Gene Expression and miRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer
Abstract
1. Introduction
2. Human Epidermal Growth Factor Receptor (EGFR) Family
3. HER2-Positive Breast Cancer
4. Treatment of HER2-Positive Breast Cancer
5. Gene Expression Profiling of HER2-Positive Breast Cancer
6. MicroRNA
7. MicroRNA and HER2-Positive Breast Cancer
8. Clinical Relevance of miRNAs in HER2-Positive Breast Cancer
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Int. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef]
- Eroles, P.; Bosch, A.; Pérez-fidalgo, J.A.; Lluch, A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat. Rev. 2012, 38, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, J.L.; Gammon, M.D.; John, E.M. Reproductive factors and breast cancer. Epidemiol. Rev. 1993, 15, 36–47. [Google Scholar] [CrossRef]
- Beral, V.; Reeves, G. Childbearing, oral contraceptive use, and breast cancer. Lancet 1993, 341, 1102. [Google Scholar] [CrossRef]
- Engin, A. Obesity-associated breast cancer: Analysis of risk factors. In Obesity and lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 571–606. [Google Scholar]
- Key, T.J.; Verkasalo, P.K. Endogenous hormones and the aetiology of breast cancer. Breast Cancer Res. 1999, 1, 18–21. [Google Scholar] [CrossRef]
- Key, T.J.; Verkasalo, P.K.; Banks, E. Epidemiology of breast cancer. Lancet Oncol. 2001, 2, 133–140. [Google Scholar] [CrossRef]
- Schnitt, S.J. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod. Pathol. 2010, 23, 60–64. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Pusztai, L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet 2011, 378, 1812–1823. [Google Scholar] [CrossRef]
- Dai, X.; Chen, A.; Bai, Z. Integrative investigation on breast cancer in ER, PR and HER2-defined subgroups using mRNA and miRNA expression profiling. Sci. Rep. 2014, 4, 6566. [Google Scholar] [CrossRef]
- Iwamoto, T.; Pusztai, L. Predicting prognosis of breast cancer with gene signatures: Are we lost in a sea of data? Genome Med. 2010, 2, 2–5. [Google Scholar] [CrossRef]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Blenkiron, C.; Goldstein, L.D.; Thorne, N.P.; Spiteri, I.; Chin, S.-F.; Dunning, M.J.; Miska, E.A. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007, 8, R214. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Nath, J.; Bandyopadhyay, S. MicroRNA signatures highlight new breast cancer subtypes. Gene 2015, 556, 192–198. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Asif, H.; Sultana, S.; Ahmed, S.; Akhtar, N.; Tariq, M. HER-2 positive breast cancer—A mini-review. Asian Pac. J. Cancer Prev. 2016, 17, 1609–1615. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef]
- Chien, A.J.; Rugo, H.S. Tyrosine kinase inhibitors for human epidermal growth factor receptor 2–positive metastatic breast cancer: Is personalizing therapy within reach? J. Clin. Oncol. 2017, 35, 3089–3091. [Google Scholar] [CrossRef]
- Brandt-Rauf, P.W.; Pincus, M.R.; Carney, W.P. The c-ERBB-2 protein in oncogenesis: Molecular structure to molecular epidemiology. Crit. Rev. Oncog. 1994, 5, 313–329. [Google Scholar] [CrossRef]
- Zhongren, Z.; Hick, D.G. HER2 amplification or overexpression in upper gi tract and breast cancer with clinical diagnosis and treatment. In Oncogene and Cancer- From Bench to Clinic; Yahwardiah, S., Ed.; IntechOpen: London, UK, 2013; pp. 68–90. ISBN 978-953-51-0858-0. [Google Scholar]
- Bertelsen, V.; Stang, E. The mysterious ways of ERBB2/HER2 trafficking. Membranes 2014, 4, 424–446. [Google Scholar] [CrossRef] [PubMed]
