The Integration of a Three-Dimensional Spheroid Cell Culture Operation in a Circulating Tumor Cell (CTC) Isolation and Purification Process: A Preliminary Study of the Clinical Significance and Prognostic Role of the CTCs Isolated from the Blood Samples of Head and Neck Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Chemotherapy Regimens
2.3. Blood Sample Processing Using the 2-Step CTC Isolation and Purification Method
2.4. Immunofluorescence Staining (IF Staining)
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results and Discussion
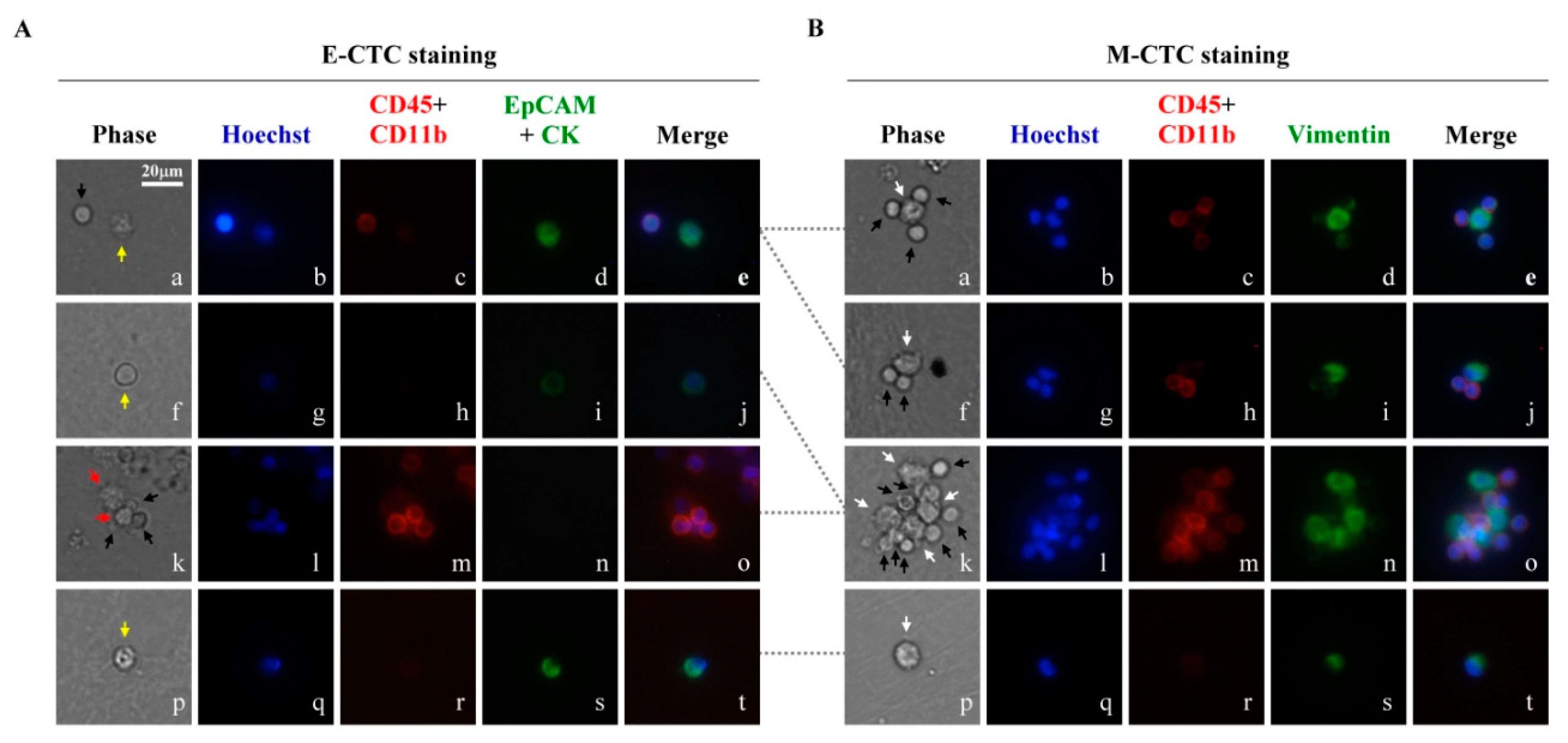
3.1. The CTCs in the Blood Samples of Head and Neck Cancer Patients Treated with the 2-Step CTC Isolation and Purification Method
3.2. A Preliminary Study of the Clinical Significance of E-CTC and M-CTC Counts in the Blood Samples Treated with the 2-Step CTC Isolation and Purification Process
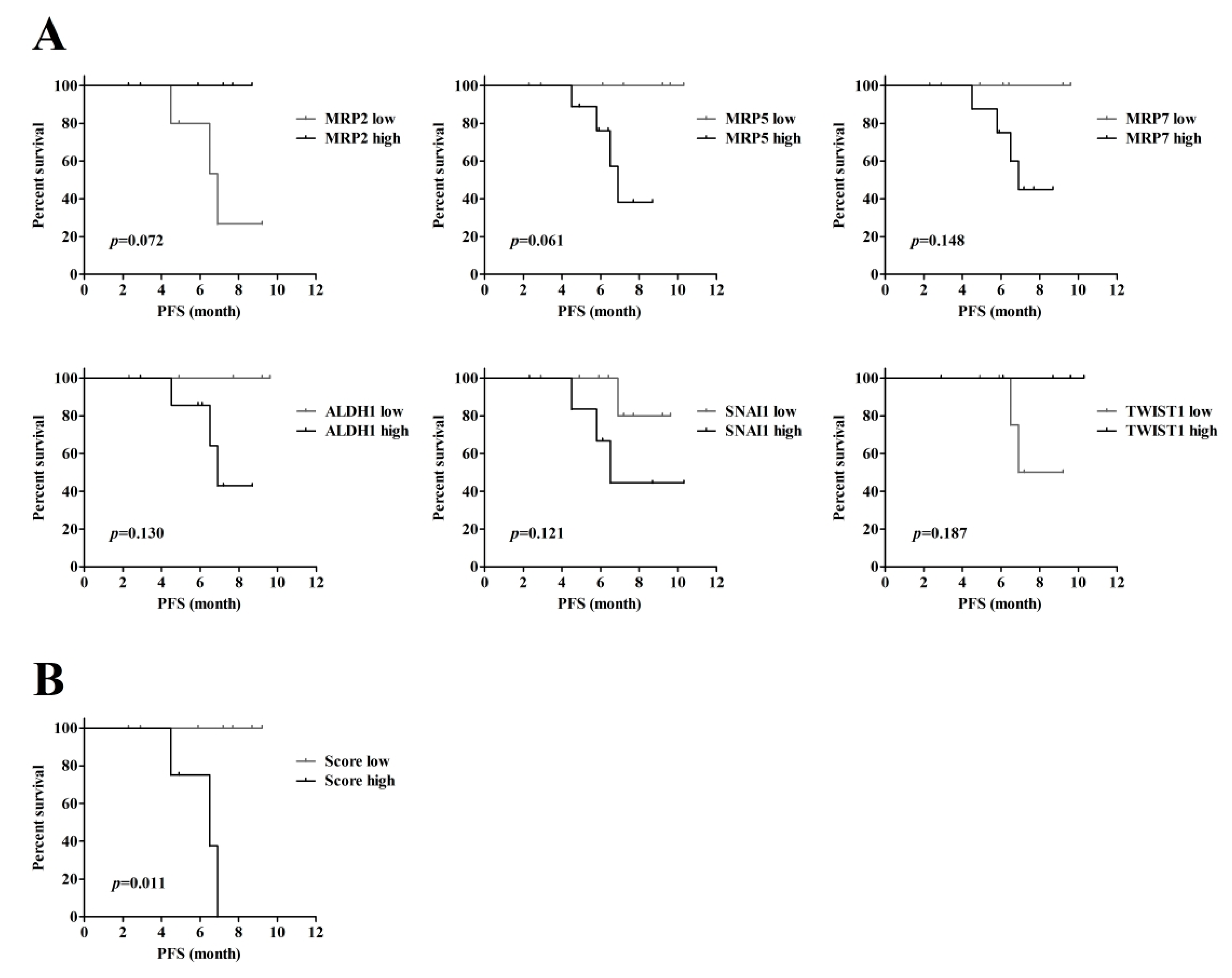
3.3. Evaluation of the Prognostic Factor of Progression-Free Survival (PFS)
3.4. The Relationship Between Gene Expression in the Cells Obtained Via the 2-step CTC Isolation Process and PFS in Cancer Patients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Colella, S.; Richards, K.L.; Bachinski, L.L.; Baggerly, K.A.; Tsavachidis, S.; Lang, J.C.; Schuller, D.E.; Krahe, R. Molecular signatures of metastasis in head and neck cancer. Head Neck 2008, 30, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Brakenhoff, R.H.; Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 2008, 8, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Tu, Q.; Faure, G.; Gallet, P.; Kohler, C.; Bittencourt Mde, C. Diagnostic and Prognostic Value of Circulating Tumor Cells in Head and Neck Squamous Cell Carcinoma: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 20210. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schutze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Gertler, R.; Rosenberg, R.; Fuehrer, K.; Dahm, M.; Nekarda, H.; Siewert, J.R. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. In Molecular Staging of Cancer; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2003; Volume 162, pp. 149–155. [Google Scholar]
- Peeters, D.J.; De Laere, B.; Van den Eynden, G.G.; Van Laere, S.J.; Rothe, F.; Ignatiadis, M.; Sieuwerts, A.M.; Lambrechts, D.; Rutten, A.; van Dam, P.A.; et al. Semiautomated isolation and molecular characterisation of single or highly purified tumour cells from CellSearch enriched blood samples using dielectrophoretic cell sorting. Br. J. Cancer 2013, 108, 1358–1367. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, A.; Chen, X. Current detection technologies for circulating tumor cells. Chem. Soc. Rev. 2017, 46, 2038–2056. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, K.; Fan, Z.H. Circulating Tumor Cell Isolation and Analysis. Adv. Clin. Chem. 2016, 75, 1–31. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Mader, S.; Pantel, K. Epithelial-mesenchymal plasticity in circulating tumor cells. J. Mol. Med. 2017, 95, 133–142. [Google Scholar] [CrossRef]
- Lustberg, M.B.; Balasubramanian, P.; Miller, B.; Garcia-Villa, A.; Deighan, C.; Wu, Y.; Carothers, S.; Berger, M.; Ramaswamy, B.; Macrae, E.R.; et al. Heterogeneous atypical cell populations are present in blood of metastatic breast cancer patients. Breast Cancer Res. 2014, 16, R23. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Malfettone, A.; Soukupova, J. New Insights into the Crossroads between EMT and Stemness in the Context of Cancer. J. Clin. Med. 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, M.; Quach, B.; Dadashian, E.L.; Mulholland, D.J.; Wu, H. Tracking and Functional Characterization of Epithelial-Mesenchymal Transition and Mesenchymal Tumor Cells during Prostate Cancer Metastasis. Cancer Res. 2015, 75, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef]
- Raimondi, C.; Gradilone, A.; Naso, G.; Vincenzi, B.; Petracca, A.; Nicolazzo, C.; Palazzo, A.; Saltarelli, R.; Spremberg, F.; Cortesi, E.; et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res. Treat. 2011, 130, 449–455. [Google Scholar] [CrossRef]
- Schneck, H.; Gierke, B.; Uppenkamp, F.; Behrens, B.; Niederacher, D.; Stoecklein, N.H.; Templin, M.F.; Pawlak, M.; Fehm, T.; Neubauer, H.; et al. EpCAM-Independent Enrichment of Circulating Tumor Cells in Metastatic Breast Cancer. PLoS ONE 2015, 10, e0144535. [Google Scholar] [CrossRef]
- Dieguez, L.; Winter, M.A.; Pocock, K.J.; Bremmell, K.E.; Thierry, B. Efficient microfluidic negative enrichment of circulating tumor cells in blood using roughened PDMS. Analyst 2015, 140, 3565–3572. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, H.; Yu, T.; Xie, J.; Chen, S.; Dong, H.; Sinko, P.J.; Lian, S.; Xu, J.; Wang, J.; et al. Isolation and characterization of living circulating tumor cells in patients by immunomagnetic negative enrichment coupled with flow cytometry. Cancer 2015, 121, 3036–3045. [Google Scholar] [CrossRef]
- Huang, S.B.; Wu, M.H.; Lin, Y.H.; Hsieh, C.H.; Yang, C.L.; Lin, H.C.; Tseng, C.P.; Lee, G.B. High-purity and label-free isolation of circulating tumor cells (CTCs) in a microfluidic platform by using optically-induced-dielectrophoretic (ODEP) force. Lab Chip 2013, 13, 1371–1383. [Google Scholar] [CrossRef]
