Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease
Abstract
1. Introduction
2. Methods
3. Results
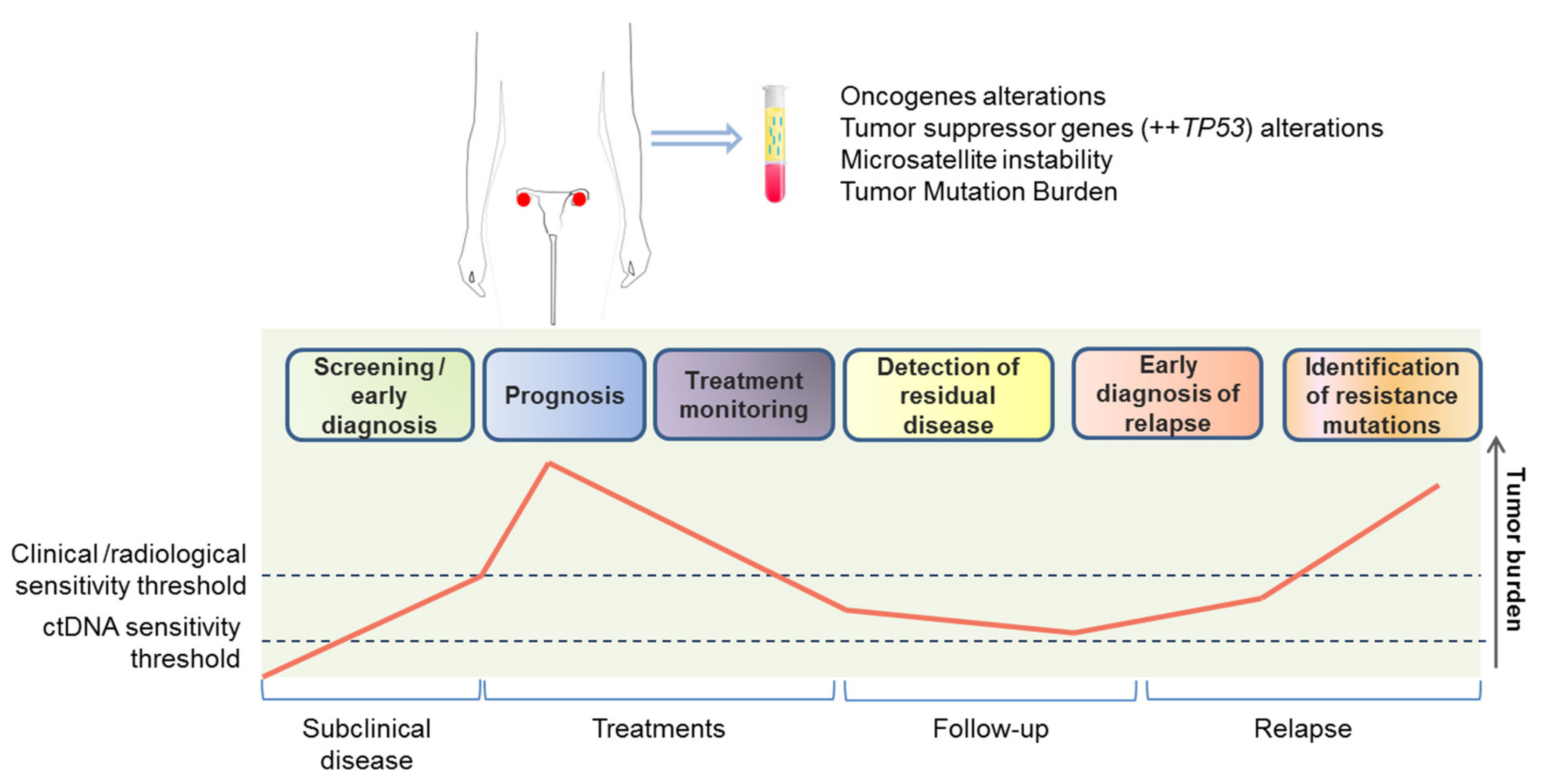
3.1. Circulating Cell-Free DNA and Circulating Tumor DNA
3.1.1. Screening and Early Diagnosis
3.1.2. Prognostic Value
3.1.3. Prediction and Monitoring of Response to Treatment
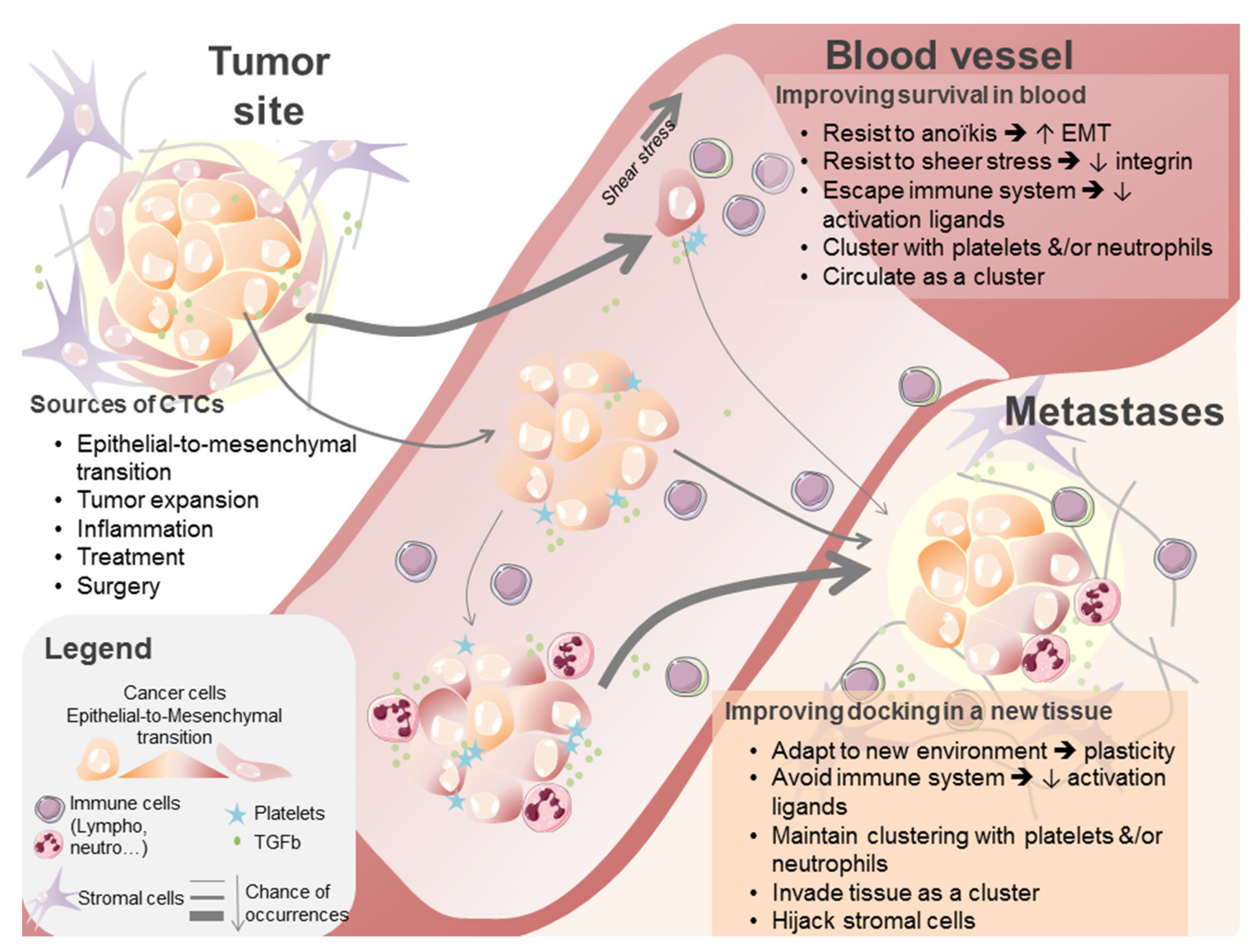
3.2. Circulating Tumor Cells
3.2.1. Quantitative Analyses
3.2.2. Qualitative Analyses
3.3. Exosomes and Circulating Cell-Free Micro RNAs
3.4. Non-Blood-Based Liquid Biopsies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network; Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; DiSaia, P.; Gabra, H.; et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar]
- Johnatty, S.E.; Tyrer, J.P.; Kar, S.; Beesley, J.; Lu, Y.; Gao, B.; Fasching, P.A.; Hein, A.; Ekici, A.B.; Beckmann, M.W.; et al. Genome-wide analysis identifies novel loci associated with ovarian cancer outcomes: findings from the ovarian cancer association consortium. Clin. Cancer Res. 2015, 21, 5264–5276. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, G.; Goranova, T.E.; De Silva, D.; Ennis, D.; Piskorz, A.M.; Eldridge, M.; Sie, D.; Lewsley, L.-A.; Hanif, A.; Wilson, C.; et al. Copy-number signatures and mutational processes in ovarian carcinoma. Nat. Genet. 2018, 50, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- Cameron, T.; Maloney, L.; Isaacson, J.; Goble, S.; Grace, C.; Harding, T.C.; Raponi, M.; Sun, J.; Lin, K.K.; Giordano, H.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar]
- Schwarz, R.F.; Ng, C.K.Y.; Cooke, S.L.; Newman, S.; Temple, J.; Piskorz, A.M.; Gale, D.; Sayal, K.; Murtaza, M.; Baldwin, P.J.; et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: A phylogenetic analysis. PLoS Med. 2015, 12, e1001789. [Google Scholar] [CrossRef]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.M.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef]
- Montagnana, M.; Benati, M.; Danese, E. Circulating biomarkers in epithelial ovarian cancer diagnosis: From present to future perspective. Ann. Transl. Med. 2017, 5, 276. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Issadore, D. Diagnostic technologies for circulating tumour cells and exosomes. Biosci. Rep. 2015, 36, e00292. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.; Cankovic, M.; Furtado, L.V.; Meier, F.; Gocke, C.D. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? J. Mol. Diagn. 2015, 17, 209–224. [Google Scholar] [CrossRef] [PubMed]
- San Lucas, F.A.; Allenson, K.; Bernard, V.; Castillo, J.; Kim, D.U.; Ellis, K.; Ehli, E.A.; Davies, G.E.; Petersen, J.L.; Li, D.; et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann. Oncol. 2015, 27, 635–641. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Marzese, D.M.; Hirose, H.; Hoon, D.S.B. Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev. Mol. Diagn. 2013, 13, 827–844. [Google Scholar] [CrossRef]
- Kuhlmann, J.D.; Schwarzenbach, H.; Wimberger, P.; Poetsch, M.; Kimmig, R.; Kasimir-Bauer, S. LOH at 6q and 10q in fractionated circulating DNA of ovarian cancer patients is predictive for tumor cell spread and overall survival. BMC Cancer 2012, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Flowers, N.; Tong, S.; Hannan, N.; Pertile, M.D.; Hui, L. Abnormal plasma DNA profiles in early ovarian cancer using a non-invasive prenatal testing platform: Implications for cancer screening. BMC Med. 2016, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 2012, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K.; et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef]
- Siena, S.; Sartore-Bianchi, A.; Garcia-Carbonero, R.; Karthaus, M.; Smith, D.; Tabernero, J.; Van Cutsem, E.; Guan, X.; Boedigheimer, M.; Ang, A.; et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann. Oncol 2018, 29, 119–126. [Google Scholar] [CrossRef]
- Vanderstichele, A.; Busschaert, P.; Smeets, D.; Landolfo, C.; Van Nieuwenhuysen, E.; Leunen, K.; Neven, P.; Amant, F.; Mahner, S.; Braicu, E.I.; et al. Chromosomal instability in cell-free DNA as a highly specific biomarker for detection of ovarian cancer in women with adnexal masses. Clin. Cancer Res. 2017, 23, 2223–2231. [Google Scholar] [CrossRef]
- Pereira, E.; Camacho-Vanegas, O.; Anand, S.; Sebra, R.; Camacho, S.C.; Garnar-Wortzel, L.; Nair, N.; Moshier, E.; Wooten, M.; Uzilov, A.; et al. Personalized circulating tumor DNA biomarkers dynamically predict treatment response and survival in gynecologic cancers. PLoS ONE 2015, 10, e0145754. [Google Scholar] [CrossRef]
- Liggett, T.E.; Melnikov, A.; Yi, Q.; Replogle, C.; Hu, W.; Rotmensch, J.; Kamat, A.; Sood, A.K.; Levenson, V. Distinctive DNA methylation patterns of cell-free plasma DNA in women with malignant ovarian tumors. Gynecol. Oncol. 2011, 120, 113–120. [Google Scholar] [CrossRef]
- Wimberger, P.; Roth, C.; Pantel, K.; Kasimir-Bauer, S.; Kimmig, R.; Schwarzenbach, H. Impact of platinum-based chemotherapy on circulating nucleic acid levels, protease activities in blood and disseminated tumor cells in bone marrow of ovarian cancer patients. Int. J. Cancer 2011, 128, 2572–2580. [Google Scholar] [CrossRef]