- Larionov, A.A. Current therapies for human epidermal growth factor receptor 2-positive metastatic breast cancer patients. Front. Oncol. 2018, 8, 89. [Google Scholar] [CrossRef]
- Connell, C.M.; Doherty, G.J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2017, 2, e000279. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Press, M.; Cordon-Cardo, C.; Slamon, D.J. Expression of the HER-2/NEU proto-oncogene in normal human adult and fetal tissues. Oncogene 1990, 5, 953–962. [Google Scholar] [PubMed]
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Nielsen, K.B.; Kjærsgaard, G.; Jepsen, A.; Mollerup, J. Gene signal distribution and HER2 amplification in gastroesophageal cancer. J. Cancer 2017, 8, 1517–1524. [Google Scholar] [CrossRef]
- Honarvar, H.; Calce, E.; Doti, N.; Langella, E.; Orlova, A.; Buijs, J.; D’Amato, V.; Bianco, R.; Saviano, M.; Tolmachev, V.; et al. Evaluation of HER2-specific peptide ligand for its employment as radiolabeled imaging probe. Sci. Rep. 2018, 8, 2998. [Google Scholar] [CrossRef]
- Langdon, S. Targeting HER2-driven cancers in non-breast cancer malignancies. J. Mol. Biomark. Diagn 2012, 3, e105. [Google Scholar] [CrossRef]
- Nassar, A.; Khoor, A.; Radhakrishnan, R.; Radhakrishnan, A.; Cohen, C. Correlation of HER2 overexpression with gene amplification and its relation to chromosome 17 aneuploidy: A 5-year experience with invasive ductal and lobular carcinomas. Int. J. Clin. Exp. Pathol. 2014, 7, 6254–6261. [Google Scholar]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mtor in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef]
- Serra, V.; Scaltriti, M.; Prudkin, L.; Eichhorn, P.J.A.; Ibrahim, Y.H.; Chandarlapaty, S.; Markman, B.; Rodriguez, O.; Guzman, M.; Rodriguez, S.; et al. Pi3k inhibition results in enhanced her signaling and acquired erk dependency in HER2-overexpressing breast cancer. Oncogene 2011, 30, 2547–2557. [Google Scholar] [CrossRef]
- Yarden, Y.; Shilo, B.-Z. Snapshot: EGFR signaling pathway. Cell 2007, 131, 1018. [Google Scholar] [CrossRef]
- Wülfing, P.; Borchard, J.; Buerger, H.; Heidl, S.; Zänker, K.S.; Kiesel, L.; Brandt, B. HER2-positive circulating tumor cells indicate poor clinical outcome in stage i to iii breast cancer patients. Clin. Cancer Res. 2006, 12, 1715–1720. [Google Scholar] [CrossRef]
- Hayes, D.F.; Walker, T.M.; Singh, B.; Vitetta, E.S.; Uhr, J.W.; Gross, S.; Rao, C.; Doyle, G.V.; Terstappen, L.W. Monitoring expression of her-2 on circulating epithelial cells in patients with advanced breast cancer. Int. J. Oncol. 2002, 21, 1111–1117. [Google Scholar] [CrossRef]
- Meng, S.; Tripathy, D.; Shete, S.; Ashfaq, R.; Haley, B.; Perkins, S.; Beitsch, P.; Khan, A.; Euhus, D.; Osborne, C.; et al. Her-2 gene amplification can be acquired as breast cancer progresses. Proc. Natl. Acad. Sci. USA 2004, 101, 9393–9398. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chang, C.-J.; Yeh, K.-Y.; Chang, P.-H.; Huang, J.-S. The prognostic value of HER2-positive circulating tumor cells in breast cancer patients: A systematic review and meta-analysis. Clin. Breast Cancer 2017, 17, 341–349. [Google Scholar] [CrossRef]
- Petrelli, F.; Tomasello, G.; Barni, S.; Lonati, V.; Passalacqua, R.; Ghidini, M. Clinical and pathological characterization of HER2 mutations in human breast cancer: A systematic review of the literature. Breast Cancer Res. Treat. 2017, 166, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, W.S.; Xiao, N.; Bender, R.; Ghazalpour, A.; Tan, Z.; Swensen, J.; Millis, S.Z.; Basu, G.; Gatalica, Z.; et al. Mutations in the kinase domain of the HER2/ERBB2 gene identified in a wide variety of human cancers. JMD 2015, 17, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discovery 2013, 3, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.; Sheng, S.; Yuan, H.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017, 108, 671–677. [Google Scholar] [CrossRef]