- Chiu, T.K.; Chou, W.P.; Huang, S.B.; Wang, H.M.; Lin, Y.C.; Hsieh, C.H.; Wu, M.H. Application of optically-induced-dielectrophoresis in microfluidic system for purification of circulating tumour cells for gene expression analysis- Cancer cell line model. Sci. Rep. 2016, 6, 32851. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Hsieh, C.H.; Wang, H.M.; Chou, W.P.; Chiu, T.K.; Chang, J.H.; Chao, A.C.; Wu, M.H. Isolation of label-free and viable circulating tumour cells (CTCs) from blood samples of cancer patients through a two-step process: Negative selection-type immunomagnetic beads and spheroid cell culture-based cell isolation. RSC Adv. 2017, 7, 29339–29349. [Google Scholar] [CrossRef]
- Rodrigues, T.; Kundu, B.; Silva-Correia, J.; Kundu, S.C.; Oliveira, J.M.; Reis, R.L.; Correlo, V.M. Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol. Ther. 2018, 184, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.W.; Lin, C.Y.; Hsieh, C.H.; Hsu, C.L.; Fan, K.H.; Huang, S.F.; Liao, C.T.; Ng, S.K.; Yen, T.C.; Chang, J.T.; et al. A phase II randomized trial comparing neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in advanced squamous cell carcinoma of the pharynx or larynx. Biomed. J. 2018, 41, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Tani, R.; Hayakawa, K.; Tanaka, S.; Shiota, K. Linker histone variant H1T targets rDNA repeats. Epigenetics 2016, 11, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, H.; Kobayashi, A.; Yokoyama, T.; Shimizu, A.; Sakai, H.; Notake, T.; Fukushima, K.; Miyagawa, S.I. Treatment with specific soluble factors promotes the functional maturation of transcription factor-mediated, pancreatic transdifferentiated cells. PLoS ONE 2018, 13, e0197175. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, J.; Alonso-Nocelo, M.; Muinelo-Romay, L.; Barbazan, J.; Vieito, M.; Abalo, A.; Gomez-Tato, A.; Maria de Los Angeles, C.C.; Garcia-Caballero, T.; Rodriguez, C.; et al. Molecular Profiling of Circulating Tumour Cells Identifies Notch1 as a Principal Regulator in Advanced Non-Small Cell Lung Cancer. Sci. Rep. 2016, 6, 37820. [Google Scholar] [CrossRef] [PubMed]
- Thalgott, M.; Rack, B.; Eiber, M.; Souvatzoglou, M.; Heck, M.M.; Kronester, C.; Andergassen, U.; Kehl, V.; Krause, B.J.; Gschwend, J.E.; et al. Categorical versus continuous circulating tumor cell enumeration as early surrogate marker for therapy response and prognosis during docetaxel therapy in metastatic prostate cancer patients. BMC Cancer 2015, 15, 458. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Oza, A.M.; Ho, T.W.; Ledermann, J.A. Intermediate clinical endpoints: A bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 2015, 121, 1737–1746. [Google Scholar] [CrossRef]