- Kamat, A.A.; Baldwin, M.; Urbauer, D.; Dang, D.; Han, L.Y.; Godwin, A.; Karlan, B.Y.; Simpson, J.L.; Gershenson, D.M.; Coleman, R.L.; et al. Plasma cell-free DNA in ovarian cancer: An independent prognostic biomarker. Cancer 2010, 116, 1918–1925. [Google Scholar] [CrossRef]
- Gifford, G.; Paul, J.; Vasey, P.A.; Kaye, S.B.; Brown, R. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin. Cancer Res. 2004, 10, 4420–4426. [Google Scholar] [CrossRef]
- Parkinson, C.A.; Gale, D.; Piskorz, A.M.; Biggs, H.; Hodgkin, C.; Addley, H.; Freeman, S.; Moyle, P.; Sala, E.; Sayal, K.; et al. Exploratory analysis of TP53 mutations in circulating tumour DNA as biomarkers of treatment response for patients with relapsed high-grade serous ovarian carcinoma: A retrospective study. PLoS Med. 2016, 13, e1002198. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, S.-W.; Lee, Y.-J.; Lee, H.-Y.; Lee, J.-E.; Choi, E.-K. Prospective study of the efficacy and utility of TP53 mutations in circulating tumor DNA as a non-invasive biomarker of treatment response monitoring in patients with high-grade serous ovarian carcinoma. J. Gynecol. Oncol. 2019, 30, e32. [Google Scholar] [CrossRef]
- Ratajska, M.; Koczkowska, M.; Żuk, M.; Gorczyński, A.; Kuźniacka, A.; Stukan, M.; Biernat, W.; Limon, J.; Wasąg, B. Detection of BRCA1/2 mutations in circulating tumor DNA from patients with ovarian cancer. Oncotarget 2017, 8, 101325–101332. [Google Scholar] [CrossRef]
- Piskorz, A.M.; Lin, K.K.; Morris, J.A.; Mann, E.; Oza, A.M.; Coleman, R.L.; O’Malley, D.M.; Friedlander, M.; Cragun, J.M.; Ma, L.; et al. Feasibility of monitoring response to PARP inhibitor rucaparib with targeted deep sequencing of circulatin tumor DNA in women with high-grade serous carcinoma on the ARIEL2 trial. J. Clin. Oncol. 2016, 34, 5549. [Google Scholar] [CrossRef]
- Christie, E.L.; Doig, K.; Pattnaik, S.; Fereday, S.; Dawson, S.-J.; Bowtell, D.D. Reversion of BRCA1/2 Germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J. Clin. Oncol. 2017, 35, 1274–1280. [Google Scholar] [CrossRef]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.T.; et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef]
- Weigelt, B.; Comino-Méndez, I.; de Bruijn, I.; Tian, L.; Meisel, J.L.; Garcia-Murillas, I.; Fribbens, C.; Cutts, R.; Martelotto, L.G.; Ng, C.K.Y.; et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin. Cancer Res. 2017, 23, 6708–6720. [Google Scholar] [CrossRef]
- Martignetti, J.A.; Camacho-Vanegas, O.; Priedigkeit, N.; Camacho, C.; Pereira, E.; Lin, L.; Garnar-Wortzel, L.; Miller, D.; Losic, B.; Shah, H.; et al. Personalized ovarian cancer disease surveillance and detection of candidate therapeutic drug target in circulating tumor DNA. Neoplasia 2014, 16, 97–103. [Google Scholar] [CrossRef]
- Harris, F.R.; Kovtun, I.V.; Smadbeck, J.; Multinu, F.; Jatoi, A.; Kosari, F.; Kalli, K.R.; Murphy, S.J.; Halling, G.C.; Johnson, S.H.; et al. Quantification of somatic chromosomal rearrangements in circulating cell-free DNA from ovarian cancers. Sci. Rep. 2016, 6, 29831. [Google Scholar] [CrossRef]
- Wittenberger, T.; Sleigh, S.; Reisel, D.; Zikan, M.; Wahl, B.; Alunni-Fabbroni, M.; Jones, A.; Evans, I.; Koch, J.; Paprotka, T.; et al. DNA methylation markers for early detection of women’s cancer: Promise and challenges. Epigenomics 2014, 6, 311–327. [Google Scholar] [CrossRef]
- Widschwendter, M.; Zikan, M.; Wahl, B.; Lempiäinen, H.; Paprotka, T.; Evans, I.; Jones, A.; Ghazali, S.; Reisel, D.; Eichner, J.; et al. The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Med. 2017, 9, 116. [Google Scholar] [CrossRef]
- Bast, R.C.; Matulonis, U.A.; Sood, A.K.; Ahmed, A.A.; Amobi, A.E.; Balkwill, F.R.; Wielgos-Bonvallet, M.; Bowtell, D.D.L.; Brenton, J.D.; Brugge, J.S.; et al. Critical questions in ovarian cancer research and treatment: Report of an American Association for Cancer Research Special Conference. Cancer 2019, 125, 1963–1972. [Google Scholar] [CrossRef]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.-J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of homologous recombination–related gene mutations across multiple cancer types. JCO Precis. Oncol. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Hodgson, D.R.; Dougherty, B.A.; Lai, Z.; Fielding, A.; Grinsted, L.; Spencer, S.; O’Connor, M.J.; Ho, T.W.; Robertson, J.D.; Lanchbury, J.S.; et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br. J. Cancer 2018, 119, 1401–1409. [Google Scholar] [CrossRef]
- Gentles, L.; Goranov, B.; Matheson, E.; Herriott, A.; Kaufmann, A.; Hall, S.; Mukhopadhyay, A.; Drew, Y.; Curtin, N.J.; O’Donnell, R.L. Exploring the frequency of homologous recombination DNA repair dysfunction in multiple cancer types. Cancers 2019, 11, 354. [Google Scholar] [CrossRef]
- Cabel, L.; Proudhon, C.; Romano, E.; Girard, N.; Lantz, O.; Stern, M.-H.; Pierga, J.-Y.; Bidard, F.-C. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2018, 15, 639–650. [Google Scholar] [CrossRef]