- Burstein, H.J. The distinctive nature of HER2-positive breast cancers. N. Engl. J. Med. 2005, 353, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother. Res. and Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef]
- Azim, H.; Piccart, M. Simultaneous targeting of estrogen receptor and HER2 in breast cancer. Expert Rev. Anticancer Ther. 2010, 10, 1255–1263. [Google Scholar] [CrossRef]
- Massarweh, S.; Schiff, R. Unraveling the mechanisms of endocrine resistance in breast cancer: New therapeutic opportunities. Clin. Cancer Res. 2007, 13, 1950–1954. [Google Scholar] [CrossRef]
- Arpino, G.; Wiechmann, L.; Osborne, C.K.; Schiff, R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocr. Rev. 2008, 29, 217–233. [Google Scholar] [CrossRef]
- Lousberg, L.; Collignon, J.; Jerusalem, G. Resistance to therapy in estrogen receptor positive and human epidermal growth factor 2 positive breast cancers: Progress with latest therapeutic strategies. Ther. Adv. Med. Oncol. 2016, 8, 429–449. [Google Scholar] [CrossRef]
- Marty, M.; Cognetti, F.; Maraninchi, D.; Snyder, R.; Mauriac, L.; Tubiana-Hulin, M.; Chan, S.; Grimes, D.; Antón, A.; Lluch, A.; et al. Randomized phase ii trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: The m77001 study group. J. Clin. Oncol. 2005, 23, 4265–4274. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef]
- Peddi, P.F.; Hurvitz, S.A. Ado-trastuzumab emtansine (t-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: From early scientific development to foundation of care. Am. J. Clin. Oncol. 2010, 33, 186–195. [Google Scholar] [CrossRef]
- Cappelletti, V.; Appierto, V.; Tiberio, P.; Fina, E.; Callari, M.; Daidone, M.G. Circulating biomarkers for prediction of treatment response. JNCI Monogr. 2015, 2015, 60–63. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Kerin, M.J. MicroRNAs as novel biomarkers for breast cancer. J. Oncol. 2010, 2009, 950201. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Sotiriou, C.; Pusztai, L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009, 360, 790–800. [Google Scholar] [CrossRef]
- Weigelt, B.; Baehner, F.L.; Reis-Filho, J.S. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: A retrospective of the last decade. J. Pathol. 2010, 220, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Vincent-Salomon, A.; Pivot, X.; Sertier, A.-S.; Thomas, E.; Tonon, L.; Boyault, S.; Mulugeta, E.; Treilleux, I.; MacGrogan, G.; et al. A whole-genome sequence and transcriptome perspective on HER2-positive breast cancers. Nat. Commun. 2016, 7, 12222. [Google Scholar] [CrossRef]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [Google Scholar]
- Darb-Esfahani, S.; Denkert, C.; Stenzinger, A.; Salat, C.; Sinn, B.; Schem, C.; Endris, V.; Klare, P.; Schmitt, W.; Blohmer, J.-U.; et al. Role of tp53 mutations in triple negative and HER2-positive breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy. Oncotarget 2016, 7, 67686–67698. [Google Scholar] [CrossRef]
