HOTAIR as a Prognostic Predictor for Diverse Human Cancers: A Meta- and Bioinformatics Analysis
Abstract
1. Introduction
2. Results
2.1. Study Selection and Charasteristics of Eligible Studies
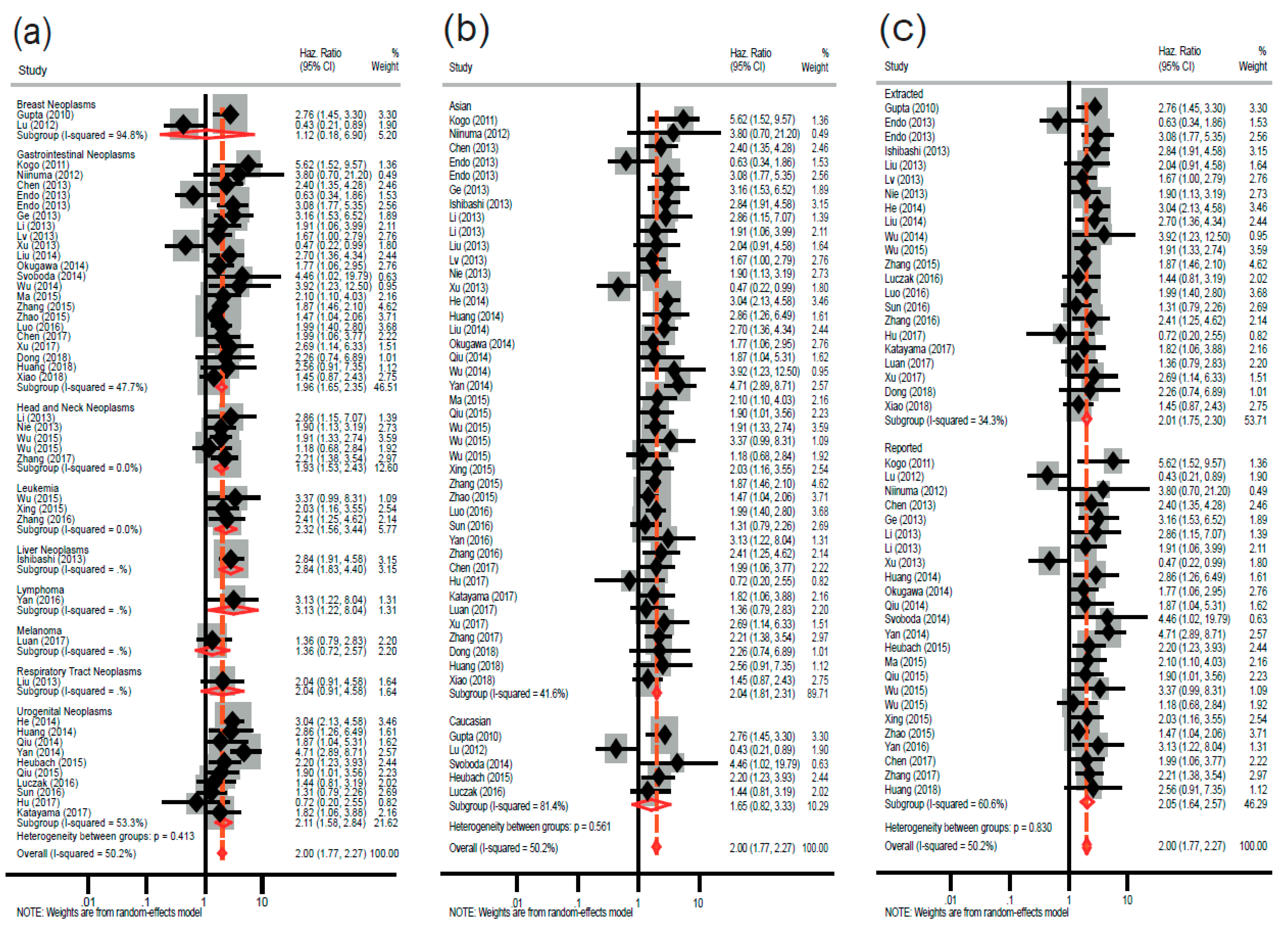
2.2. Association between High HOTAIR Expression and Overall Survival in Diverse Cancers
2.3. HOTAIR Overexpression Is Associated with Cancer Recurrence and Progression
2.4. Publication Bias
2.5. Sensitivity Analysis
2.6. TCGA-Derived Survival Curves
3. Discussion
4. Materials and Methods
4.1. Search Strategy and Study Eligibility Criteria
4.2. Study Selection, Data Extraction, and Quality Assessment
4.3. Statistical Analyses
4.4. Bioinformatics Analysis
4.4.1. Survival Analysis
4.4.2. Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagano, T.; Fraser, P. No-nonsense functions for long noncoding RNAs. Cell 2011, 145, 178–181. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Coller, J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 2013, 14, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Chinnaiyan, A.M. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011, 1, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M.; Chang, H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human hox loci by noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Achuthan, P.; Akanni, W.; Allen, J.; Amode, M.R.; Armean, I.M.; Bennett, R.; Bhai, J.; Billis, K.; Boddu, S.; et al. Ensembl 2019. Nucleic Acids Res. 2019, 47, D745–D751. [Google Scholar] [CrossRef]
- Tsai, M.C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef]
- Cai, B.; Song, X.Q.; Cai, J.P.; Zhang, S. Hotair: A cancer-related long non-coding RNA. Neoplasma 2014, 61, 379–391. [Google Scholar] [CrossRef]
- Woo, C.J.; Kingston, R.E. Hotair lifts noncoding RNAs to new levels. Cell 2007, 129, 1257–1259. [Google Scholar] [CrossRef]
- Hao, S.; Shao, Z. Hotair is upregulated in acute myeloid leukemia and that indicates a poor prognosis. Int. J. Clin. Exp. Pathol. 2015, 8, 7223–7228. [Google Scholar] [PubMed]
- Qu, X.; Alsager, S.; Zhuo, Y.; Shan, B. Hox transcript antisense RNA (hotair) in cancer. Cancer Lett. 2019, 454, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Mandal, S.S. LncRNA hotair: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta 2015, 1856, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA hotair reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Yin, Z.; Wu, Y.; Xu, Y.; Luo, Y.; Zhang, T.C. LncRNA hotair promotes cell migration and invasion by regulating mkl1 via inhibition mir206 expression in hela cells. Cell Commun. Signal. 2018, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; Zhang, L.; Wang, Y.; Li, H.; Ren, X.; Wei, F.; Yu, W.; Liu, T.; Wang, X.; et al. Long non-coding RNA hotair promotes tumor cell invasion and metastasis by recruiting ezh2 and repressing e-cadherin in oral squamous cell carcinoma. Int. J. Oncol. 2015, 46, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Shiroki, T.; Nakagawa, T.; Yokoyama, M.; Tamai, K.; Yamanami, H.; Fujiya, T.; Sato, I.; Yamaguchi, K.; Tanaka, N.; et al. Enhanced expression of long non-coding RNA hotair is associated with the development of gastric cancer. PLoS ONE 2013, 8, e77070. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Sun, M.; Nie, F.Q.; Ge, Y.B.; Zhang, E.B.; Yin, D.D.; Kong, R.; Xia, R.; Lu, K.H.; Li, J.H.; et al. Lnc RNA hotair functions as a competing endogenous RNA to regulate her2 expression by sponging mir-331-3p in gastric cancer. Mol. Cancer 2014, 13, 92. [Google Scholar] [CrossRef]
- Hajjari, M.; Salavaty, A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biol. Med. 2015, 12, 1–9. [Google Scholar]
- Tang, Q.; Hann, S.S. Hotair: An oncogenic long non-coding RNA in human cancer. Cell. Physiol. Biochem. 2018, 47, 893–913. [Google Scholar] [CrossRef]