- Van Berckelaer, C.; Brouwers, A.J.; Peeters, D.J.E.; Tjalma, W.; Trinh, X.B.; van Dam, P.A. Current and future role of circulating tumor cells in patients with epithelial ovarian cancer. Eur J. Surg. Oncol. 2016, 42, 1772–1779. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.-F.; Norton, L.; Massagué, J. Tumor self-seeding by circulating cancer cells. Cell 2009, 139, 1315–1326. [Google Scholar] [CrossRef]
- Larson, C.J.; Moreno, J.G.; Pienta, K.J.; Gross, S.; Repollet, M.; O’Hara, S.M.; Russell, T.; Terstappen, L.W.M.M. Apoptosis of circulating tumor cells in prostate cancer patients. Cytom. Part. J. Int. Soc. Anal. Cytol. 2004, 62, 46–53. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, X.; Shi, H.; Han, X.; Liu, W.; Tian, X.; Zeng, X. Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumour Biol. 2016, 37, 5397–5404. [Google Scholar] [CrossRef]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef]
- Smith, H.A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 2013, 91, 411–429. [Google Scholar] [CrossRef]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Kolostova, K.; Pinkas, M.; Jakabova, A.; Pospisilova, E.; Svobodova, P.; Spicka, J.; Cegan, M.; Matkowski, R.; Bobek, V. Molecular characterization of circulating tumor cells in ovarian cancer. Am. J. Cancer Res. 2016, 6, 973–980. [Google Scholar]
- Phillips, K.G.; Velasco, C.R.; Li, J.; Kolatkar, A.; Luttgen, M.; Bethel, K.; Duggan, B.; Kuhn, P.; Mccarty, O.J.T. Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Front. Oncol. 2012, 2, 72. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Marth, C.; Kisic, J.; Kaern, J.; Trope, C.; Fodstad, Ø. Circulating tumor cells in the peripheral blood and bone marrow of patients with ovarian carcinoma do not predict prognosis. Cancer 2002, 94, 707–712. [Google Scholar] [CrossRef]
- Judson, P.L.; Geller, M.A.; Bliss, R.L.; Boente, M.P.; Downs, L.S., Jr.; Argenta, P.A.; Carson, L.F. Preoperative detection of peripherally circulating cancer cells and its prognostic significance in ovarian cancer. Gynecol. Oncol. 2003, 91, 389–394. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Koo, K.H.; Sung, J.Y.; Yun, U.-J.; Kim, H. Anoikis resistance: An essential prerequisite for tumor metastasis. Int. J. Cell Biol. 2012, 2012, 306879. [Google Scholar] [CrossRef]
- Cayrefourcq, L.; Mazard, T.; Joosse, S.; Solassol, J.; Ramos, J.; Assenat, E.; Schumacher, U.; Costes, V.; Maudelonde, T.; Pantel, K.; et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015, 75, 892–901. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Obermayr, E.; Castillo-Tong, D.C.; Pils, D.; Speiser, P.; Braicu, I.; Van Gorp, T.; Mahner, S.; Sehouli, J.; Vergote, I.; Zeillinger, R. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance—A study of the OVCAD consortium. Gynecol. Oncol. 2013, 128, 15–21. [Google Scholar] [CrossRef]
- Pearl, M.L.; Zhao, Q.; Yang, J.; Dong, H.; Tulley, S.; Zhang, Q.; Golightly, M.; Zucker, S.; Chen, W.-T.; Zhang, Q. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 581–590. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.; Choi, J.Y.; Bu, J.; Kang, Y.-T.; Kwon, B.S.; Lee, B.; Kim, K.; No, J.H.; Kim, Y.-B.; et al. Circulating tumor cells in the differential diagnosis of adnexal masses. Oncotarget 2017, 8, 77195–77206. [Google Scholar] [CrossRef]
- Pearl, M.L.; Dong, H.; Tulley, S.; Zhao, Q.; Golightly, M.; Zucker, S.; Chen, W.-T. Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs). Gynecol. Oncol. 2015, 137, 229–238. [Google Scholar] [CrossRef]
- Fan, T.; Zhao, Q.; Chen, J.J.; Chen, W.-T.; Pearl, M.L. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol. Oncol. 2009, 112, 185–191. [Google Scholar] [CrossRef]
- Aktas, B.; Kasimir-Bauer, S.; Heubner, M.; Kimmig, R.; Wimberger, P. Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy. Int. J. Gynecol. Cancer 2011, 21, 822–830. [Google Scholar] [CrossRef]
- Kuhlmann, J.D.; Wimberger, P.; Bankfalvi, A.; Keller, T.; Schöler, S.; Aktas, B.; Buderath, P.; Hauch, S.; Otterbach, F.; Kimmig, R.; et al. ERCC1-positive circulating tumor cells in the blood of ovarian cancer patients as a predictive biomarker for platinum resistance. Clin. Chem. 2014, 60, 1282–1289. [Google Scholar] [CrossRef]
- Poveda, A.; Kaye, S.B.; McCormack, R.; Wang, S.; Parekh, T.; Ricci, D.; Lebedinsky, C.A.; Tercero, J.C.; Zintl, P.; Monk, B.J. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol. Oncol. 2011, 122, 567–572. [Google Scholar] [CrossRef]
- Lee, M.; Kim, E.J.; Cho, Y.; Kim, S.; Chung, H.H.; Park, N.H.; Song, Y.-S. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 361–365. [Google Scholar] [CrossRef]
- Behbakht, K.; Sill, M.W.; Darcy, K.M.; Rubin, S.C.; Mannel, R.S.; Waggoner, S.; Schilder, R.J.; Cai, K.Q.; Godwin, A.K.; Alpaugh, R.K. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: A gynecologic oncology group study. Gynecol. Oncol. 2011, 123, 19–26. [Google Scholar] [CrossRef]