- Rahmatpanah, F.B.; Jia, Z.; Chen, X.; Char, J.E.; Men, B.; Franke, A.-C.; Jones, F.E.; McClelland, M.; Mercola, D. A class of genes in the HER2 regulon that is poised for transcription in breast cancer cell lines and expressed in human breast tumors. Oncotarget 2014, 6, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-H.; Liu, Z.-B.; Yang, C.; Qin, W.; Shao, Z.-M. Expression of dickkopf-1 and beta-catenin related to the prognosis of breast cancer patients with triple negative phenotype. PLoS ONE 2012, 7, e37624. [Google Scholar] [CrossRef] [PubMed]
- Kasoha, M.; Bohle, R.M.; Seibold, A.; Gerlinger, C.; Juhasz-Böss, I.; Solomayer, E.-F. Dickkopf-1 (dkk1) protein expression in breast cancer with special reference to bone metastases. Clin. Exp. Metastasis 2018, 35, 763–775. [Google Scholar] [CrossRef]
- McGowan, P.M.; Duffy, M.J. Matrix metalloproteinase expression and outcome in patients with breast cancer: Analysis of a published database. Ann. Oncol. 2008, 19, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, S.M.; Dasari, P.; Walsh, D.; Townsend, A.R.; Price, T.J.; Ingman, W.V. Hormonal modulation of breast cancer gene expression: Implications for intrinsic subtyping in premenopausal women. Front. Oncol. 2016, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Mohammadizadeh, F.; Hani, M.; Ranaee, M.; Bagheri, M. Role of cyclin d1 in breast carcinoma. J. Res. Med. Sci. 2013, 18, 1021–1025. [Google Scholar]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Melhem-Bertrandt, A.; Bojadzieva, J.; Ready, K.J.; Obeid, E.; Liu, D.D.; Gutierrez-Barrera, A.M.; Litton, J.K.; Olopade, O.I.; Hortobagyi, G.N.; Strong, L.C.; et al. Early onset HER2-positive breast cancer is associated with germline tp53 mutations. Cancer 2012, 118, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.H.; Kim, H.S.; Lee, A.; Song, B.J.; Chae, B.J. BCL2 as a subtype-specific prognostic marker for breast cancer. J. Breast Cancer 2016, 19, 252–260. [Google Scholar] [CrossRef]
- Hsu, J.L.; Hung, M.-C. The role of HER2, egfr, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016, 35, 575–588. [Google Scholar] [CrossRef]
- Stern, H.M.; Gardner, H.; Burzykowski, T.; Elatre, W.; O’Brien, C.; Lackner, M.R.; Pestano, G.A.; Santiago, A.; Villalobos, I.; Eiermann, W.; et al. Pten loss is associated with worse outcome in HER2-amplified breast cancer patients but is not associated with trastuzumab resistance. Clin. Cancer Res. 2015, 21, 2065–2074. [Google Scholar] [CrossRef]
- Cizkova, M.; Vacher, S.; Meseure, D.; Trassard, M.; Susini, A.; Mlcuchova, D.; Callens, C.; Rouleau, E.; Spyratos, F.; Lidereau, R.; et al. Pik3r1 underexpression is an independent prognostic marker in breast cancer. BMC Cancer 2013, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Rangel, N.; Villegas, V.E.; Rondón-Lagos, M. Profiling of gene expression regulated by 17β-estradiol and tamoxifen in estrogen receptor-positive and estrogen receptor-negative human breast cancer cell lines. Breast Cancer 2017, 9, 537–550. [Google Scholar]
- Wirapati, P.; Sotiriou, C.; Kunkel, S.; Farmer, P.; Pradervand, S.; Haibe-Kains, B.; Desmedt, C.; Ignatiadis, M.; Sengstag, T.; Schütz, F.; et al. Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008, 10, R65. [Google Scholar] [CrossRef]
- Savci-Heijink, C.D.; Halfwerk, H.; Koster, J.; Horlings, H.M.; van de Vijver, M.J. A specific gene expression signature for visceral organ metastasis in breast cancer. BMC Cancer 2019, 19, 333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, W.; Cheng, H. Co-expression network analysis of gene expression profiles of HER2+ breast cancer-associated brain metastasis. Oncol. Lett. 2018, 16, 7008–7019. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.D.; Carey, L.A.; Ahmed, A.A.; Caldas, C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J. Clin. Oncol. 