- Nakagawa, T.; Endo, H.; Yokoyama, M.; Abe, J.; Tamai, K.; Tanaka, N.; Sato, I.; Takahashi, S.; Kondo, T.; Satoh, K. Large noncoding RNA hotair enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2013, 436, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Caponio, V.C.A.; Boldrup, L.; Gu, X.; Muzio, L.L.; Sgaramella, N.; Wang, L.; Nylander, K. Expression of the long non-coding RNA hotair as a prognostic factor in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncotarget 2017, 8, 73029–73036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Xu, T.; Tian, W.; Yang, C.; Wang, X.; Zhong, S.; Ran, Q.; Yang, H.; Zhu, S. Clinical value of long noncoding RNA hotair as a novel biomarker in digestive cancers: A meta-analysis. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Sun, M.; Li, S.Q.; Wu, Q.Q.; Ji, L.; Liu, Z.L.; Zhou, G.Z.; Cao, G.; Jin, L.; Xie, H.W.; et al. Upregulation of the long non-coding RNA hotair promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol. Carcinog. 2013, 52, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, W.; Zhao, S.; Wang, J.; Chen, J.; Wang, Y.; Zhang, Q. Prognostic role of hotair in four estrogen-dependent malignant tumors: A meta-analysis. Oncotargets Ther. 2015, 8, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA hotair as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, T.; Wang, M.; Zhao, J.; Li, X.; Zhang, Z.; Zhou, Y.; Liu, J.; Jia, L.; Han, Y. Long non-coding RNAs function as novel predictors and targets of non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2018, 9, 11377–11386. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Sun, H.; He, B.; Pan, Y.; Gao, T.; Chen, J.; Ying, H.; Liu, X.; Wang, F.; Xu, Y.; et al. Prognostic value of long non-coding RNA hotair in various cancers. PLoS ONE 2014, 9, e110059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, S.; Yang, G.; Gu, F.; Li, M.; Zhong, B.; Hu, J.; Hoffman, A.; Chen, M. Long noncoding RNA hotair as an independent prognostic marker in cancer: A meta-analysis. PLoS ONE 2014, 9, e105538. [Google Scholar] [CrossRef]
- Geng, Y.J.; Xie, S.L.; Li, Q.; Ma, J.; Wang, G.Y. Large intervening non-coding RNA hotair is associated with hepatocellular carcinoma progression. J. Int. Med. Res. 2011, 39, 2119–2128. [Google Scholar] [CrossRef]
- Kogo, R.; Shimamura, T.; Mimori, K.; Kawahara, K.; Imoto, S.; Sudo, T.; Tanaka, F.; Shibata, K.; Suzuki, A.; Komune, S.; et al. Long noncoding RNA hotair regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011, 71, 6320–6326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, L.; Wu, L.M.; Lai, M.C.; Xie, H.Y.; Zhang, F.; Zheng, S.S. Overexpression of long non-coding RNA hotair predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann. Surg. Oncol. 2011, 18, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhu, G.; Zhang, C.; Deng, Q.; Katsaros, D.; Mayne, S.T.; Risch, H.A.; Mu, L.; Canuto, E.M.; Gregori, G.; et al. Association of large noncoding RNA hotair expression and its downstream intergenic cpg island methylation with survival in breast cancer. Breast Cancer Res. Treat. 2012, 136, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Niinuma, T.; Suzuki, H.; Nojima, M.; Nosho, K.; Yamamoto, H.; Takamaru, H.; Yamamoto, E.; Maruyama, R.; Nobuoka, T.; Miyazaki, Y.; et al. Upregulation of mir-196a and hotair drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012, 72, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.S.; Ma, H.J.; Zheng, X.H.; Ruan, H.L.; Liao, X.Y.; Xue, W.Q.; Chen, Y.B.; Zhang, Y.; Jia, W.H. Hotair, a prognostic factor in esophageal squamous cell carcinoma, inhibits wif-1 expression and activates wnt pathway. Cancer Sci. 2013, 104, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Kogo, R.; Shibata, K.; Sawada, G.; Takahashi, Y.; Kurashige, J.; Akiyoshi, S.; Sasaki, S.; Iwaya, T.; Sudo, T.; et al. Clinical significance of the expression of long non-coding RNA hotair in primary hepatocellular carcinoma. Oncol. Rep. 2013, 29, 946–950. [Google Scholar] [CrossRef]
