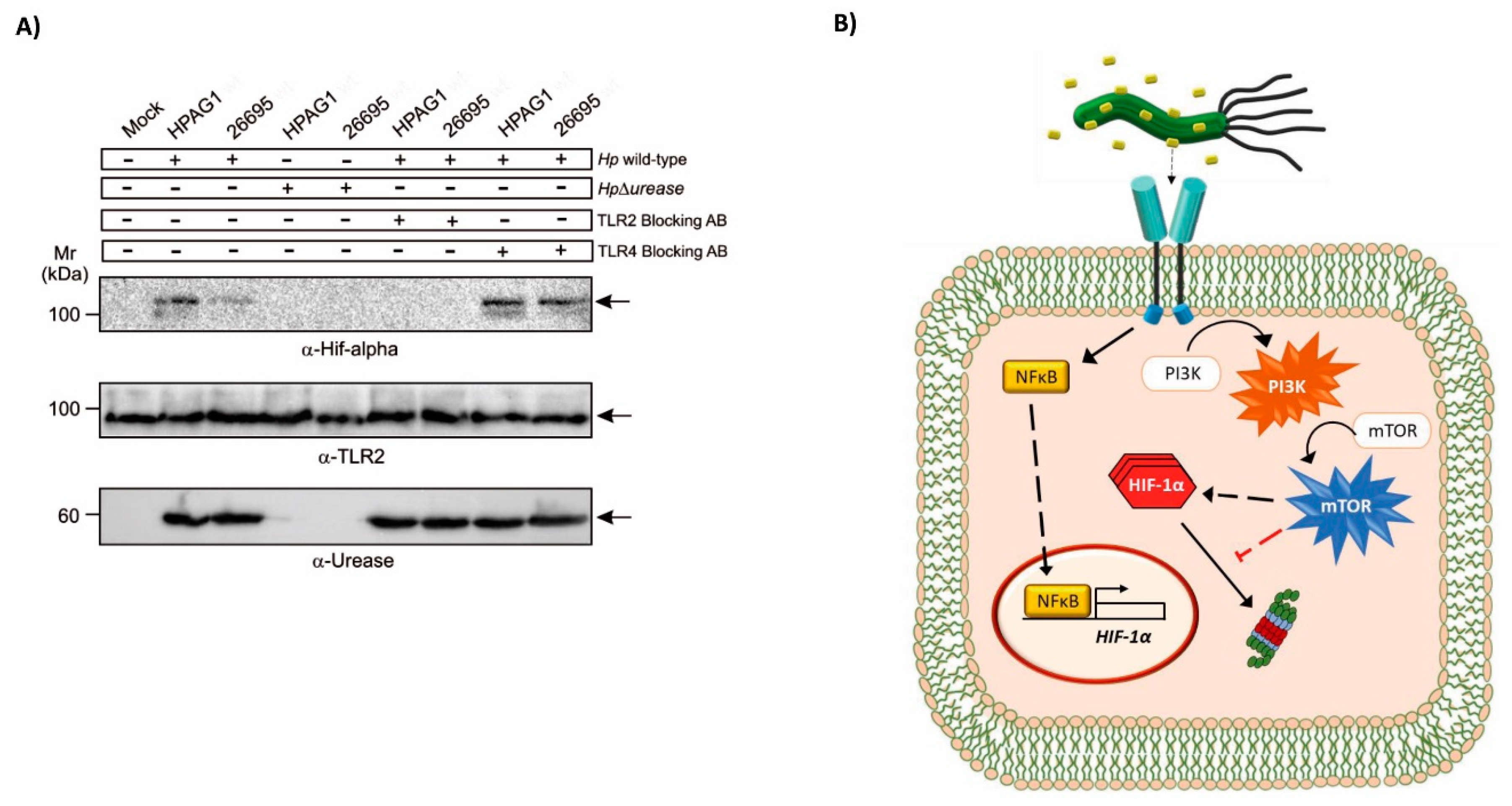
2.4. Enzymatic Urease Activity Appears Not to Be Required for HIF-1α Induction
2.5. TLR2 Is Implicated in the Induction of HIF-1α by the H. pylori Urease
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. Bacterial Culture
4.3. Construction of H. pylori CagA and Urease Mutants
4.4. Experiments with TLR2 and TLR4 Blocking Antibodies
4.5. Infection of Gastric Cells
4.6. Luciferase Reporter Assay
4.7. Western Blotting
4.8. Determination of Urease Activity
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atherton, J.C.; Blaser, M.J. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J. Clin. Investig. 2009, 119, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.A.; Canales, J.; Corvalan, A.H.; Quest, A.F. Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis. World J. Gastroenterol. 2015, 21, 12742–12756. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Sokic-Milutinovic, A.; Alempijevic, T.; Milosavljevic, T. Role of Helicobacter pylori infection in gastric carcinogenesis: Current knowledge and future directions. World J. Gastroenterol. 2015, 21, 11654–11672. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. North Am. 2013, 42, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.R.; Marcus, E.A.; Weeks, D.L.; Sachs, G. Mechanisms of acid resistance due to the urease system of Helicobacter pylori. Gastroenterology 2002, 123, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Dehzad, N.; Reuter, S.; Martin, H.; Becher, B.; Taube, C.; Muller, A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J. Clin. Investig. 2011, 121, 3088–3093. [Google Scholar] [CrossRef]
- Gebert, B.; Fischer, W.; Weiss, E.; Hoffmann, R.; Haas, R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 2003, 301, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Oertli, M.; Noben, M.; Engler, D.B.; Semper, R.P.; Reuter, S.; Maxeiner, J.; Gerhard, M.; Taube, C.; Muller, A. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc. Natl. Acad. Sci. USA 2013, 110, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: A paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.; Perez-Perez, G.; Corvalan, A.H.; Carrasco, G.; Urra, H.; Bravo, D.; Toledo, H.; Quest, A.F. Helicobacter pylori-induced loss of the inhibitor-of-apoptosis protein survivin is linked to gastritis and death of human gastric cells. J. Infect. Dis. 2010, 202, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Karita, M.; Tsuda, M.; Nakazawa, T. Essential role of urease in vitro and in vivo Helicobacter pylori colonization study using a wild-type and isogenic urease mutant strain. J. Clin. Gastroenterol. 1995, 21 (Suppl. 1), S160–S163. [Google Scholar]
- Schoep, T.D.; Fulurija, A.; Good, F.; Lu, W.; Himbeck, R.P.; Schwan, C.; Choi, S.S.; Berg, D.E.; Mittl, P.R.; Benghezal, M.; et al. Surface properties of Helicobacter pylori urease complex are essential for persistence. PLoS ONE 2010, 5, e15042. [Google Scholar] [CrossRef] [PubMed]
- Weeks, D.L.; Eskandari, S.; Scott, D.R.; Sachs, G. A H+-gated urea channel: The link between Helicobacter pylori urease and gastric colonization. Science 2000, 287, 482–485. [Google Scholar] [CrossRef]