2005, 23, 7350–7360. [Google Scholar] [CrossRef]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Hauptmann, M.; et al. A functional genetic approach identifies the pi3k pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Taveira, M.; Nabavi, S.; Wang, Y.; Tonellato, P.; Esteva, F.J.; Cantley, L.C.; Wulf, G.M. Genomic characteristics of trastuzumab-resistant HER2-positive metastatic breast cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Lan, K.-H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. Pten activation contributes to tumor inhibition by trastuzumab, and loss of pten predicts trastuzumab resistance in patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef]
- Morrow, P.K.; Wulf, G.M.; Ensor, J.; Booser, D.J.; Moore, J.A.; Flores, P.R.; Xiong, Y.; Zhang, S.; Krop, I.E.; Winer, E.P.; et al. Phase i/ii study of trastuzumab in combination with everolimus (rad001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J. Clin. Oncol. 2011, 29, 3126–3132. [Google Scholar] [CrossRef]
- Omarini, C.; Bettelli, S.; Caprera, C.; Manfredini, S.; Barbolini, M.; Moscetti, L.; Isca, C.; Toss, A.; Barbieri, E.; Cortesi, L.; et al. Differential molecular pathways expression in HER2 positive early breast cancer according to hormone receptor status. J. Cancer Res. Clin. Oncol. 2019, 145, 821–828. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The c. Elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef]
- Mendell, J.T.; Olson, E.N. MicroRNAs in stress signaling and human disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef]
- Song, S.; Ajani, J.A. The role of microRNAs in cancers of the upper gastrointestinal tract. Nature Rev. Gastroenterol. Hepatol. 2013, 10, 109–118. [Google Scholar] [CrossRef]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. Oncomir or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef]
- Kahraman, M.; Röske, A.; Laufer, T.; Fehlmann, T.; Backes, C.; Kern, F.; Kohlhaas, J.; Schrörs, H.; Saiz, A.; Zabler, C.; et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci. Rep. 2018, 8, 11584. [Google Scholar] [CrossRef]
- Van Schooneveld, E.; Wouters, M.C.; Van der Auwera, I.; Peeters, D.J.; Wildiers, H.; Van Dam, P.A.; Vergote, I.; Vermeulen, P.B.; Dirix, L.Y.; Van Laere, S.J. Expression profiling of cancerous and normal breast tissues identifies microRNAs that are differentially expressed in serum from patients with (metastatic) breast cancer and healthy volunteers. Breast Cancer Res. 2012, 14, R34. [Google Scholar] [CrossRef]
- Dave, V.P.; Ngo, T.A.; Pernestig, A.-K.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Bang, D.D. MicroRNA amplification and detection technologies: Opportunities and challenges for point of care diagnostics. Lab. Invest. 2019, 99, 452–469. [Google Scholar] [CrossRef]
- Kost, G.J.; Tran, N.K.; Louie, R.F. Point-of-care testing: Principles, practice, and critical-emergency-disaster medicine. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Huang, T.; Yang, J.; Liu, G.; Jin, W.; Liu, Z.; Zhao, S.; Yao, M. Quantification of mature microRNAs using pincer probes and real-time pcr amplification. PLoS ONE 2015, 10, e0120160. [Google Scholar] [CrossRef] [PubMed]
- Vedarethinam, I.; Shah, P.; Dimaki, M.; Tumer, Z.; Tommerup, N.; Svendsen, W.E. Metaphase fish on a chip: Miniaturized microfluidic device for fluorescence in situ hybridization. Sensors 2010, 10, 9831–9846. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Yang, Z.; Lu, L.; Yin, H.; Ai, S. Electrochemical biosensor for microRNA detection based on hybridization protection against nuclease s1 digestion. J. Solid State Electrochem. 2016, 20, 413–419. [Google Scholar] [CrossRef]