- Chebouti, I.; Kuhlmann, J.D.; Buderath, P.; Weber, S.; Wimberger, P.; Bokeloh, Y.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. ERCC1-expressing circulating tumor cells as a potential diagnostic tool for monitoring response to platinum-based chemotherapy and for predicting post-therapeutic outcome of ovarian cancer. Oncotarget 2017, 8, 24303–24313. [Google Scholar] [CrossRef]
- Obermayr, E.; Bednarz-Knoll, N.; Orsetti, B.; Weier, H.-U.; Lambrechts, S.; Castillo-Tong, D.C.; Reinthaller, A.; Braicu, E.I.; Mahner, S.; Sehouli, J.; et al. Circulating tumor cells: Potential markers of minimal residual disease in ovarian cancer? A study of the OVCAD consortium. Oncotarget 2017, 8, 106415–106428. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Yu, X.; Li, S.; Lei, Z.; Li, C.; Zhang, Q.; Han, Q.; Li, Y.; Zhang, K.; et al. Analysis of circulating tumor cells in ovarian cancer and their clinical value as a biomarker. Cell. Physiol. Biochem. 2018, 48, 1983–1994. [Google Scholar] [CrossRef]
- Gasparri, M.L.; Savone, D.; Besharat, R.A.; Farooqi, A.A.; Bellati, F.; Ruscito, I.; Panici, P.B.; Papadia, A. Circulating tumor cells as trigger to hematogenous spreads and potential biomarkers to predict the prognosis in ovarian cancer. Tumour Biol. 2016, 37, 71–75. [Google Scholar] [CrossRef]
- Giannopoulou, L.; Kasimir-Bauer, S.; Lianidou, E.S. Liquid biopsy in ovarian cancer: Recent advances on circulating tumor cells and circulating tumor DNA. Clin. Chem. Lab. Med. 2018, 56, 186–197. [Google Scholar] [CrossRef]
- Romero-Laorden, N.; Olmos, D.; Fehm, T.; García-Donas, J.; Diaz-Padilla, I. Circulating and disseminated tumor cells in ovarian cancer: A systematic review. Gynecol. Oncol. 2014, 133, 632–639. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, B.; Yuan, X.; Xie, G.; Ma, Y.; Shen, L. Prognostic value of circulating tumor cells in ovarian cancer: a meta-analysis. PLoS ONE 2015, 10, e0130873. [Google Scholar] [CrossRef]
- Zeng, L.; Liang, X.; Liu, Q.; Yang, Z. The predictive value of circulating tumor cells in ovarian cancer: a meta analysis. Int. J. Gynecol. Cancer 2017, 27, 1109–1117. [Google Scholar] [CrossRef]
- Cui, L.; Kwong, J.; Wang, C.C. Prognostic value of circulating tumor cells and disseminated tumor cells in patients with ovarian cancer: A systematic review and meta-analysis. J. Ovarian Res. 2015, 8, 38. [Google Scholar] [CrossRef]
- Bartkowiak, K.; Pantel, K.; Alix-Panabières, C. Functional studies on circulating and disseminated tumor cells in carcinoma patients. Mol. Oncol. 2016, 10, 443–449. [Google Scholar]
- O’Shannessy, D.J.; Davis, D.W.; Anderes, K.; Somers, E.B. Isolation of circulating tumor cells from multiple epithelial cancers with apostream® for detecting (or monitoring) the expression of folate receptor alpha. Biomark. Insights 2016, 11, 7–18. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 2019, 176, 98–112. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Chebouti, I.; Kasimir-Bauer, S.; Buderath, P.; Wimberger, P.; Hauch, S.; Kimmig, R.; Kuhlmann, J.D. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 2017, 8, 48820–48831. [Google Scholar] [CrossRef]
- Mitra, T.; Prasad, P.; Mukherjee, P.; Chaudhuri, S.R.; Chatterji, U.; Roy, S.S. Stemness and chemoresistance are imparted to the OC cells through TGFβ1 driven EMT. J. Cell. Biochem. 2018, 119, 5775–5787. [Google Scholar] [CrossRef]
- Kolostova, K.; Spicka, J.; Matkowski, R.; Bobek, V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am. J. Transl. Res. 2015, 7, 1203–1213. [Google Scholar]
- Kolostova, K.; Matkowski, R.; Jędryka, M.; Soter, K.; Cegan, M.; Pinkas, M.; Jakabova, A.; Pavlasek, J.; Spicka, J.; Bobek, V. The added value of circulating tumor cells examination in ovarian cancer staging. Am. J. Cancer Res. 2015, 5, 3363–3375. [Google Scholar]
- Liu, J.F.; Kindelberger, D.; Doyle, C.; Lowe, A.; Barry, W.T.; Matulonis, U.A. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol. Oncol. 2013, 131, 352–356. [Google Scholar] [CrossRef]
- Brouwer, A.; De Laere, B.; Peeters, D.; Peeters, M.; Salgado, R.; Dirix, L.; Van Laere, S. Evaluation and consequences of heterogeneity in the circulating tumor cell compartment. Oncotarget 2016, 7, 48625–48643. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Characterization of single circulating tumor cells. FEBS Lett. 2017, 591, 2241–2250. [Google Scholar] [CrossRef]
- Gawad, C.; Koh, W.; Quake, S.R. Single-cell genome sequencing: Current state of the science. Nat. Rev. Genet. 2016, 17, 175–188. [Google Scholar] [CrossRef]
- Blassl, C.; Kuhlmann, J.D.; Webers, A.; Wimberger, P.; Fehm, T.; Neubauer, H. Gene expression profiling of single circulating tumor cells in ovarian cancer—Establishment of a multi-marker gene panel. Mol. Oncol. 2016, 10, 1030–1042. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Mader, S.; Pantel, K. Liquid biopsy: Current status and future perspectives. Oncol. Res. Treat. 2017, 40, 404–408. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A Microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Zeng, Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip 2016, 16, 3033–3042. [Google Scholar] [CrossRef]