- Akada, J.K.; Shirai, M.; Takeuchi, H.; Tsuda, M.; Nakazawa, T. Identification of the urease operon in Helicobacter pylori and its control by mRNA decay in response to pH. Mol. Microbiol. 2000, 36, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.G.; Wang, D.X.; Hao, M.C.; Sun, X.S. Nickel trafficking system responsible for urease maturation in Helicobacter pylori. World J. Gastroenterol. 2013, 19, 8211–8218. [Google Scholar] [CrossRef]
- Sachs, G.; Weeks, D.L.; Wen, Y.; Marcus, E.A.; Scott, D.R.; Melchers, K. Acid acclimation by Helicobacter pylori. Physiology 2005, 20, 429–438. [Google Scholar] [CrossRef]
- Gobert, A.P.; Mersey, B.D.; Cheng, Y.; Blumberg, D.R.; Newton, J.C.; Wilson, K.T. Cutting edge: Urease release by Helicobacter pylori stimulates macrophage inducible nitric oxide synthase. J. Immunol. 2002, 168, 6002–6006. [Google Scholar] [CrossRef]
- Vanet, A.; Labigne, A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect. Immun. 1998, 66, 1023–1027. [Google Scholar] [PubMed]
- Dunn, B.E.; Vakil, N.B.; Schneider, B.G.; Miller, M.M.; Zitzer, J.B.; Peutz, T.; Phadnis, S.H. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect. Immun. 1997, 65, 1181–1188. [Google Scholar] [PubMed]
- Phadnis, S.H.; Parlow, M.H.; Levy, M.; Ilver, D.; Caulkins, C.M.; Connors, J.B.; Dunn, B.E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 1996, 64, 905–912. [Google Scholar] [PubMed]
- Harris, P.R.; Mobley, H.L.; Perez-Perez, G.I.; Blaser, M.J.; Smith, P.D. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology 1996, 111, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.N.; Muller, A. Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory T-cells, establish persistent infection and promote tolerance to allergens. Gut Microbes 2015, 6, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Severo, D.; Uberti, A.F.; Marques, M.S.; Pinto, M.T.; Gomez-Lazaro, M.; Figueiredo, C.; Leite, M.; Carlini, C.R. A New Role for Helicobacter pylori Urease: Contributions to Angiogenesis. Front. Microbiol. 2017, 8, 1883. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Fang, W.G. Hypoxia-inducible factor-1 in tumour angiogenesis. World J. Gastroenterol. 2004, 10, 1082–1087. [Google Scholar] [CrossRef]
- Goda, N.; Ryan, H.E.; Khadivi, B.; McNulty, W.; Rickert, R.C.; Johnson, R.S. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol. Cell. Biol. 2003, 23, 359–369. [Google Scholar] [CrossRef]
- Kitajima, Y.; Miyazaki, K. The Critical Impact of HIF-1a on Gastric Cancer Biology. Cancers 2013, 5, 15–26. [Google Scholar] [CrossRef]
- Canales, J.; Valenzuela, M.; Bravo, J.; Cerda-Opazo, P.; Jorquera, C.; Toledo, H.; Bravo, D.; Quest, A.F. Helicobacter pylori Induced Phosphatidylinositol-3-OH Kinase/mTOR Activation Increases Hypoxia Inducible Factor-1alpha to Promote Loss of Cyclin D1 and G0/G1 Cell Cycle Arrest in Human Gastric Cells. Front. Cell. Infect. Microbiol. 2017, 7, 92. [Google Scholar] [CrossRef]