- Li, D.; Feng, J.; Wu, T.; Wang, Y.; Sun, Y.; Ren, J.; Liu, M. Long intergenic noncoding RNA hotair is overexpressed and regulates pten methylation in laryngeal squamous cell carcinoma. Am. J. Pathol. 2013, 182, 64–70. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Mei, Q.; Li, X.; Guo, M.; Fu, X.; Han, W. Long non-coding RNA hotair, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br. J. Cancer 2013, 109, 2266–2278. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Z.L.; Sun, M.; Liu, J.; Wang, Z.X.; De, W. The long non-coding RNA hotair indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 2013, 13, 464. [Google Scholar] [CrossRef]
- Lv, X.B.; Lian, G.Y.; Wang, H.R.; Song, E.; Yao, H.; Wang, M.H. Long noncoding RNA hotair is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS ONE 2013, 8, e63516. [Google Scholar] [CrossRef]
- Nie, Y.; Liu, X.; Qu, S.; Song, E.; Zou, H.; Gong, C. Long non-coding RNA hotair is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013, 104, 458–464. [Google Scholar] [CrossRef]
- Sorensen, K.P.; Thomassen, M.; Tan, Q.; Bak, M.; Cold, S.; Burton, M.; Larsen, M.J.; Kruse, T.A. Long non-coding RNA hotair is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast Cancer Res. Treat. 2013, 142, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Yu, Q.M.; Du, Y.A.; Yang, L.T.; Dong, R.Z.; Huang, L.; Yu, P.F.; Cheng, X.D. Knockdown of long non-coding RNA hotair suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int. J. Biol. Sci. 2013, 9, 587–597. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bao, W.; Li, X.; Chen, Z.; Che, Q.; Wang, H.; Wan, X.P. The long non-coding RNA hotair is upregulated in endometrial carcinoma and correlates with poor prognosis. Int. J. Mol. Med. 2014, 33, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liao, L.M.; Liu, A.W.; Wu, J.B.; Cheng, X.L.; Lin, J.X.; Zheng, M. Overexpression of long noncoding RNA hotair predicts a poor prognosis in patients with cervical cancer. Arch. Gynecol. Obstet. 2014, 290, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Lee, J.H.; Park, C.H.; Yu, D.; Lee, Y.C.; Cheong, J.H.; Noh, S.H.; Lee, S.K. Long non-coding RNA hotair promotes carcinogenesis and invasion of gastric adenocarcinoma. Biochem. Biophys. Res. Commun. 2014, 451, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Hur, K.; Toden, S.; Saigusa, S.; Tanaka, K.; Inoue, Y.; Mohri, Y.; Kusunoki, M.; Boland, C.R.; et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis 2014, 35, 2731–2739. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, Y.Y.; Ye, L.C.; Ding, J.X.; Feng, W.W.; Jin, H.Y.; Zhang, Y.; Li, Q.; Hua, K.Q. Overexpression of long non-coding RNA hotair predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 121–128. [Google Scholar] [CrossRef]
- Svoboda, M.; Slyskova, J.; Schneiderova, M.; Makovicky, P.; Bielik, L.; Levy, M.; Lipska, L.; Hemmelova, B.; Kala, Z.; Protivankova, M.; et al. Hotair long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis 2014, 35, 1510–1515. [Google Scholar] [CrossRef]
- Wu, Z.H.; Wang, X.L.; Tang, H.M.; Jiang, T.; Chen, J.; Lu, S.; Qiu, G.Q.; Peng, Z.H.; Yan, D.W. Long non-coding RNA hotair is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol. Rep. 2014, 32, 395–402. [Google Scholar] [CrossRef]
- Yan, T.H.; Lu, S.W.; Huang, Y.Q.; Que, G.B.; Chen, J.H.; Chen, Y.P.; Zhang, H.B.; Liang, X.L.; Jiang, J.H. Upregulation of the long noncoding RNA hotair predicts recurrence in stage ta/t1 bladder cancer. Tumour Biol. 2014, 35, 10249–10257. [Google Scholar] [CrossRef] [PubMed]