- Kabe, Y.; Suematsu, M.; Sakamoto, S.; Hirai, M.; Koike, I.; Hishiki, T.; Matsuda, A.; Hasegawa, Y.; Tsujita, K.; Ono, M.; et al. Development of a highly sensitive device for counting the number of disease-specific exosomes in human sera. Clin. Chem. 2018, 64, 1463–1473. [Google Scholar] [CrossRef]
- Gercel-Taylor, C.; Atay, S.; Tullis, R.H.; Kesimer, M.; Taylor, D.D. Nanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patients. Anal. Biochem. 2012, 428, 44–53. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef]
- Meng, X.; Müller, V.; Milde-Langosch, K.; Trillsch, F.; Pantel, K.; Schwarzenbach, H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget 2016, 7, 16923–16935. [Google Scholar] [CrossRef]
- Whiteside, T.L. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar]
- Tang, M.K.; Wong, A.S. Exosomes: Emerging biomarkers and targets for ovarian cancer. Cancer Lett. 2015, 367, 26–33. [Google Scholar] [CrossRef]
- Li, W.; Lu, Y.; Yu, X.; Yong, M.; Ma, D.; Gao, Q. Detection of exosomal tyrosine receptor kinase B as a potential biomarker in ovarian cancer. J. Cell Biochem. 2019, 120, 6361–6369. [Google Scholar] [CrossRef]
- Qiu, J.-J.; Lin, X.-J.; Tang, X.-Y.; Zheng, T.-T.; Lin, Y.-Y.; Hua, K.-Q. Exosomal metastasis-associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int. J. Biol. Sci. 2018, 14, 1960–1973. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Alharbi, M.; Zuñiga, F.; Elfeky, O.; Guanzon, D.; Lai, A.; E Rice, G.; Perrin, L.C.; Hooper, J.; Salomon, C.; Rice, G. The potential role of miRNAs and exosomes in chemotherapy in ovarian cancer. Endocr. Relat. Cancer 2018, 25, R663–R685. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Giannopoulou, L.; Zavridou, M.; Kasimir-Bauer, S.; Lianidou, E.S. Liquid biopsy in ovarian cancer: The potential of circulating miRNAs and exosomes. Transl. Res. J. Lab. Clin. Med. 2018, 205, 77–91. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Resnick, K.E.; Alder, H.; Hagan, J.P.; Richardson, D.L.; Croce, C.M.; Cohn, D.E. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 2009, 112, 55–59. [Google Scholar] [CrossRef]
- Häusler, S.F.M.; Keller, A.; Chandran, A.P.; Ziegler, K.; Zipp, K.; Heuer, S.; Krockenberger, M.; Engel, J.B.; Hönig, A.; Scheffler, M.; et al. Whole blood-derived miRNA profiles as potential new tools for ovarian cancer screening. Br. J. Cancer 2010, 103, 693–700. [Google Scholar] [CrossRef]
- Kan, C.W.; Hahn, A.M.; Gard, G.B.; Maidens, J.; Huh, J.Y.; Marsh, D.J.; Howell, V.M. Elevated levels of circulating microRNA-200 family members correlate with serous epithelial ovarian cancer. BMC Cancer 2012, 12, 627. [Google Scholar] [CrossRef]
- Chung, Y.-W.; Bae, H.-S.; Song, J.-Y.; Lee, J.K.; Lee, N.W.; Kim, T.; Lee, K.-W. Detection of MicroRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patient. Int. J. Gynecol. Cancer 2013, 23, 673–679. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, L.; Zhao, Y.; Yang, D.; Song, F.; Wen, Y.; Hao, Q.; Hu, Z.; Zhang, W.; Chen, K. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS ONE 2013, 8, e77853. [Google Scholar] [CrossRef]
- Suryawanshi, S.; Vlad, A.M.; Lin, H.-M.; Mantia-Smaldone, G.; Laskey, R.; Lee, M.; Lin, Y.; Donnellan, N.; Klein-Patel, M.; Lee, T.; et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin. Cancer Res. 2013, 19, 1213–1224. [Google Scholar] [CrossRef]
- Meng, X.; Joosse, A.S.; Müller, V.; Trillsch, F.; Milde-Langosch, K.; Mahner, S.; Geffken, M.; Pantel, K.; Schwarzenbach, H. Diagnostic and prognostic potential of serum miR-7, miR-16, miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer patients. Br. J. Cancer 2015, 113, 1358–1366. [Google Scholar] [CrossRef]
- Yoshimura, A.; Sawada, K.; Nakamura, K.; Kinose, Y.; Nakatsuka, E.; Kobayashi, M.; Miyamoto, M.; Ishida, K.; Matsumoto, Y.; Kodama, M.; et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC Cancer 2018, 18, 1065. [Google Scholar] [CrossRef]
- Wang, X.; Kong, D.; Wang, C.; Ding, X.; Zhang, L.; Zhao, M.; Chen, J.; Xu, X.; Hu, X.; Yang, J.; et al. Circulating microRNAs as novel potential diagnostic biomarkers for ovarian cancer: A systematic review and updated meta-analysis. J. Ovarian Res. 2019, 12, 24. [Google Scholar] [CrossRef]
- Shapira, I.; Oswald, M.; Lovecchio, J.; Khalili, H.; Menzin, A.; Whyte, J.; Dos Santos, L.; Liang, S.; Bhuiya, T.; Keogh, M.; et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br. J. Cancer 2014, 110, 976–983. [Google Scholar] [CrossRef]
- Liang, H.; Jiang, Z.; Xie, G.; Lu, Y. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumor Biol. 2015, 36, 5305–5313. [Google Scholar] [CrossRef]
- Guo, F.; Tian, J.; Lin, Y.; Jin, Y.; Wang, L.; Cui, M. Serum microRNA-92 expression in patients with ovarian epithelial carcinoma. J. Int. Med. Res. 2013, 41, 1456–1461. [Google Scholar] [CrossRef]
- Xu, Y.-Z.; Xi, Q.-H.; Ge, W.-L.; Zhang, X.-Q. Identification of serum MicroRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pac. J. Cancer Prev. 2013, 14, 1057–1060. [Google Scholar] [CrossRef]
- Vaksman, O.; Tropé, C.; Davidson, B.; Reich, R. Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis 2014, 35, 2113–2120. [Google Scholar] [CrossRef]
