Pulmonary Carcinoid Surface Receptor Modulation Using Histone Deacetylase Inhibitors
Abstract
1. Introduction
2. Results
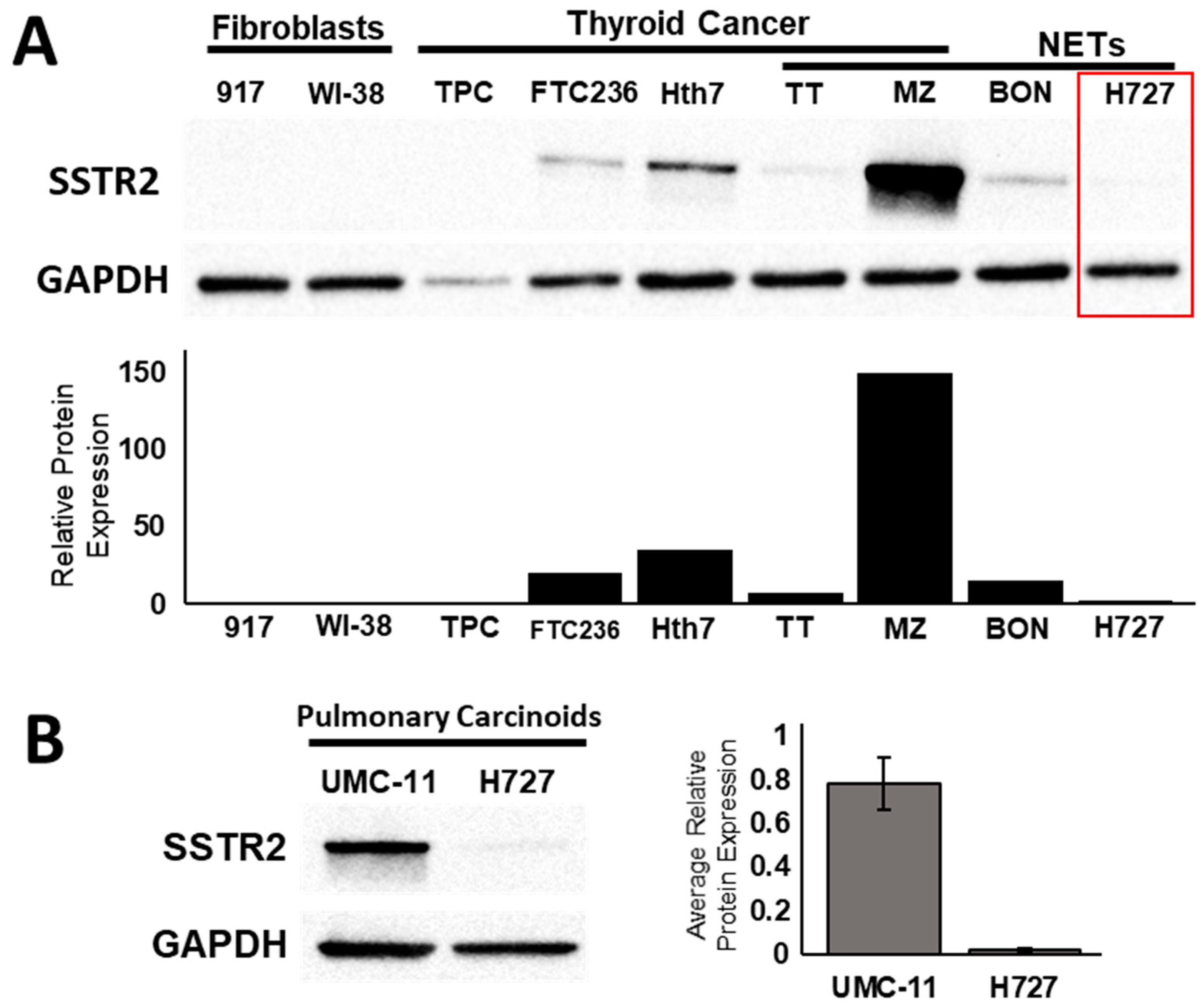
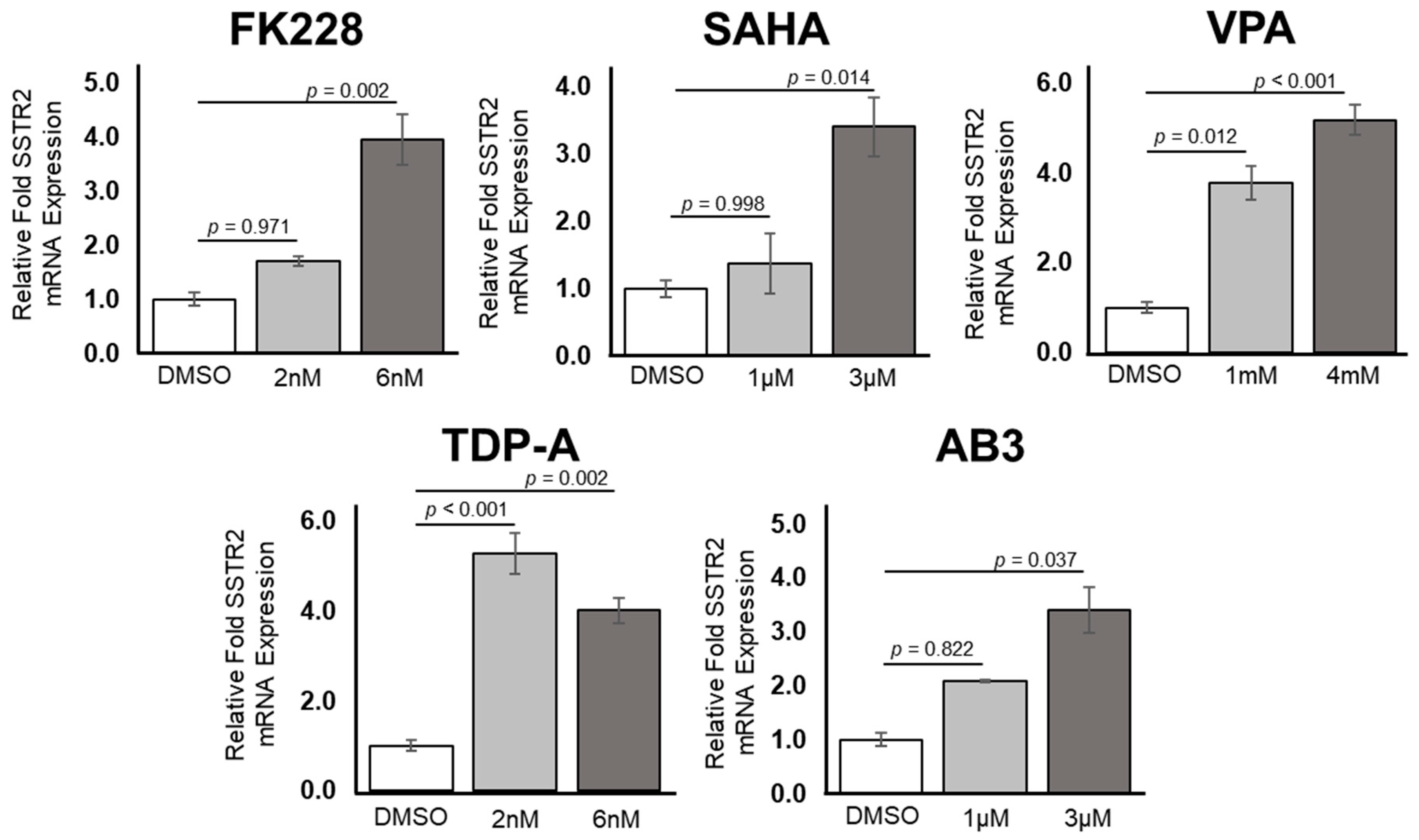
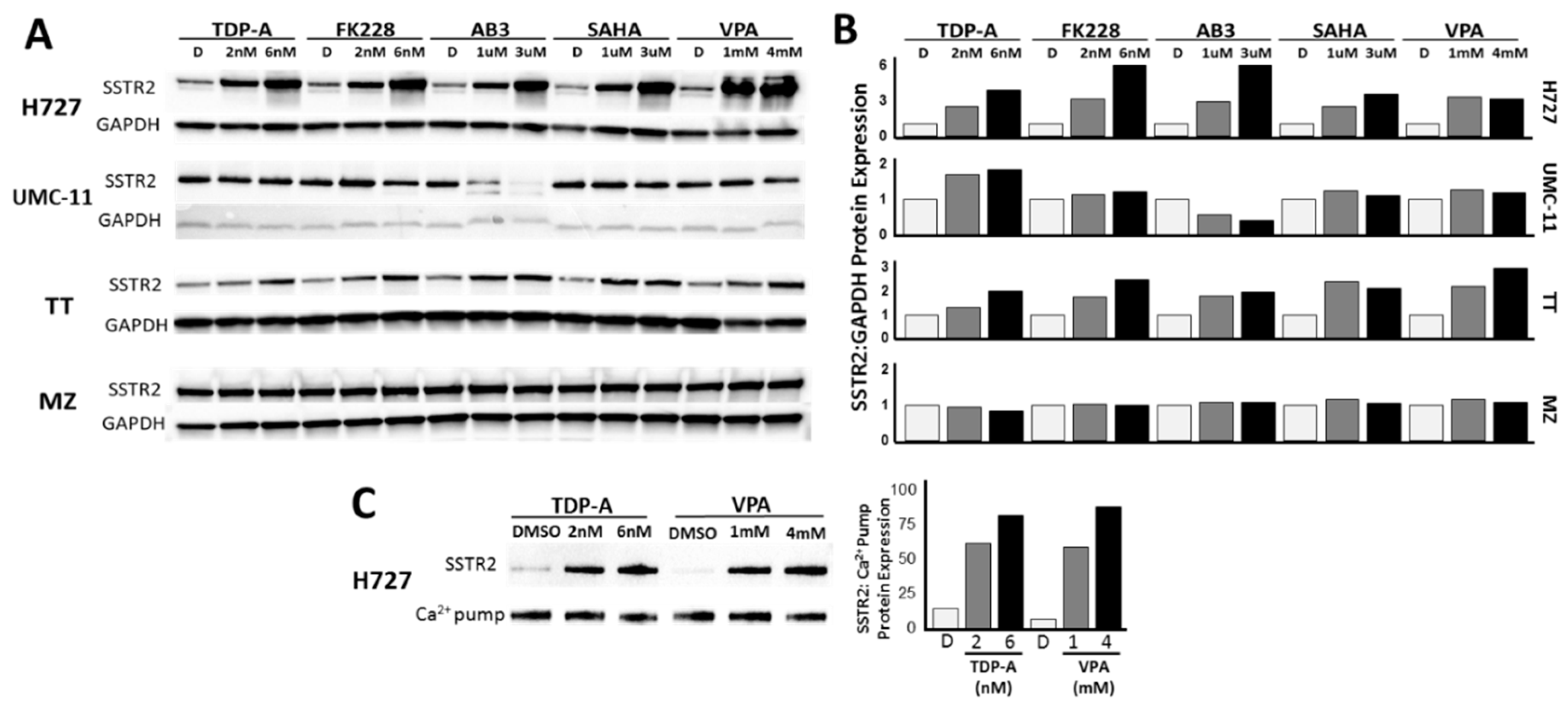
2.1. Transcriptional and Translational Induction of SSTR2
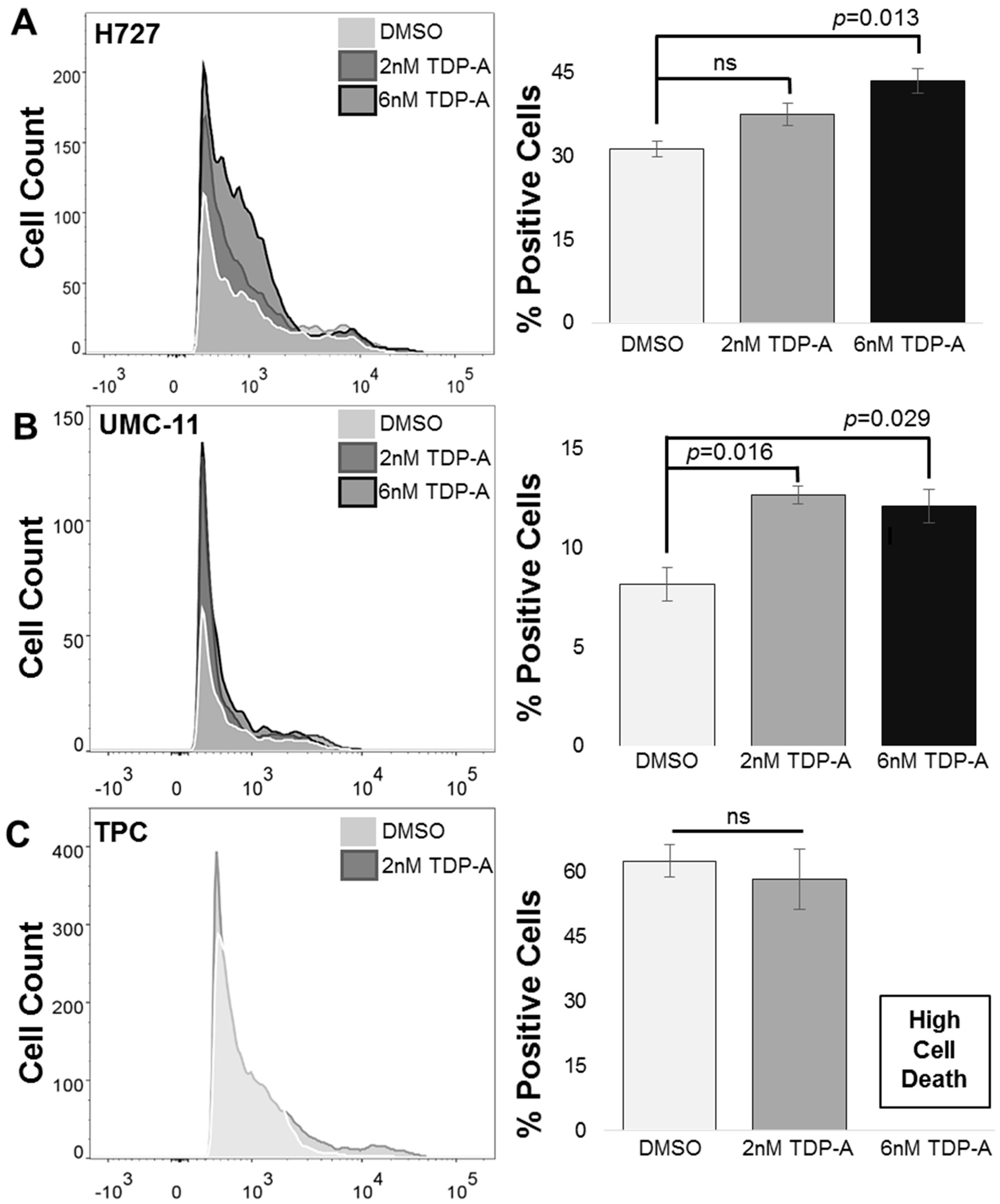
2.2. Detection of Functional SSTR2 Expression in Pulmonary Carcinoids
2.3. Cell Uptake of [68Ga]DOTATATE PET/CT In Vitro
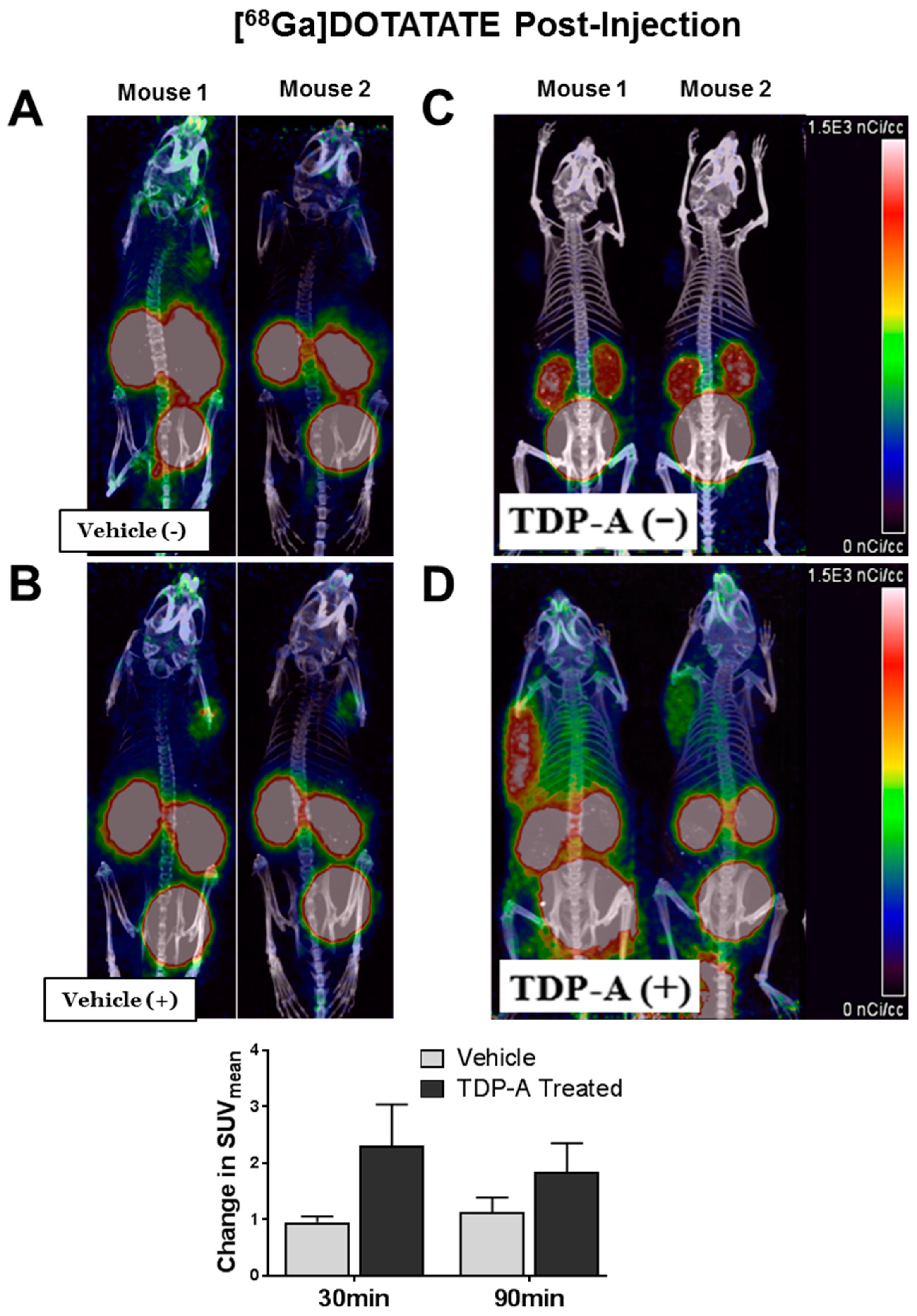
2.4. [68Ga]DOTATATE PET/CT Small Animal Imaging
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Real Time Quantitative PCR
4.3. Western Blot Analysis
4.4. Flow Cytometry
4.5. In Vitro [68Ga]DOTATATE PET/CT Uptake
4.6. Small Animal [68Ga]-DOTATATE PET/CT Imaging
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Travis, W.D. Lung tumours with neuroendocrine differentiation. Eur. J. Cancer 2009, 45, 251–266. [Google Scholar] [CrossRef]
- Chen, L.C.; Travis, W.D.; Krug, L.M. Pulmonary neuroendocrine tumors: What (little) do we know? J. Natl. Compr. Cancer Netw. 2006, 4, 623–630. [Google Scholar] [CrossRef]
- Lim, E. Surigical management of bronchopulmonary and thymic neuroendocrine tumours. Gastroenteropancreatic and thoracic neuroendocrine tumors. BioScienifica 2011, 185–189, in print. [Google Scholar]