- Heubach, J.; Monsior, J.; Deenen, R.; Niegisch, G.; Szarvas, T.; Niedworok, C.; Schulz, W.A.; Hoffmann, M.J. The long noncoding RNA hotair has tissue and cell type-dependent effects on hox gene expression and phenotype of urothelial cancer cells. Mol. Cancer 2015, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, D.W.; Yim, G.W.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Long non-coding RNA hotair is associated with human cervical cancer progression. Int. J. Oncol. 2015, 46, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Sun, M.; Xia, R.; Zhang, E.B.; Liu, X.H.; Zhang, Z.H.; Xu, T.P.; De, W.; Liu, B.R.; Wang, Z.X. Linchotair epigenetically silences mir34a by binding to prc2 to promote the epithelial-to-mesenchymal transition in human gastric cancer. Cell Death Dis. 2015, 6, e1802. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, Q.; Lv, C.; Qiang, F.; Hua, Q.; Chu, H.; Du, M.; Tong, N.; Jiang, Y.; Wang, M.; et al. The prognostic significance of hotair for predicting clinical outcome in patients with digestive system tumors. J. Cancer Res. Clin. Oncol. 2015, 141, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Martinez-FeRNAndez, M.; Feber, A.; Duenas, M.; Segovia, C.; Rubio, C.; FeRNAndez, M.; Villacampa, F.; Duarte, J.; Lopez-Calderon, F.F.; Gomez-Rodriguez, M.J.; et al. Analysis of the polycomb-related lncRNAs hotair and anril in bladder cancer. Clin. Epigenet. 2015, 7, 109. [Google Scholar] [CrossRef]
- Qiu, J.J.; Wang, Y.; Ding, J.X.; Jin, H.Y.; Yang, G.; Hua, K.Q. The long non-coding RNA hotair promotes the proliferation of serous ovarian cancer cells through the regulation of cell cycle arrest and apoptosis. Exp. Cell Res. 2015, 333, 238–248. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H. Expression of long noncoding RNA-hox transcript antisense intergenic RNA in oral squamous cell carcinoma and effect on cell growth. Tumour Biol. 2015, 36, 8573–8578. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, C.; Chen, S.; Cai, X.; Shi, Y.; Lin, B.; Chen, Y. Overexpression of long non-coding RNA hotair predicts a poor prognosis in patients with acute myeloid leukemia. Oncol. Lett. 2015, 10, 2410–2414. [Google Scholar] [CrossRef]
- Xing, C.Y.; Hu, X.Q.; Xie, F.Y.; Yu, Z.J.; Li, H.Y.; Bin, Z.; Wu, J.B.; Tang, L.Y.; Gao, S.M. Long non-coding RNA hotair modulates c-kit expression through sponging mir-193a in acute myeloid leukemia. FEBS Lett. 2015, 589, 1981–1987. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Shen, Z.Y.; Shen, Y.Y.; Zhao, E.H.; Wang, M.; Wang, C.J.; Cao, H.; Xu, J. Hotair long noncoding RNA promotes gastric cancer metastasis through suppression of poly r(c)-binding protein (pcbp) 1. Mol. Cancer Ther. 2015, 14, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Dong, S.; Duan, B.; Chen, P.; Shi, L.; Gao, H.; Qi, H. Hotair is a predictive and prognostic biomarker for patients with advanced gastric adenocarcinoma receiving fluorouracil and platinum combination chemotherapy. Am. J. Transl. Res. 2015, 7, 1295–1302. [Google Scholar] [PubMed]
- Luczak, A.; SupeRNAt, A.; Lapinska-Szumczyk, S.; Jachimowicz, D.; Majewska, H.; Gulczynski, J.; Zaczek, A.J. Hotair in relation to epithelial-mesenchymal transition and cancer stem cells in molecular subtypes of endometrial cancer. Int. J. Biol. Markers 2016, 31, e245–e251. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.F.; Zhao, D.; Li, X.Q.; Cui, Y.X.; Ma, N.; Lu, C.X.; Liu, M.Y.; Zhou, Y. Clinical significance of hotair expression in colon cancer. World J. Gastroenterol. 2016, 22, 5254–5259. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, H.; Ji, J.; Huo, G.; Song, Q.; Zhang, X. Long non-coding RNA hotair modulates hla-g expression by absorbing mir-148a in human cervical cancer. Int. J. Oncol. 2016, 49, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Han, J.; Li, Z.; Yang, H.; Sui, Y.; Wang, M. Elevated RNA expression of long noncoding hotair promotes cell proliferation and predicts a poor prognosis in patients with diffuse large b cell lymphoma. Mol. Med. Rep. 2016, 13, 5125–5131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Huang, S.H.; Zhou, H.R.; Chen, C.J.; Tian, L.H.; Shen, J.Z. Role of hotair in the diagnosis and prognosis of acute leukemia. Oncol. Rep. 2016, 36, 3113–3122. [Google Scholar] [CrossRef]