- Kian, R.; Moradi, S.; Ghorbian, S. Role of components of microRNA machinery in carcinogenesis. Exp. Oncol. 2018, 40, 2–9. [Google Scholar] [CrossRef]
- Van Schooneveld, E.; Wildiers, H.; Vergote, I.; Vermeulen, P.B.; Dirix, L.Y.; Van Laere, S.J. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015, 17, 21. [Google Scholar] [CrossRef]
- Inns, J.; James, V. Circulating microRNAs for the prediction of metastasis in breast cancer patients diagnosed with early stage disease. Breast 2015, 24, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Serpico, D.; Molino, L.; Di Cosimo, S. MicroRNAs in breast cancer development and treatment. Cancer Treat. Rev. 2014, 40, 595–604. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Atasoy, U.; Sauter, E.R. Circulating microRNAs in breast cancer and healthy subjects. BMC Res. Notes 2009, 2, 89. [Google Scholar] [CrossRef]
- Freiesleben, S.; Hecker, M.; Zettl, U.K.; Fuellen, G.; Taher, L. Analysis of microRNA and gene expression profiles in multiple sclerosis: Integrating interaction data to uncover regulatory mechanisms. Sci. Rep. 2016, 6, 34512. [Google Scholar] [CrossRef]
- Khalighfard, S.; Alizadeh, A.M.; Irani, S.; Omranipour, R. Plasma Mir-21, Mir-155, Mir-10b, and Let-7a as the potential biomarkers for the monitoring of breast cancer patients. Sci. Rep. 2018, 8, 17981. [Google Scholar] [CrossRef]
- Fkih M’hamed, I.; Privat, M.; Trimeche, M.; Penault-Llorca, F.; Bignon, Y.-J.; Kenani, A. Mir-10b, Mir-26a, Mir-146a and Mir-153 expression in triple negative vs. non triple negative breast cancer: Potential biomarkers. Pathol. Oncol. Res. 2017, 23, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, H.; Shah, N.; Patel, Y.; Chen, H. Identification of new miRNA biomarkers associated with HER2-positive breast cancers. Oncoscience 2015, 2, 924–929. [Google Scholar]
- Uhlmann, S.; Mannsperger, H.; Zhang, J.D.; Horvat, E.-Á.; Schmidt, C.; Küblbeck, M.; Henjes, F.; Ward, A.; Tschulena, U.; Zweig, K.; et al. Global microRNA level regulation of egfr-driven cell-cycle protein network in breast cancer. Mol. Syst. Biol. 2012, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Leivonen, S.-K.; Sahlberg, K.K.; Mäkelä, R.; Due, E.U.; Kallioniemi, O.; Børresen-Dale, A.-L.; Perälä, M. High-throughput screens identify microRNAs essential for HER2 positive breast cancer cell growth. Mol. Oncol. 2014, 8, 93–104. [Google Scholar] [CrossRef]
- Mattie, M.D.; Benz, C.C.; Bowers, J.; Sensinger, K.; Wong, L.; Scott, G.K.; Fedele, V.; Ginzinger, D.; Getts, R.; Haqq, C. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol. Cancer 2006, 5, 24. [Google Scholar] [CrossRef]
- Lowery, A.J.; Miller, N.; Devaney, A.; McNeill, R.E.; Davoren, P.A.; Lemetre, C.; Benes, V.; Schmidt, S.; Blake, J.; Ball, G.; et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/NEU receptor status in breast cancer. Breast cancer Res. 2009, 11, R27. [Google Scholar] [CrossRef]
- Grelier, G.; Voirin, N.; Ay, A.S.; Cox, D.G.; Chabaud, S.; Treilleux, I.; Léon-Goddard, S.; Rimokh, R.; Mikaelian, I.; Venoux, C.; et al. Prognostic value of dicer expression in human breast cancers and association with the mesenchymal phenotype. Br. J. Cancer 2009, 101, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Dedes, K.J.; Natrajan, R.; Lambros, M.B.; Geyer, F.C.; Lopez-Garcia, M.A.; Savage, K.; Jones, R.L.; Reis-Filho, J.S. Down-regulation of the miRNA master regulators drosha and dicer is associated with specific subgroups of breast cancer. Eur. J. Cancer 2011, 47, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Cava, C.; Bertoli, G.; Ripamonti, M.; Mauri, G.; Zoppis, I.; Della Rosa, P.A.; Gilardi, M.C.; Castiglioni, I. Integration of mrna expression profile, copy number alterations, and microRNA expression levels in breast cancer to improve grade definition. PLoS ONE 2014, 9, e97681. [Google Scholar] [CrossRef]