- Caplin, M.E.; Pavel, M.; Ruszniewski, P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Otte, A.; Herrmann, R.; Heppeler, A.; Behe, M.; Jermann, E.; Powell, P.; Maecke, H.R.; Muller, J.; Powell, P. Yttrium-90 DOTATOC: First clinical results. Eur. J. Nucl. Med. 1999, 26, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Marincek, N.; Radojewski, P.; Dumont, R.A.; Brunner, P.; Müller-Brand, J.; Maecke, H.R.; Briel, M.; Walter, M.A. Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: Long-term results of a phase II clinical trial. J. Nucl. Med. 2015, 56, 171–176. [Google Scholar] [CrossRef]
- Oberg, K. Molecular imaging radiotherapy: Theranostics for personalized patient management of neuroendocrine tumors (NETs). Theranostics 2012, 2, 448–458. [Google Scholar] [CrossRef]
- Karaca, B.; Degirmenci, M.; Ozveren, A.; Atmaca, H.; Bozkurt, E.; Karabulut, B.; Sanli, U.A.; Uslu, R. Docetaxel in combination with octreotide shows synergistic apoptotic effect by increasing SSTR2 and SSTR5 expression levels in prostate and breast cancer cell lines. Cancer Chemother. Pharmacol. 2015, 75, 1273–1280. [Google Scholar] [CrossRef]
- He, Y.; Yuan, X.; Lei, P. The antiproliferative effects of somatostatin receptor subtype 2 in breast cancer cells. Acta Pharamacol. Sin. 2009, 30, 1053–1059. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin receptor PET ligands—The next generation for clinical practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar]