- Chen, W.M.; Chen, W.D.; Jiang, X.M.; Jia, X.F.; Wang, H.M.; Zhang, Q.J.; Shu, Y.Q.; Zhao, H.B. Hox transcript antisense intergenic RNA represses e-cadherin expression by binding to ezh2 in gastric cancer. World J. Gastroenterol. 2017, 23, 6100–6110. [Google Scholar] [CrossRef]
- Hu, G.; Dong, B.; Zhang, J.; Zhai, W.; Xie, T.; Huang, B.; Huang, C.; Yao, X.; Zheng, J.; Che, J.; et al. The long noncoding RNA hotair activates the hippo pathway by directly binding to sav1 in renal cell carcinoma. Oncotarget 2017, 8, 58654–58667. [Google Scholar] [CrossRef]
- Katayama, H.; Tamai, K.; Shibuya, R.; Nakamura, M.; Mochizuki, M.; Yamaguchi, K.; Kawamura, S.; Tochigi, T.; Sato, I.; Okanishi, T.; et al. Long non-coding RNA hotair promotes cell migration by upregulating insulin growth factor-binding protein 2 in renal cell carcinoma. Sci. Rep. 2017, 7, 12016. [Google Scholar] [CrossRef]
- Luan, W.; Li, R.; Liu, L.; Ni, X.; Shi, Y.; Xia, Y.; Wang, J.; Lu, F.; Xu, B. Long non-coding RNA hotair acts as a competing endogenous RNA to promote malignant melanoma progression by sponging mir-152-3p. Oncotarget 2017, 8, 85401–85414. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, J. Long non-coding RNA hotair functions as miRNA sponge to promote the epithelial to mesenchymal transition in esophageal cancer. Biomed. Pharmacother. 2017, 90, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, S.; Jiang, L.; Wang, X.; Song, X. Hotair is a promising novel biomarker in patients with thyroid cancer. Exp. Ther. Med. 2017, 13, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; He, X.; Guan, A.; Huang, W.; Jia, H.; Huang, Y.; Chen, S.; Zhang, Z.; Gao, J.; Wang, H. Long non-coding RNA hotair promotes gastric cancer progression via mir-217-gpc5 axis. Life Sci. 2019, 217, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.B.; Zhang, S.P.; Zhu, Y.J.; Guo, C.H.; Yang, M.; Liu, J.; Xia, L.G.; Zhang, J.F. Hotair mediates tumorigenesis through recruiting ezh2 in colorectal cancer. J. Cell. Biochem. 2019, 120, 6071–6077. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Qu, Z.; Chen, Z.; Fang, Z.; Zhou, K.; Huang, Z.; Guo, X.; Zhang, Y. LncRNA hotair is a prognostic biomarker for the proliferation and chemoresistance of colorectal cancer via mir-203a-3p-mediated wnt/ss-catenin signaling pathway. Cell. Physiol. Biochem. 2018, 46, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Coordinators, N.R. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.J.; Li, W.F.; Zhang, X.; Yang, X.J. The prognostic value of hotair for predicting long-term prognosis of patients with gastrointestinal cancers. Medicine 2018, 97, e11139. [Google Scholar] [CrossRef] [PubMed]
- Abdeahad, H.; Avan, A.; Pashirzad, M.; Khazaei, M.; Soleimanpour, S.; Ferns, G.A.; Fiuji, H.; Ryzhikov, M.; Bahrami, A.; Hassanian, S.M. The prognostic potential of long noncoding RNA hotair expression in human digestive system carcinomas: A meta-analysis. J. Cell. Physiol. 2019, 234, 10926–10933. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; An, Y.; Chen, X.; Sun, D.; Chen, T.; Peng, Y.; Zhu, F.; Jiang, Y.; He, X. Epigenetic inhibition of mir-663b by long non-coding RNA hotair promotes pancreatic cancer cell proliferation via up-regulation of insulin-like growth factor 2. Oncotarget 2016, 7, 86857–86870. [Google Scholar] [CrossRef]
- Song, Y.; Wang, R.; Li, L.W.; Liu, X.; Wang, Y.F.; Wang, Q.X.; Zhang, Q. Long non-coding RNA hotair mediates the switching of histone h3 lysine 27 acetylation to methylation to promote epithelial-to-mesenchymal transition in gastric cancer. Int. J. Oncol. 