- Yap, T.A.; Lorente, D.; Omlin, A.; Olmos, D.; de Bono, J.S. Circulating tumor cells: A multifunctional biomarker. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 2553–2568. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; di Tomaso, E.; McDonald, D.M.; Jones, R.; Jain, R.K.; Munn, L.L. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 2000, 97, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017, 31, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307. [Google Scholar] [CrossRef]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, B.; Wu, J.; Zhang, C.; Zhou, Y.; Yang, X.; Zhou, J.; Guo, W.; Fan, J. Association of preoperative EpCAM Circulating Tumor Cells and peripheral Treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection. BMC Cancer 2016, 16, 506. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Sarioglu, A.F.; Aceto, N.; Kojic, N.; Donaldson, M.C.; Zeinali, M.; Hamza, B.; Engstrom, A.; Zhu, H.; Sundaresan, T.K.; Miyamoto, D.T.; et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods 2015, 12, 685–691. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Micalizzi, D.S.; Haber, D.A.; Maheswaran, S. Cancer metastasis through the prism of epithelial-to-mesenchymal transition in circulating tumor cells. Mol. Oncol. 2017, 11, 770–780. [Google Scholar] [CrossRef]
- Li, Y.M.; Xu, S.C.; Li, J.; Han, K.Q.; Pi, H.F.; Zheng, L.; Zuo, G.H.; Huang, X.B.; Li, H.Y.; Zhao, H.Z.; et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013, 4, e831. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Kallergi, G.; Zafeiriou, Z.; Manouras, L.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Co-expression of putative stemness and epithelial-to-mesenchymal transition markers on single circulating tumour cells from patients with early and metastatic breast cancer. BMC Cancer 2014, 14, 651. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Tewes, M.; Fehm, T.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009, 11, R46. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Mitra, A.; Brownlee, Z.; Xia, X.; Bellister, S.; Overman, M.J.; Kopetz, S.; Ellis, L.M.; Meng, Q.H.; Li, S. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Chen, Z.S.; Tiwari, A.K. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011, 278, 3226–3245. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, G.B.; Wildeman, M.; Rasch, C.R.; Armstrong, N.; Schuuring, E.; Begg, A.C.; Looijenga, L.H.; Scheper, R.; van der Wal, J.E.; Menkema, L.; et al. Molecular markers predict outcome in squamous cell carcinoma of the head and neck after concomitant cisplatin-based chemoradiation. Int. J. Cancer 2009, 124, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Theile, D.; Ketabi-Kiyanvash, N.; Herold-Mende, C.; Dyckhoff, G.; Efferth, T.; Bertholet, V.; Haefeli, W.E.; Weiss, J. Evaluation of drug transporters’ significance for multidrug resistance in head and neck squamous cell carcinoma. Head Neck 2011, 33, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Warta, R.; Theile, D.; Mogler, C.; Herpel, E.; Grabe, N.; Lahrmann, B.; Plinkert, P.K.; Herold-Mende, C.; Weiss, J.; Dyckhoff, G. Association of drug transporter expression with mortality and progression-free survival in stage IV head and neck squamous cell carcinoma. PLoS ONE 2014, 9, e108908. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Verdin, S.; Lavalle-Carrasco, J.; Carreon-Burciaga, R.G.; Serafin-Higuera, N.; Molina-Frechero, N.; Gonzalez-Gonzalez, R.; Bologna-Molina, R. Molecular Markers of Anticancer Drug Resistance in Head and Neck Squamous Cell Carcinoma: A Literature Review. Cancers 2018, 10, 376. [Google Scholar] [CrossRef] [PubMed]

| Variable | Category | No | % |
|---|---|---|---|
| Age, year | Median (range) | 52.5 (39.5–64.4) | |
| Gender | Male | 17 | 85 |
| Female | 3 | 15 | |
| Tumor site | Oral cavity | 2 | 10 |
| Oropharynx | 9 | 45 | |
| Hypopharynx | 7 | 35 | |
| Larynx | 2 | 10 | |
| ECOG #1 performance status | 0 | 2 | 10 |
| 1 | 14 | 70 | |
| 2 | 4 | 20 | |
| AJCC stage, 8th edition | II | 4 | 20 |
| IV | 16 | 80 | |
| Distant metastasis | No | 15 | 75 |
| Yes | 5 | 25 | |
| Disease progression | No | 13 | 65 |
| Yes | 5 | 25 | |
| Missing | 2 | 10 | |
| Treatment after blood sample collection | CCRT #2 or CT #3 | 18 | 90 |
| CCRT+ salvage surgery | 2 | 10 |
| Variable | Category | n | Post-Culture Cell/mL (Mean ± s.d.) | ||
|---|---|---|---|---|---|
| E-CTCs #1 | M-CTCs #2 | All CTCs #3 | |||
| Age | <50.0 | 8 | 4.7 ± 8.1 | 8.3 ± 9.2 | 13.0 ± 11.6 |
| ≥50.0 | 12 | 3.9 ± 7.3 | 5.2 ± 5.2 | 9.1 ± 8.9 | |
| Gender | Male | 17 | 4.7 ± 8.0 | 7.0 ± 7.5 | 11.7 ± 10.5 |
| Female | 3 | 1.5 ± 1.3 | 3.3 ± 1.1 | 4.8 ± 2.1 | |
| Tumor site | Oral cavity | 2 | 4.2 ± 3.5 | 9.2 ± 5.2 | 13.3 ± 1.7 |
| Oropharynx | 9 | 1.6 ± 1.1 | 7.6 ± 8.9 | 9.2 ± 9.1 | |
| Hypopharynx | 7 | 5.2 ± 9.7 | 4.8 ± 6.1 | 10.0 ± 11.3 | |
| Larynx | 2 | 12.3 ± 16.5 | 4.5 ± 2.1 | 16.8 ± 18.7 | |
| AJCC stage, 8th edition | II | 4 | 1.4 ± 1.1 | 3.0 ± 1.1 | 4.4 ± 1.9 |
| IV | 16 | 4.9 ± 8.2 | 7.3 ± 7.7 | 12.2 ± 10.6 | |
| Distance metastasis | No | 15 | 3.4 ± 6.7 | 5.3 ± 6.8 | 8.7 ± 9.3 |