- Du, F.; Yuan, P.; Zhao, Z.T.; Yang, Z.; Wang, T.; Zhao, J.D.; Luo, Y.; Ma, F.; Wang, J.Y.; Fan, Y.; et al. A miRNA-based signature predicts development of disease recurrence in HER2 positive breast cancer after adjuvant trastuzumab-based treatment. Sci. Rep. 2016, 6, 33825. [Google Scholar] [CrossRef]
- Yin, Q.-W.; Sun, X.-F.; Yang, G.-T.; Li, X.-B.; Wu, M.-S.; Zhao, J. Increased expression of microRNA-150 is associated with poor prognosis in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 842–846. [Google Scholar]
- Sugita, B.M.; Zabala, Y.; Fonseca, A.; Almeida, R.; Gusev, Y.; Boca, S.; Cavalli, I.J.; Ribeiro, E.M.; Cavalli, L.R. Abstract 3431: The oncogenic role of Mir-150-5p in triple-negative breast cancer. Cancer Res. 2017, 77, 3431. [Google Scholar] [CrossRef]
- Persson, H.; Kvist, A.; Rego, N.; Staaf, J.; Vallon-Christersson, J.; Luts, L.; Loman, N.; Jonsson, G.; Naya, H.; Hoglund, M.; et al. Identification of new microRNAs in paired normal and tumor breast tissue suggests a dual role for the ERBB2/HER2 gene. Cancer Res. 2011, 71, 78–86. [Google Scholar] [CrossRef]
- Brow, D.A.; Guthrie, C. Spliceosomal rna u6 is remarkably conserved from yeast to mammals. Nature 1988, 334, 213–218. [Google Scholar] [CrossRef]
- Zohreh, R.; Ahmadreza, S.; Mohammad, K.-T.D.; Kazem, D. Involvement of the dysregulation of Mir-23b-3p, Mir-195-5p, Mir-656-5p, and Mir-340-5p in trastuzumab resistance of HER2-positive breast cancer cells and system biology approach to predict their targets involved in resistance. DNA Cell Biol. 2019, 38, 184–192. [Google Scholar]
- Wang, B.; Zhang, Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J. Cancer Res. Clin. Oncol. 2012, 138, 1659–1666. [Google Scholar] [CrossRef]
- Han, J.-G.; Jiang, Y.-D.; Zhang, C.-H.; Yang, Y.-M.; Pang, D.; Song, Y.-N.; Zhang, G.-Q. A novel panel of serum Mir-21/Mir-155/Mir-365 as a potential diagnostic biomarker for breast cancer. Ann. Surg. Treat. Res. 2017, 92, 55–66. [Google Scholar] [CrossRef]
- Fujita, S.; Ito, T.; Mizutani, T.; Minoguchi, S.; Yamamichi, N.; Sakurai, K.; Iba, H. Mir-21 gene expression triggered by ap-1 is sustained through a double-negative feedback mechanism. J. Mol. Biol. 2008, 378, 492–504. [Google Scholar] [CrossRef] [PubMed]
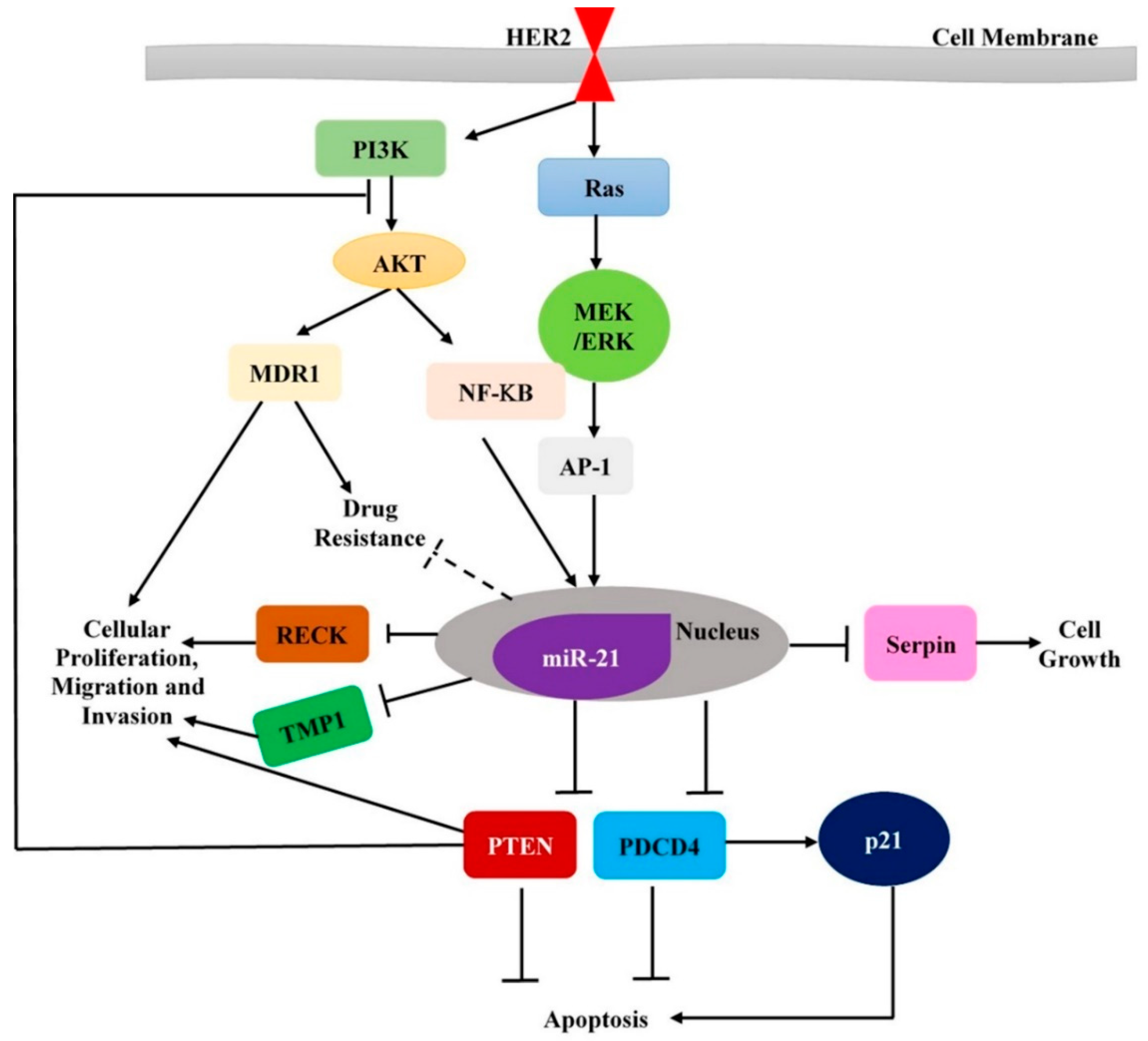
- Pan, X.; Wang, Z.X.; Wang, R. MicroRNA-21: A novel therapeutic target in human cancer. Cancer Biol. Ther. 2010, 10, 1224–1232. [Google Scholar] [CrossRef]
- Huang, T.-H.; Wu, F.; Loeb, G.B.; Hsu, R.; Heidersbach, A.; Brincat, A.; Horiuchi, D.; Lebbink, R.J.; Mo, Y.-Y.; Goga, A.; et al. Up-regulation of Mir-21 by HER2/NEU signaling promotes cell invasion. J. Biol. Chem. 2009, 284, 18515–18524. [Google Scholar] [CrossRef] [PubMed]
- Frankel, L.B.; Christoffersen, N.R.; Jacobsen, A.; Lindow, M.; Krogh, A.; Lund, A.H. Programmed cell death 4 (pdcd4) is an important functional target of the microRNA Mir-21 in breast cancer cells. J. Biol. Chem. 2008, 283, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Si, M.-L.; Wu, H.; Mo, Y.-Y. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (tpm1). J. Biol. Chem. 2007, 282, 14328–14336. [Google Scholar] [CrossRef]