2019, 54, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Kim, S.H.; Yang, W.I.; Ko, Y.H.; Yoon, S.O. Long non-coding RNA hotair expression in diffuse large b-cell lymphoma: In relation to polycomb repressive complex pathway proteins and h3k27 trimethylation. J. Pathol. Transl. Med. 2016, 50, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yang, Y.; Yang, Y.; Guo, L.; Huang, J.; Liu, X.; Wu, C.; Zou, J. Long noncoding RNA (lncRNA) hotair affects tumorigenesis and metastasis of non-small cell lung cancer by upregulating mir-613. Oncol. Res. 2018, 26, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, J.; Wu, Y.; Qiu, J.; Sun, Y.; Tong, X. LncRNA hotair controls the expression of rab22a by sponging mir-373 in ovarian cancer. Mol. Med. Rep. 2016, 14, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. P21: A two-faced genome guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Galanos, P.; Vougas, K.; Walter, D.; Polyzos, A.; Maya-Mendoza, A.; Haagensen, E.J.; Kokkalis, A.; Roumelioti, F.M.; Gagos, S.; Tzetis, M.; et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat. Cell Biol. 2016, 18, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Zhai, N.; Xia, Y.; Yin, R.; Liu, J.; Gao, F. A negative regulation loop of long noncoding RNA hotair and p53 in non-small-cell lung cancer. Oncotargets Ther. 2016, 9, 5713–5720. [Google Scholar]
- Liu, H.; Li, Z.; Wang, C.; Feng, L.; Huang, H.; Liu, C.; Li, F. Expression of long non-coding RNA-hotair in oral squamous cell carcinoma tca8113 cells and its associated biological behavior. Am. J. Transl. Res. 2016, 8, 4726–4734. [Google Scholar]
- Gao, J.Z.; Li, J.; Du, J.L.; Li, X.L. Long non-coding RNA hotair is a marker for hepatocellular carcinoma progression and tumor recurrence. Oncol. Lett. 2016, 11, 1791–1798. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Wu, C.; Huang, J.; Zhang, Y.; Xie, M.; Zhang, M.; Zhou, L. The role of long non-coding RNA hotair in the progression and development of laryngeal squamous cell carcinoma interacting with ezh2. Acta Oto-Laryngol. 2017, 137, 90–98. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H.J.; Kim, S.W.; Park, S.A.; Chun, K.H.; Cho, N.H.; Song, Y.S.; Kim, Y.T. The long non-coding RNA hotair increases tumour growth and invasion in cervical cancer by targeting the notch pathway. Oncotarget 2016, 7, 44558–44571. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.C.; Wang, Y.L.; Lin, P.L.; Zhang, X.; Cheng, W.C.; Liu, S.H.; Chen, C.J.; Hung, Y.; Jan, C.I.; Chang, L.C.; et al. Long noncoding RNA hotair promotes invasion of breast cancer cells through chondroitin sulfotransferase chst15. Int. J. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Li, Q.; Shen, W.; Guo, H.; Zhao, S. The long non-coding RNA hotair promotes thyroid cancer cell growth, invasion and migration through the mir-1-ccnd2 axis. Am. J. Cancer Res. 2017, 7, 1298–1309. [Google Scholar] [PubMed]
- Li, Q.; Feng, Y.; Chao, X.; Shi, S.; Liang, M.; Qiao, Y.; Wang, B.; Wang, P.; Zhu, Z. Hotair contributes to cell proliferation and metastasis of cervical cancer via targetting mir-23b/mapk1 axis. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Fiorini, N.; Lipman, D.J.; Lu, Z. Towards pubmed 2.0. eLife 2017, 6, e28801. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. Gepia: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]



| Author, Year | Country | Cancer | Max. Follow-Up (Months) | Sample | Case Number | OS | DFS/RFS | MFS/PFS | Assay Method | Data Extraction Method | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High Expression | Low Expression | Total | HR (95% CI) | p-Value | HR (95%CI) | p-Value | HR (95% CI) | p-Value | |||||||
| Gupta, 2010 [14] | USA | Breast Cancer | 240 | Tissue | 44 | 88 | 132 | 2.76 (1.45–3.3) | 0.036 | NM | NM | 3.53 (2.78–4.89) | 0.017 | qRT-PCR | K-M |
| Geng, 2011 [30] | China | HCC | 36 | Tissue | NM | NM | 50 | NM | NM | 2.24 (1.49–3.36) | 0,049 | NM | NM | qRT-PCR | K-M |
| Kogo, 2011 [31] | Japan | CRC | 60 | Tissue | 20 | 80 | 100 | 5.62 (1.52–9.57) | 0.008 | NM | NM | NM | NM | qRT-PCR | reported |
| Yang, 2011 [32] | China | HCC | 45 | Tissue | 32 | 28 | 60 | NM | NM | 3.56 (1.67–7.63) | 0.001 | NM | NM | qRT-PCR | reported |
| Lu, 2012 [33] | Italy | Breast Cancer | 108 | Tissue | NM | NM | 336 | 0.43 (0.21–0.89) | 0.022 | 0.47 (0.26–0.87) | 0.016 | NM | NM | qRT-PCR | reported |