- Gao, Y.-C.; Wu, J. MicroRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer. Tumor Biol. 2015, 36, 4843–4850. [Google Scholar] [CrossRef]
- Kapetanakis, N.-I.; Uzan, C.; Jimenez-Pailhes, A.-S.; Gouy, S.; Bentivegna, E.; Morice, P.; Caron, O.; Gourzones-Dmitriev, C.; Le Teuff, G.; Busson, P. Plasma miR-200b in ovarian carcinoma patients: Distinct pattern of pre/post-treatment variation compared to CA-125 and potential for prediction of progression-free survival. Oncotarget 2015, 6, 36815–36824. [Google Scholar] [CrossRef]
- Kuhlmann, J.D.; Baraniskin, A.; Hahn, S.A.; Mosel, F.; Bredemeier, M.; Wimberger, P.; Kimmig, R.; Kasimir-Bauer, S. Circulating U2 small nuclear RNA fragments as a novel diagnostic tool for patients with epithelial ovarian cancer. Clin. Chem. 2014, 60, 206–213. [Google Scholar] [CrossRef]
- Zhou, J.; Gong, G.; Tan, H.; Dai, F.; Zhu, X.; Chen, Y.; Wang, J.; Liu, Y.; Chen, P.; Wu, X.; et al. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol. Rep. 2015, 33, 2915–2923. [Google Scholar] [CrossRef]
- Záveský, L.; Jandáková, E.; Turyna, R.; Langmeierová, L.; Weinberger, V.; Záveská Drábková, L.; Hůlková, M.; Hořínek, A.; Dušková, D.; Feyereisl, J.; et al. Evaluation of Cell-Free Urine microRNAs expression for the use in diagnosis of ovarian and endometrial cancers. A pilot study. Pathol. Oncol. Res. POR 2015, 21, 1027–1035. [Google Scholar] [CrossRef]
- Yamamoto, C.M.; Oakes, M.L.; Murakami, T.; Muto, M.G.; Berkowitz, R.S.; Ng, S.-W. Comparison of benign peritoneal fluid- and ovarian cancer ascites-derived extracellular vesicle RNA biomarkers. J. Ovarian Res. 2018, 11, 20. [Google Scholar] [CrossRef]
- Gasparri, M.L.; Casorelli, A.; Bardhi, E.; Besharat, A.R.; Savone, D.; Ruscito, I.; Farooqi, A.A.; Papadia, A.; Mueller, M.D.; Ferretti, E.; et al. Beyond circulating microRNA biomarkers: Urinary microRNAs in ovarian and breast cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Swisher, E.M.; Wollan, M.; Mahtani, S.M.; Willner, J.B.; Garcia, R.; Goff, B.A.; King, M.-C. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am. J. Obstet. Gynecol. 2005, 193, 662–667. [Google Scholar] [CrossRef]
- Barquín, M.; Maximiano, C.; Pérez-Barrios, C.; Sanchez-Herrero, E.; Soriano, M.; Colmena, M.; García-Espantaleón, M.; González, E.T.; Gutierrez, L.; Ruiz, A.C.S.; et al. Peritoneal washing is an adequate source for somatic BRCA1/2 mutation testing in ovarian malignancies. Pathol. Res. Pract. 2019, 215, 392–394. [Google Scholar] [CrossRef]
- Ahmed, N.; Greening, D.; Samardzija, C.; Escalona, R.M.; Chen, M.; Findlay, J.K.; Kannourakis, G. Unique proteome signature of post-chemotherapy ovarian cancer ascites-derived tumor cells. Sci. Rep. 2016, 6, 30061. [Google Scholar] [CrossRef]
- Kinde, I.; Bettegowda, C.; Wang, Y.; Wu, J.; Agrawal, N.; Shih, I.-M.; Kurman, R.; Dao, F.; Levine, D.A.; Giuntoli, R.; et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci. Transl. Med. 2013, 5, 167ra4. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Douville, C.; Cohen, J.D.; Yen, T.-T.; Kinde, I.; Sundfelt, K.; Kjær, S.K.; Hruban, R.H.; Shih, I.-M.; et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci. Transl. Med. 2018, 10, eaap8793. [Google Scholar] [CrossRef]
- Maritschnegg, E.; Wang, Y.; Pecha, N.; Horvat, R.; Van Nieuwenhuysen, E.; Vergote, I.; Heitz, F.; Sehouli, J.; Kinde, I.; Diaz, L.A.; et al. Lavage of the uterine cavity for molecular detection of müllerian duct carcinomas: a proof-of-concept study. J. Clin. Oncol. 2015, 33, 4293–4300. [Google Scholar] [CrossRef]
| Reference | Areas of Interest | ctDNA /cfDNA | Methods | Molecular Alterations | N | FIGO Stage | Results |
|---|---|---|---|---|---|---|---|
| [28] | Screening /Diagnosis | cfDNA | Low coverage whole genome sequencing | Chromosomal instability | 68 adnexal masses 44 benign cases | I–II = 8 III–IV = 46 Borderline = 3 Benign = 11 | Chromosomal instability significantly more elevated in tumor cases than healthy and benign cases |
| [22] | Screening /Diagnosis | cfDNA | Low coverage whole genome sequencing | Copy number alterations | 32 OvC 32 benign cases | Detection rate 40% (13/32) Sensitivity 40.6% Specificity 93.8% | |
| [29] | Screening /Diagnosis Prognosis | ctDNA | Droplet digital PCR | Tumor specific mutations | 44 gynecological cancers including 22 OvC | Detection rate 93.8% Sensitivity 91%, Specificity 60% Undetectable ctDNA 6 months after treatment completion was associated to a better PFS (p = 0.0011) and OS (p = 0.0194). | |
| [30] | Screening /Diagnosis | cfDNA | DNA microarray | Methylation profiling PGR-PROX and RASSF1A | 30 OvC 30 benign cases | III–IV | Sensitivity 80% and Specificity 73% |
| [31] | Prognosis | cfDNA | Fluorescence | Fluorimetry | 62 | I–II = 18% III = 69% IV = 13% | Pre-operative ctDNA is correlated to PFS (p = 0.02) and OS (p = 0.01) |
| [32] | Prognosis | cfDNA | RT-PCR | Beta-globin | 164 | I–II = 38 III–IV = 126 | Pre-treatment ctDNA levels are correlated to OS (median 3.1 years for high ctDNA levels vs. 4 years for patients with low ctDNA rate. |