- Koch, K.N.; Hartung, M.L.; Urban, S.; Kyburz, A.; Bahlmann, A.S.; Lind, J.; Backert, S.; Taube, C.; Muller, A. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J. Clin. Investig. 2015, 125, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F.; Watanabe, E.; Nakagawa, Y.; Yamanishi, S.; Norose, Y.; Fukunaga, Y.; Takahashi, H. Production of autoantibodies by murine B-1a cells stimulated with Helicobacter pylori urease through toll-like receptor 2 signaling. Infect. Immun. 2011, 79, 4791–4801. [Google Scholar] [CrossRef] [PubMed]
- Imtiyaz, H.Z.; Simon, M.C. Hypoxia-inducible factors as essential regulators of inflammation. Curr. Top. Microbiol. Immunol. 2010, 345, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Chavarria-Velazquez, C.O.; Torres-Martinez, A.C.; Montano, L.F.; Rendon-Huerta, E.P. TLR2 activation induced by H. pylori LPS promotes the differential expression of claudin-4, -6, -7 and -9 via either STAT3 and ERK1/2 in AGS cells. Immunobiology 2018, 223, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Ceponis, P.J.; Lebel, S.; Huynh, H.; Sherman, P.M. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect. Immun. 2003, 71, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; (University of Erlangen-Nuremberg, Erlangen, Germany). Personal communication, 2019.
- Perez-Perez, G.I.; Olivares, A.Z.; Cover, T.L.; Blaser, M.J. Characteristics of Helicobacter pylori variants selected for urease deficiency. Infect. Immun. 1992, 60, 3658–3663. [Google Scholar]
- Kempf, V.A.; Lebiedziejewski, M.; Alitalo, K.; Walzlein, J.H.; Ehehalt, U.; Fiebig, J.; Huber, S.; Schutt, B.; Sander, C.A.; Muller, S.; et al. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: Evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 2005, 111, 1054–1062. [Google Scholar] [CrossRef]
- Werth, N.; Beerlage, C.; Rosenberger, C.; Yazdi, A.S.; Edelmann, M.; Amr, A.; Bernhardt, W.; von Eiff, C.; Becker, K.; Schafer, A.; et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE 2010, 5, e11576. [Google Scholar] [CrossRef]
- Van Uden, P.; Kenneth, N.S.; Rocha, S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 2008, 412, 477–484. [Google Scholar] [CrossRef]
- Beswick, E.J.; Pinchuk, I.V.; Minch, K.; Suarez, G.; Sierra, J.C.; Yamaoka, Y.; Reyes, V.E. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-kappaB activation and interleukin-8 production. Infect. Immun. 2006, 74, 1148–1155. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; El-Zataari, M.; Owyang, S.Y.; Eaton, K.A.; Liu, M.; Chang, Y.M.; Zou, W.; Kao, J.Y. TLR2 mediates Helicobacter pylori-induced tolerogenic immune response in mice. PLoS ONE 2013, 8, e74595. [Google Scholar] [CrossRef] [PubMed]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF transcription factors, inflammation, and immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Spirig, R.; Djafarzadeh, S.; Regueira, T.; Shaw, S.G.; von Garnier, C.; Takala, J.; Jakob, S.M.; Rieben, R.; Lepper, P.M. Effects of TLR agonists on the hypoxia-regulated transcription factor HIF-1alpha and dendritic cell maturation under normoxic conditions. PLoS ONE 2010, 5, e0010983. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, M.; Song, L.; Qi, P.; Chen, C.; Wang, X.; Li, N.; Peng, J.; Wu, D.; Hu, G.; et al. The contribution of toll-like receptor 2 on Helicobacter pylori activation of the nuclear factor-kappa B signaling pathway in gastric epithelial cells. Microb. Pathog. 2016, 98, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kronsteiner, B.; Bassaganya-Riera, J.; Philipson, C.; Viladomiu, M.; Carbo, A.; Abedi, V.; Hontecillas, R. Systems-wide analyses of mucosal immune responses to Helicobacter pylori at the interface between pathogenicity and symbiosis. Gut Microbes 2016, 7, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xi, P.; Wang, Z.; Han, X.; Xu, Y.; Zhang, Y.; Miao, J. PI3K/Akt/mTOR signaling pathway participates in Streptococcus uberis-induced inflammation in mammary epithelial cells in concert with the classical TLRs/NF-kB pathway. Vet. Microbiol. 2018, 227, 103–111. [Google Scholar] [CrossRef]
- Wang, Y.; Taylor, D.E. Chloramphenicol resistance in Campylobacter coli: Nucleotide sequence, expression, and cloning vector construction. Gene 1990, 94, 23–28. [Google Scholar] [CrossRef]
- Valenzuela, M.; Bravo, D.; Canales, J.; Sanhueza, C.; Diaz, N.; Almarza, O.; Toledo, H.; Quest, A.F. Helicobacter pylori-induced loss of survivin and gastric cell viability is attributable to secreted bacterial gamma-glutamyl transpeptidase activity. J. Infect. Dis. 2013, 208, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Zimman, A.; Gao, D.; Byzova, T.V.; Podrez, E.A. TLR2 Plays a Key Role in Platelet Hyperreactivity and Accelerated Thrombosis Associated With Hyperlipidemia. Circ. Res. 2017, 121, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Valderrama, M.; Cerda-Opazo, P.; Backert, S.; González, M.F.; Carrasco-Véliz, N.; Jorquera-Cordero, C.; Wehinger, S.; Canales, J.; Bravo, D.; Quest, A.F.G. The Helicobacter pylori Urease Virulence Factor Is Required for the Induction of Hypoxia-Induced Factor-1α in Gastric Cells. Cancers 2019, 11, 799. https://doi.org/10.3390/cancers11060799
Valenzuela-Valderrama M, Cerda-Opazo P, Backert S, González MF, Carrasco-Véliz N, Jorquera-Cordero C, Wehinger S, Canales J, Bravo D, Quest AFG. The Helicobacter pylori Urease Virulence Factor Is Required for the Induction of Hypoxia-Induced Factor-1α in Gastric Cells. Cancers. 2019; 11(6):799. https://doi.org/10.3390/cancers11060799
Chicago/Turabian StyleValenzuela-Valderrama, Manuel, Paulina Cerda-Opazo, Steffen Backert, María Fernanda González, Nicolás Carrasco-Véliz, Carla Jorquera-Cordero, Sergio Wehinger, Jimena Canales, Denisse Bravo, and Andrew F. G. Quest. 2019. "The Helicobacter pylori Urease Virulence Factor Is Required for the Induction of Hypoxia-Induced Factor-1α in Gastric Cells" Cancers 11, no. 6: 799. https://doi.org/10.3390/cancers11060799
APA StyleValenzuela-Valderrama, M., Cerda-Opazo, P., Backert, S., González, M. F., Carrasco-Véliz, N., Jorquera-Cordero, C., Wehinger, S., Canales, J., Bravo, D., & Quest, A. F. G. (2019). The Helicobacter pylori Urease Virulence Factor Is Required for the Induction of Hypoxia-Induced Factor-1α in Gastric Cells. Cancers, 11(6), 799. https://doi.org/10.3390/cancers11060799