| Niinuma, 2012 [34] | Japan | GIST | 200 | Tissue | 11 | 28 | 39 | 3.8 (0.7–21.2) | 0.123 | NM | NM | NM | NM | qRT-PCR | reported |
| Chen, 2013 [24] | China | ESCC | 60 | Tissue | 27 | 51 | 78 | 2.40 (1.35–4.28) | 0.003 | NM | NM | 2.34 (1.22–4.48) | 0.01 | qRT-PCR | reported |
| Endo, 2013 [17] | Japan | IGC | 68 | Tissue | 23 | 13 | 36 | 0.63 (0.34–1.86) | 0.137 | NM | NM | NM | NM | qRT-PCR | K-M |
| Endo, 2013 [17] | Japan | DGC | 60 | Tissue | 20 | 12 | 32 | 3.08 (1.77–5.35) | <0.01 | NM | NM | NM | NM | qRT-PCR | K-M |
| Ge, 2013 [35] | China | ESCC | 100 | Tissue | 90 | 47 | 137 | 3.16 (1.53–6.52) | 0.002 | NM | NM | 4.47 (1.99–10.06) | 0.001 | qRT-PCR | reported |
| Ishibashi, 2013 [36] | Japan | HCC | 36 | Tissue | 13 | 51 | 64 | 2.84 (1.91–4.58) | 0.041 | NM | NM | NM | NM | qRT-PCR | K-M |
| Li, 2013 [37] | China | LSCC | 60 | Tissue | 33 | 39 | 72 | 2.86 (1.15–7.07) | 0.023 | NM | NM | NM | NM | qRT-PCR | reported |
| Li, 2013 [38] | China | ESCC | 60 | Tissue | 30 | 70 | 100 | 1.91 (1.06–3.99) | 0.033 | NM | NM | NM | NM | qRT-PCR | reported |
| Liu, 2013 [39] | China | NSCLC | 60 | Tissue | 21 | 21 | 42 | 2.043 (0.91–4.58) | 0.048 | NM | NM | NM | NM | qRT-PCR | K-M |
| Lv, 2013 [40] | China | ESCC | 70 | Tissue | 49 | 44 | 93 | 1.67 (1.02–2.79) | 0.049 | NM | NM | NM | NM | qRT-PCR | K-M |
| Nakagawa, 2013 [21] | Japan | NSCLC | 50 | Tissue | 17 | 60 | 77 | NM | NM | 1.81 (1.09–3.74) | 0,047 | NM | NM | qRT-PCR | K-M |
| Nie, 2013 [41] | China | NPC | 82 | Tissue | 91 | 69 | 160 | 1.9 (1.13–3.19) | 0.012 | 1.41 (0.95–2.09) | 0.47 | 1.92 (1.11–3.31) | 0.018 | qRT-PCR | K-M |
| Sorensen, 2013 [42] | Denmark | Breast Cancer | 276 | Tissue | 79 | 85 | 164 | NM | NM | NM | NM | 1.75 (1.13–2.71) | 0.012 | Microarray | reported |
| Xu, 2013 [43] | China | Gastric cancer | 75 | Tissue | 56 | 27 | 83 | 0.47 (0.22–0.99) | 0.04 | NM | NM | NM | NM | qRT-PCR | reported |
| He, 2014 [44] | China | EC | 48 | Tissue | 62 | 83 | 145 | 3.04 (2.13–4.58) | 0.026 | NM | NM | NM | NM | qRT-PCR | K-M |
| Huang, 2014 [45] | China | Cervical cancer | 55 | Tissue | 109 | 109 | 218 | 2.86 (1.26–6.49) | 0.012 | NM | NM | NM | NM | qRT-PCR | reported |
| Lee, 2014 [46] | Korea | Gastric cancer | 48 | Tissue | 28 | 20 | 48 | NM | NM | 2.21 (0.53–9.16) | 0.141 | NM | NM | qRT-PCR | reported |
| Liu, 2014 [18] | China | Gastric cancer | 48 | Tissue | 39 | 39 | 78 | 2.7 (1.36–4.34) | 0.023 | NM | NM | NM | NM | qRT-PCR | K-M |
| Okugawa, 2014 [47] | Japan | Gastric cancer | 60 | Tissue | 77 | 73 | 150 | 1.77 (1.06–2.95) | 0.028 | NM | NM | NM | NM | qRT-PCR | reported |
| Qiu, 2014 [48] | China | EOC | 79 | Tissue | 32 | 32 | 64 | 1.87 (1.04–5.31) | 0.041 | 2.54 (1.18–5.45) | 0.034 | NM | NM | qRT-PCR | reported |
| Svoboda, 2014 [49] | Czech Republic | Colorectal cancer | 54 | Tissue | 36 | 37 | 73 | 4.46 (1.02–19.79) | 0.048 | NM | NM | NM | NM | qRT-PCR | reported |
| Wu, 2014 [50] | China | Colon Cancer | 72 | Tissue | 40 | 80 | 120 | 3.92 (1.23–12.50) | 0.021 | NM | NM | 3.88 (1.37–10.98) | 0.011 | qRT-PCR | K-M |
| Yan, 2014 [51] | China | Bladder Cancer | 60 | Tissue | 90 | 20 | 110 | 4.71 (2.89–8.71) | <0.001 | NM | NM | NM | NM | qRT-PCR | reported |
| Heubach, 2015 [52] | Germany | UHC | 200 | Tissue | 27 | 81 | 108 | 2.20 (1.23–3.93) | 0.008 | NM | NM | NM | NM | qRT-PCR | reported |
| Kim, 2015 [53] | Korea | Cervical cancer | 60 | Tissue | 89 | 22 | 111 | NM | NM | 5.28 (1.01–27.74) | 0,049 | NM | NM | qRT-PCR | reported |
| Liu, 2015 [54] | China | Gastric cancer | 40 | Tissue | 24 | 37 | 61 | NM | NM | 2.6 (1.74–3.89) | <0.001 | NM | NM | qRT-PCR | K-M |
| Ma, 2015 [55] | China | Gastric cancer | 60 | Tissue | 18 | 53 | 71 | 2.10 (1.10–4.03) | 0.022 | NM | NM | NM | NM | qRT-PCR | reported |
| Martinez-Fernandez, 2015 [56] | Spain | NMIBC | 38 | Tissue | 17 | 16 | 33 | NM | NM | NM | NM | 1.86 (0.58–5.96) | 0.296 | qRT-PCR | K-M |
| Martinez-Fernandez, 2015 [56] | Spain | NMIBC | 38 | Tissue | 30 | 33 | 63 | NM | NM | 3.78 (2.40–5.96) | <0.001 | NM | NM | qRT-PCR | K-M |