| [33] | Prognosis | ctDNA | PCR | Methylation profiling | 138 | Correlation between hMLH1 methylation acquisition after chemotherapy and post-progression survival (HR = 99; p = 0.007) | |
| [34] | Prediction and monitoring of response to treatment | ctDNA | Digital PCR | TP53 mutations | 40 (mostly relapses) | I = 3 III = 27 IV = 10 | TP53 mutant allele fraction associated to tumor burden and time to progression (HR = 0.22, p = 0.008, multivariate analysis). ctDNA is an earlier marker of response to treatment (median time to nadir of 37 days vs. 84 days for CA125) |
| [35] | Prediction and monitoring of response to treatment | ctDNA | Droplet digital PCR | TP53 mutations | 41 | III–IV | Correlation between plasma TP53 mutant allele count 3 months after chemotherapy completion and time to progression (p = 0.038) |
| [36] | Prediction and monitoring of response to treatment | ctDNA | NGS | BRCA1/2 mutations | 121 | Mutations detected for 24.8% | |
| [37] | Prediction and monitoring of response to treatment | ctDNA | NGS, Targeted amplicon deep sequencing | TP53 mutant allele fraction | 18 | Relapses | 0/5 patients low ctDNA decrease achieved radiological response. 7/9 patients with high ctDNA decrease were responder |
| [38] | Prediction and monitoring of response | ctDNA | NGS, Targeted amplicon-sequencing | BRCA1/2 and TP53 | 30 | II = 2 III = 23 IV = 5 | BRCA1/2 reversion mutation identified in 5 tumors and 3 plasma samples |
| [39] | Prediction and monitoring of response | ctDNA | NGS | Panels of genes (Guardant360 and FundationACT) | 112 | BRCA1/2 mutation detected in 97 patients. BRCA1/2 reversion mutation found in pre-treatment ctDNA from 8 patients. Correlation to PFS after rucaparib (median 9 months vs. 1.8 months (p < 0.0001). | |
| [40] | Prediction of response | ctDNA | NGS | 143-gene panel | 19 | Platinum-resistant | BRCA1/2 reversion mutations identified for 4 (21%) patients with OvC |
| [41] | Prediction and monitoring of response | ctDNA | qRT-PCR | FGFR2 transcript fusion | 1 | IIIc | Systematic increase of transcript at each relapse with better sensitivity than CA125 |
| [42] | Monitoring of response | ctDNA | qPCR | Tumor specific chromosomal rearrangements | 10 | IIIc and IV | 8 cases with rearrangements identified in plasma at diagnosis. Correlation between persistence of detectable rearrangements and post-operative residual disease. |
| Reference | Areas of Interest | CTC Enrichment Methods | CTC Detection Methods | N | FIGO Stage | Results |
|---|---|---|---|---|---|---|
| [66] | Diagnosis | Density gradient centrifugation | RT-qPCR | 39 healthy subjects$216 OvC | II = 8 III = 152 IV = 40 | 24.5% of patients with OvC were CTC+ vs. 1/39 of healthy subjects |
| [67] | Diagnosis Prognosis | Cell adhesion matrix-based | Microscopy and flow cytometry (EPCAM, CA125, DPP4, CD44, seprase, CD45, cytokeratins) | 41 benign masses 88 OvC | I = 13 II = 4 III = 50 IV = 21 | 83% sensitivity 97.3% positive predictive value Correlation with OS and PFS higher than CA125 |
| [68] | Screening /Diagnosis | Physical properties (TSF platform) | Immunostaining (DAPI+, CD45−, CK+ or EpCAM+) | 43 benign cases 13 border line tumors 31 OvC | I = 6 II = 4 III-IV = 21 | 77.4% sensitivity and 100% specificity to discriminate benign from malignant tumors |
| [69] | Prediction of response Early diagnosis of relapse | Cell adhesion matrix-based | Microscopy and flow cytometry (EPCAM, CA125, DPP4, CD44, seprase, CD45, cytokeratins) | 64 healthy subjects 49 benign cases 123 OvC (including 31 with serial time points) | I = 9 II = 1 III = 13 IV = 8 | Higher correlation to duration of response than CA125 CTCs more sensitive than CA125 to predict relapse |
| [70] | Staging Prognosis | Density gradient centrifugation | Immunostaining (CD45−, CK+, ESA+ or EpCAM+) | 66 OvC 5 benign masses | I–II = 10 II = 37 IV = 15 | Sensitivity from 10% (stage I–II) to 73% (III–IV) Correlation with DFS (median 15 months for CTC+ vs. 35 months for CTC−, p = 0.042 |
| [71] | Prognosis | Immunomagnetic assay (EpCAM/MUC1) | RT-qPCR (EpCAM/MUC1/ERBB2) | 122 OvC | 86 pre-chemo 70 post-chemo | CTC+ correlated with OS, both for pre-chemo samples (p = 0.0054) and after chemotherapy (p = 0.047). |
| [72] | Prognosis | Immunomagnetic assay (EpCAM/MUC1) | RT-PCR (EpCAM/MUC1/ MUC16/ERCC1) | 143 | I–II = 26 II = 87 IV = 30 | CTC+ correlated to OS. CTC ERCC1+ correlated to OS, PFS, and platinum resistance. |
| [73] | Prognosis | CellSearch | Immunostaining (DAPI+, EpCAM+, CD45−) | 216 | Relapses (66.2% platinum-sensitive) | Trend for CTC correlation to PFS (p = 0.058) and OS (p = 0.096) |
| [74] | Prognosis | Polymer-deposited microfluidic device | Immunostaining (DAPI+, EpCAM+, CD45−) | 54 | 24 primaries 30 relapses | ≥3 CTCs correlated to PFS CTC-cluster+ correlated with platinum resistance |
| [75] | Prognosis | CellSearch | Cellsearch (DAPI+, EpCAM+, cytokeratin+, CD45−) | 54 | All relapses | CTC+ correlated to lack of response to temsirolimus |