- Reubi, J.C.; Schar, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Macke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Wild, D.; Macke, H.R.; Waser, B.; Reubi, J.C.; Ginj, M.; Rasch, H.; Muller-Brand, J.; Hofmann, M. 68Ga-DOTANOC: A first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 724. [Google Scholar] [CrossRef]
- Righi, L.; Volante, M.; Tavaglione, V.; Billè, A.; Daniele, L.; Angusti, T.; Inzani, F.; Pelosi, G.; Rindi, G.; Papotti, M. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: A clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann. Oncol. 2010, 21, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Taelman, V.F.; Radojewski, P.; Marincek, N.; Ben-Shlomo, A.; Grotzky, A.; Olariu, C.; Perren, A.; Stettler, C.; Krause, T.; Meier, L.P.; et al. Upregulation of Key Molecules for Targeted Imaging and Therapy. J. Nucl. Med. 2016, 57, 1805–1810. [Google Scholar] [CrossRef]
- Jaskula-Sztul, R.; Eide, J.; Tesfazghi, S.; Dammalapati, A.; Harrison, A.D.; Yu, M.N.; Scheinebeck, C.; Winston-McPherson, G.; Kupcho, K.R.; Robers, M.B.; et al. Tumor suppressor role of Notch3 in Medullary Thyroid Carcinoma revealed by genetic and pharmacological induction. Mol. Cancer Ther. 2015, 14, 499–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daniels, C.E.; Lowe, V.J.; Aubry, M.C.; Allen, M.S.; Jett, J.R. The Utility of Fluorodeoxyglucose Positron Emission Tomography