| Yes | 5 | 6.6 ± 9.8 | 9.9 ± 7.2 * | 16.5 ± 10.6 | |
| Treatment after blood sample collection | CCRT #4 | 16 | 4.7 ± 8.3 | 5.3 ± 6.6 | 10.0 ± 10.4 |
| CT #5 | 4 | 2.3 ± 1.1 | 10.9 ± 8.0 | 13.1 ± 8.6 | |
| Clusters in cultures | No | 18 | 3.2 ± 6.2 | 5.9 ± 6.9 | 9.0 ± 9.0 |
| Yes | 2 | 13.5 ± 14.8 | 11.8 ± 8.1 | 25.3 ± 6.7 ** | |
| Variable | HR #1 | 95% CI #2 | p |
|---|---|---|---|
| Age | 0.875 | 0.716–1.068 | 0.189 |
| Gender | 0.039 | 0.000–7309.796 | 0.601 |
| Tumor site | 0.904 | 0.314–2.599 | 0.851 |
| ECOG #3 performance status | 8.271 | 0.583–117.370 | 0.119 |
| AJCC stage, 8th edition | 5.633 | 0.045–698.776 | 0.482 |
| Treatment after blood collection | 0.372 | 0.073–1.883 | 0.232 |
| E-CTCs | 1.045 | 0.951–1.147 | 0.360 |
| M-CTCs | 1.153 | 1.015–1.310 | 0.029 * |
| All CTCs | 1.101 | 0.999–1.213 | 0.053 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-J.; Hsieh, C.-H.; Hung, F.-C.; Wang, H.-M.; Chou, W.-P.; Wu, M.-H. The Integration of a Three-Dimensional Spheroid Cell Culture Operation in a Circulating Tumor Cell (CTC) Isolation and Purification Process: A Preliminary Study of the Clinical Significance and Prognostic Role of the CTCs Isolated from the Blood Samples of Head and Neck Cancer Patients. Cancers 2019, 11, 783. https://doi.org/10.3390/cancers11060783
Liao C-J, Hsieh C-H, Hung F-C, Wang H-M, Chou W-P, Wu M-H. The Integration of a Three-Dimensional Spheroid Cell Culture Operation in a Circulating Tumor Cell (CTC) Isolation and Purification Process: A Preliminary Study of the Clinical Significance and Prognostic Role of the CTCs Isolated from the Blood Samples of Head and Neck Cancer Patients. Cancers. 2019; 11(6):783. https://doi.org/10.3390/cancers11060783
Chicago/Turabian StyleLiao, Chia-Jung, Chia-Hsun Hsieh, Feng-Chun Hung, Hung-Ming Wang, Wen-Pin Chou, and Min-Hsien Wu. 2019. "The Integration of a Three-Dimensional Spheroid Cell Culture Operation in a Circulating Tumor Cell (CTC) Isolation and Purification Process: A Preliminary Study of the Clinical Significance and Prognostic Role of the CTCs Isolated from the Blood Samples of Head and Neck Cancer Patients" Cancers 11, no. 6: 783. https://doi.org/10.3390/cancers11060783
APA StyleLiao, C.-J., Hsieh, C.-H., Hung, F.-C., Wang, H.-M., Chou, W.-P., & Wu, M.-H. (2019). The Integration of a Three-Dimensional Spheroid Cell Culture Operation in a Circulating Tumor Cell (CTC) Isolation and Purification Process: A Preliminary Study of the Clinical Significance and Prognostic Role of the CTCs Isolated from the Blood Samples of Head and Neck Cancer Patients. Cancers, 11(6), 783. https://doi.org/10.3390/cancers11060783