| [76] | Minimal residual disease | AdnaTest Ovarian Cancer Select | RT-PCR (AdnaTest Ovarian Cancer Detect, ERCC1) | 65 | I–II = 11 III = 41 IV = 13 | ERCC1 + CTCs after chemotherapy correlated with platinum-resistance (p = 0.01), PFS (p = 0.0293) and OS (p = 0.0008) |
| [77] | Prognosis Prediction of response | Density gradient centrifugation+ | IHC+ fusion genes associated to stem-cell like phenotype (MECOM, HHLA1) | Blood collected at diagnosis (n = 102) + 6 months after treatment completion (n= 78) | II = 4 III = 67 IV = 31 | 26.5% CTC+ at baseline 7.7% CTC+ after treatment CTC status at baseline associated to survival for patient with complete debulking surgery CTC status after treatment correlated to response to platinum |
| [78] | Prediction of platinum resistance Prognosis | Immunocytological staining | Multiplex RT-PCR | 109 at diagnosis, including 51 at serial time points | I = 23 II = 13 III = 58 IV = 15 | 90% CTC+ at baseline Higher sensitivity than CA125 for early stages EpCAM and ERBB2 correlated to platinum resistance EpCAM correlated to survival |
| Heading | Circulating Tumor DNA | Circulating Tumor Cells | Extracellular Vesicles (Exosomes) | Tumor Micro-RNA |
|---|---|---|---|---|
| Origins | DNA released by apoptotic or necrotic cells, carrying specific molecular alterations of the tumor. Complementary to tumor genomic profiling (due to spatial heterogeneity and possibility of serial blood sampling). | Cells released in the bloodstream by the primary tumor or metastatic sites. Some of them are able to colonize distant sites after epithelial to mesenchymal transition. | Physiologically secreted by cells and released in the extracellular space and the bloodstream. Contain nucleic acids (DNA, RNA, micro-RNA), proteins, and metabolites | Carried by exosomes or isolated in the plasma (cf-miRNA). Highly stable in serum/plasma |
| Settings of interest | Screening/early diagnosis Prognosis Identification of minimal residual disease and monitoring of response to treatment | Screening/early diagnosis Prognosis Response to treatment Evaluation of minimal residual disease Functional studies (phenotypic and genotypic studies) | Diagnosis Prognosis | Screening/diagnosis Prognosis |
| Methods | Blood sample collection and processing • EDTA tubes - Widely available, low cost - Risk of degradation of DNA and release of genomic DNA from hemotopoietic cells. Warrant rapid processing (1 to 2 h from collection) • cfDNA preservative tubes: - More expensive - Maintain the quality of DNA for multiple days at room temperature. DNA extraction from plasma (kits designed for fragmented DNA) Analysis • Targeted methods: Droplet digital PCR, BEAMing (beads, emulsion, amplification, magnetics) - Focus on individual predefined alterations. Highest sensitivity • Untargeted methods: NGS - Sequencing of large panel of genes. Ability to detect several alterations simultaneously. - High sensitivity with adapted technologies (barcoding) and specific computational pipelines (ex: TecSeq (Targeted error correction Sequencing), TamSeq (Tagged amplicon Sequencing), or CAPP-Seq (CAncer Personalized Profiling by deep Sequencing). | Blood sample collection CTC’s half-life is shorter than ctDNA after blood collection (around 4 h). Blood samples have to be processed quickly. Enrichment • Biophysical features: size, density, electric charges, deformability, invasive capacity • Biological features: expression of epithelial markers and absence of expression of hematopoietic markers (CD45 for example) Detection • Immune-cytological assays (epithelial protein expression) • Genomic assays (epithelial mRNA) • Functional methods Limits: Warrant specific platforms, limiting its use in routine practice. | Isolation • Ultracentrifugation • Density-based separation • Magnetic beads coated with antibodies for surface antigens • Laser-light scattering • Nano-plasmonic sensors Issue: High logistic complexity limiting routine clinical use | Isolation • Commercially available kits based on TRIzol or column-based extraction Detection • qRT-PCR • microarrays • NGS Issue: lack of standardized pre-analytic and analytic procedures |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mari, R.; Mamessier, E.; Lambaudie, E.; Provansal, M.; Birnbaum, D.; Bertucci, F.; Sabatier, R. Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease. Cancers 2019, 11, 774. https://doi.org/10.3390/cancers11060774
Mari R, Mamessier E, Lambaudie E, Provansal M, Birnbaum D, Bertucci F, Sabatier R. Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease. Cancers. 2019; 11(6):774. https://doi.org/10.3390/cancers11060774
Chicago/Turabian StyleMari, Roxane, Emilie Mamessier, Eric Lambaudie, Magali Provansal, Daniel Birnbaum, François Bertucci, and Renaud Sabatier. 2019. "Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease" Cancers 11, no. 6: 774. https://doi.org/10.3390/cancers11060774
APA StyleMari, R., Mamessier, E., Lambaudie, E., Provansal, M., Birnbaum, D., Bertucci, F., & Sabatier, R. (2019). Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease. Cancers, 11(6), 774. https://doi.org/10.3390/cancers11060774