in the Evaluation of Carcinoid Tumors Presenting as Pulmonary Nodules. Chest 2006, 131, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Henkes, L.M.; Doughty, L.B.; He, M.; Wang, D.; Meyer-Almes, F.J.; Cheng, Y.Q. Thailandepsins: Bacterial products with potent histone deacetylase inhibitory activities and broad-spectrum antiproliferative activities. J. Nat. Prod. 2011, 74, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, Y.Q. Thailandepsin A. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o2948–o2949. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.P.; Wang, C.; Ahmad, S.; Vu, M.; Kuma, K.; Cheng, Y.Q.; Lam, K.S. Disulfide cross-linked micelles of novel HDAC inhibitor thailandepsin A for the treatment of breast cancer. Biomaterials 2015, 67, 183–193. [Google Scholar] [CrossRef]
- Weinlander, E.; Somnay, Y.; Harrison, A.D.; Wang, C.; Cheng, Y.Q.; Jaskula-Sztul, R.; Yu, X.M.; Chen, H. The novel histone deacetylase inhibitor thailandepsin A inhibits anaplastic thyroid cancer growth. J. Surg. Res. 2014, 190, 191–197. [Google Scholar] [CrossRef]
- Wilson, A.J.; Cheng, Y.Q.; Khabele, D. Thailandepsins are new small molecule class I HDAC inhibitors with potent cytotoxic activity in ovarian cancer cells: A preclinical study of epigenetic ovarian cancer therapy. J. Ovarian Res. 2012, 5, 12. [Google Scholar] [CrossRef]