| Qiu, 2015 [57] | China | SOC | 96 | Tissue | 34 | 34 | 64 | 1.90 (1.01–3.56) | 0.046 | NM | NM | NM | NM | qRT-PCR | reported |
| Wu, 2015 [58] | China | OSCC | 60 | Tissue | 25 | 25 | 50 | 1.91 (1.33–2.74) | <0.001 | NM | NM | NM | NM | qRT-PCR | K-M |
| Wu, 2015 [59] | China | AML | 40 | Tissue | 52 | 33 | 85 | 3.37 (0.99–8.31) | 0.008 | 4.68 (2.81–7.79) | <0.001 | NM | NM | qRT-PCR | reported |
| Wu, 2015 [16] | China | OSCC | 96 | Tissue | 38 | 38 | 76 | 1.18 (0.68–2.84) | 0.03 | 1.11 (0.78–2.54) | 0.044 | NM | NM | qRT-PCR | reported |
| Xing, 2015 [60] | China | AML | 36 | Tissue | 68 | 68 | 136 | 2.03 (1.16–3.55) | 0.007 | 0.61 (0.37–1.00) | 0.034 | NM | NM | qRT-PCR | reported |
| Zhang, 2015 [61] | China | Gastric cancer | 45 | Tissue | 35 | 15 | 50 | 1.87 (1.46–2.1) | 0.028 | NM | NM | NM | NM | qRT-PCR | K-M |
| Zhao, 2015 [62] | China | Gastric cancer | 65 | Tissue | 84 | 84 | 168 | 1.47 (1.04–2.06) | 0.027 | NM | NM | NM | NM | qRT-PCR | reported |
| Luczak, 2016 [63] | Poland | EC | 96 | Tissue | 56 | 100 | 156 | 1.44 (0.81–3.19) | 0.03 | NM | NM | NM | NM | qRT-PCR | K-M |
| Luo, 2016 [64] | China | Colon cancer | 70 | Tissue | NM | NM | 80 | 1.99 (1.4–2.8) | <0.001 | NM | NM | NM | NM | qRT-PCR | K-M |
| Sun, 2016 [65] | China | Cervical cancer | 50 | Tissue | 49 | 10 | 59 | 1.31 (0.79–2.26) | 0.02 | NM | NM | NM | NM | qRT-PCR | K-M |
| Yan, 2016 [66] | China | DLBCL | 120 | Tissue | 25 | 25 | 50 | 3.13 (1.22–8.04) | 0.018 | NM | NM | NM | NM | qRT-PCR | reported |
| Zhang, 2016 [67] | China | Acute leukemia | 40 | Tissue | 19 | 77 | 96 | 2.41 (1.25–4.62) | 0.005 | NM | NM | NM | NM | qRT-PCR | K-M |
| Chen, 2017 [68] | China | Gastric cancer | 62 | Tissue | 33 | 32 | 65 | 1.99 (1.06–3.77) | 0.033 | NM | NM | NM | NM | qRT-PCR | reported |
| Hu, 2017 [69] | China | RCC | 50 | Tissue | 32 | 11 | 43 | 0.72 (0.20–2.55) | 0.62 | NM | NM | NM | NM | qRT-PCR | K-M |
| Katayama, 2017 [70] | Japan | RCC | 100 | Tissue | 21 | 43 | 64 | 1.82 (1.06–3.88) | 0.02 | NM | NM | NM | NM | qRT-PCR | K-M |
| Luan, 2017 [71] | China | MM | 60 | Tissue | 30 | 30 | 60 | 1.36 (0.79–2.83) | 0.01 | NM | NM | NM | NM | qRT-PCR | K-M |
| Xu, 2017 [72] | China | * EC | 36 | Tissue | 20 | 20 | 40 | 2.69 (1.14–6.33) | 0.032 | NM | NM | NM | NM | qRT-PCR | K-M |
| Zhang, 2017 [73] | China | Thyroid cancer | 60 | Tissue | NM | NM | 35 | 2.21 (1.38–3.54) | 0.001 | NM | NM | NM | NM | qRT-PCR | reported |
| Dong, 2018 [74] | China | Gastric cancer | 60 | Tissue | 22 | 10 | 32 | 2.26 (0.74–6.89) | 0.158 | NM | NM | NM | NM | qRT-PCR | K-M |
| Huang, 2018 [75] | China | Colorectal cancer | 110 | Tissue | 26 | 26 | 52 | 2.56 (0.91–7.35) | <0.01 | NM | NM | NM | NM | qRT-PCR | reported |
| Xiao, 2018 [76] | China | Colorectal cancer | 60 | Tissue | 52 | 52 | 104 | 1.45 (0.87–2.43) | 0.041 | NM | NM | NM | NM | qRT-PCR | K-M |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toy, H.I.; Okmen, D.; Kontou, P.I.; Georgakilas, A.G.; Pavlopoulou, A. HOTAIR as a Prognostic Predictor for Diverse Human Cancers: A Meta- and Bioinformatics Analysis. Cancers 2019, 11, 778. https://doi.org/10.3390/cancers11060778
Toy HI, Okmen D, Kontou PI, Georgakilas AG, Pavlopoulou A. HOTAIR as a Prognostic Predictor for Diverse Human Cancers: A Meta- and Bioinformatics Analysis. Cancers. 2019; 11(6):778. https://doi.org/10.3390/cancers11060778
Chicago/Turabian StyleToy, Halil Ibrahim, Didem Okmen, Panagiota I. Kontou, Alexandros G. Georgakilas, and Athanasia Pavlopoulou. 2019. "HOTAIR as a Prognostic Predictor for Diverse Human Cancers: A Meta- and Bioinformatics Analysis" Cancers 11, no. 6: 778. https://doi.org/10.3390/cancers11060778
APA StyleToy, H. I., Okmen, D., Kontou, P. I., Georgakilas, A. G., & Pavlopoulou, A. (2019). HOTAIR as a Prognostic Predictor for Diverse Human Cancers: A Meta- and Bioinformatics Analysis. Cancers, 11(6), 778. https://doi.org/10.3390/cancers11060778