- Jang, S.; Janssen, A.; Aburjania, Z.; Robers, M.B.; Harrison, A.; Dammalapati, A.; Cheng, Y.Q.; Chen, H.; Jaskula-Sztul, R. Histone deacetylase inhibitor thailandepsin-A activates Notch signaling and suppresses neuroendocrine cancer cell growth in vivo. Oncotarget 2017, 8, 70828–70840. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, G.; Grimelius, L.; Spathis, A.; Tringidou, R.; Rassidakis, G.Z.; Öberg, K.; Kaltsas, G.; Tsolakis, A.V. Expression of Somatostatin Receptors 1-5 and Dopamine Receptor 2 in Lung Carcinoids: Implications for a Therapeutic Role. Neuroendocrinology 2015, 101, 211–222. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Triple-Peptide Receptor Targeting In Vitro Allows Detection of All Tested Gut and Bronchial NETs. J. Nucl. Med. 2015, 56, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.; Ejeskar, K.; Kogner, P.; Martinsson, T. Gain of chromosome arm 17q is associated with unfavourable prognosis in neuroblastoma, but does not involve mutations in the somatostatin receptor 2 (SSTR2) gene at 17q24. Br. J. Cancer 1999, 81, 1402–1409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daskalakis, K.; Kaltsas, G.; Öberg, K.; Tsolakis, A.V. Lung Carcinoids: Long-Term Surgical Results and the Lack of Prognostic Value of Somatostatin Receptors and Other Novel Immunohistochemical Markers. Neuroendocrinology 2018, 107, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Sestini, R.; Orlando, C.; Peri, A.; Tricarico, C.; Pazzagli, M.; Serio, M.; Pagani, A.; Bussolati, G.; Granchi, S.; Maggi, M. Quantitation of somatostatin receptor type 2 gene expression in neuroblastoma cell lines and primary tumors using competitive reverse transcription-polymerase chain reaction. Clin. Cancer Res. 1996, 2, 1757–1765. [Google Scholar] [PubMed]
- Jaskula-Sztul, R.; Xu, W.; Chen, G.; Harrison, A.; Dammalapati, A.; Nair, R.; Cheng, Y.; Gong, S.; Chen, H. Thailandepsin A-loaded and octreotide-functionalized unimolecular micelles for targeted neuroendocrine cancer therapy. Biomaterials 2016, 91, 1–10. [Google Scholar] [CrossRef]
- Tirosh, A.; Kebebew, E. The utility of 68Ga-DOTATATE positron-emission tomography/computed tomography in the diagnosis, management, follow-up and prognosis of neuroendocrine tumors. Future Oncol. 2018, 14, 111–122. [Google Scholar] [CrossRef]
- Yoon, S.; Eom, G.H. HDAC and HDAC inhibitor: From cancer to cardiovascular diseases. Chonnam. Med. J. 2016, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Somnay, Y.R.; Dull, B.Z.; Eide, J.; Jaskula-Sztul, R.; Chen, H. Chrysin suppresses the achaete-scute complex-like1 and alters the neuroendocrine phenotype of carcinoids. Cancer Gene. Ther. 2015, 22, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Somnay, Y.R.; Yu, X.M.; Lloyd, R.; Leverson, G.; Aburjania, Z.; Jang, S.; Jaskula-Sztul, R.; Chen, H. Notch3 Expression Correlates with Thyroid Cancer Differentiation, Induces Apoptosis, and Predicts Disease Prognosis. Cancer 2017, 123, 769–782. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guenter, R.E.; Aweda, T.; Carmona Matos, D.M.; Whitt, J.; Chang, A.W.; Cheng, E.Y.; Liu, X.M.; Chen, H.; Lapi, S.E.; Jaskula-Sztul, R. Pulmonary Carcinoid Surface Receptor Modulation Using Histone Deacetylase Inhibitors. Cancers 2019, 11, 767. https://doi.org/10.3390/cancers11060767
Guenter RE, Aweda T, Carmona Matos DM, Whitt J, Chang AW, Cheng EY, Liu XM, Chen H, Lapi SE, Jaskula-Sztul R. Pulmonary Carcinoid Surface Receptor Modulation Using Histone Deacetylase Inhibitors. Cancers. 2019; 11(6):767. https://doi.org/10.3390/cancers11060767
Chicago/Turabian StyleGuenter, Rachael E., Tolulope Aweda, Danilea M. Carmona Matos, Jason Whitt, Alexander W. Chang, Eric Y. Cheng, X. Margaret Liu, Herbert Chen, Suzanne E. Lapi, and Renata Jaskula-Sztul. 2019. "Pulmonary Carcinoid Surface Receptor Modulation Using Histone Deacetylase Inhibitors" Cancers 11, no. 6: 767. https://doi.org/10.3390/cancers11060767
APA StyleGuenter, R. E., Aweda, T., Carmona Matos, D. M., Whitt, J., Chang, A. W., Cheng, E. Y., Liu, X. M., Chen, H., Lapi, S. E., & Jaskula-Sztul, R. (2019). Pulmonary Carcinoid Surface Receptor Modulation Using Histone Deacetylase Inhibitors. Cancers, 11(6), 767. https://doi.org/10.3390/cancers11060767