- Asangani, I.A.; Rasheed, S.A.K.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (Mir-21) post-transcriptionally downregulates tumor suppressor pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2007, 27, 2128–2136. [Google Scholar] [CrossRef]
- Göke, R.; Barth, P.; Schmidt, A.; Samans, B.; Lankat-Buttgereit, B. Programmed cell death protein 4 suppresses cdk1/cdc2 via induction of p21waf1/cip1. Am. J. Physiol. Cell Physiol. 2004, 287, C1541. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Bottai, G.; Nuciforo, P.G.; Di Tommaso, L.; Giovannetti, E.; Peg, V.; Losurdo, A.; Pérez-Garcia, J.; Masci, G.; Corsi, F.; et al. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget 2015, 6, 37269–37280. [Google Scholar] [CrossRef]
- Davis, B.N.; Hilyard, A.C.; Lagna, G.; Hata, A. Smad proteins control drosha-mediated microRNA maturation. Nature 2008, 454, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fang, M.; Li, S.; Yan, Y.; Zhong, Y.; Du, B. Mir-21 is involved in transforming growth factor β1-induced chemoresistance and invasion by targeting pten in breast cancer. Oncol. Lett. 2017, 14, 6929–6936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hatley, M.E.; Patrick, D.M.; Garcia, M.R.; Richardson, J.A.; Bassel-Duby, R.; van Rooij, E.; Olson, E.N. Modulation of k-ras-dependent lung tumorigenesis by microRNA-21. Cancer Cell 2010, 18, 282–293. [Google Scholar] [CrossRef]
- Fong, S.; Itahana, Y.; Sumida, T.; Singh, J.; Coppe, J.-P.; Liu, Y.; Richards, P.C.; Bennington, J.L.; Lee, N.M.; Debs, R.J.; et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. USA 2003, 100, 13543–13548. [Google Scholar] [CrossRef]
- Migliaccio, A.; Piccolo, D.; Castoria, G.; Di Domenico, M.; Bilancio, A.; Lombardi, M.; Gong, W.; Beato, M.; Auricchio, F. Activation of the src/p21ras/erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998, 17, 2008–2018. [Google Scholar] [CrossRef]
- Quinn, J.A.; Bland, K.I.; Filardo, E.J.; Frackelton, A.R., Jr. Estrogen-induced activation of erk-1 and erk-2 requires the g protein-coupled receptor homolog, gpr30, and occurs via trans-activation of the epidermal growth factor receptor through release of hb-egf. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar]
- Niu, J.; Shi, Y.; Tan, G.; Yang, C.H.; Fan, M.; Pfeffer, L.M.; Wu, Z.-H. DNA damage induces nf-κb-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. J. Biol. Chem. 2012, 287, 21783–21795. [Google Scholar] [CrossRef]
- Gong, C.; Yao, Y.; Wang, Y.; Liu, B.; Wu, W.; Chen, J.; Su, F.; Yao, H.; Song, E. Up-regulation of Mir-21 mediates resistance to trastuzumab therapy for breast cancer. J. biol. Chem. 2011, 286, 19127–19137. [Google Scholar] [CrossRef]
- Huang, X.; Le, Q.-T.; Giaccia, A.J. Mir-210--micromanager of the hypoxia pathway. Trends Mol. Med. 2010, 16, 230–237. [Google Scholar] [CrossRef]
- Liu, D.; Xia, H.; Wang, F.; Chen, C.; Long, J. MicroRNA-210 interacts with fbxo31 to regulate cancer proliferation cell cycle and migration in human breast cancer. OncoTargets Ther. 2016, 9, 5245–5255. [Google Scholar]
- Graveel, C.; Calderone, H.; Westerhuis, J.; Winn, M.; Sempere, L. Critical analysis of the potential for microRNA biomarkers in breast cancer management. Breast Cancer (Dove Med. Press) 2015, 7, 59–79. [Google Scholar]
- Volinia, S.; Galasso, M.; Sana, M.E.; Wise, T.F.; Palatini, J.; Huebner, K.; Croce, C.M. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proc. Natl. Acad. Sci. USA 2012, 109, 3024–3029. [Google Scholar] [CrossRef]
- Jung, E.-J.; Santarpia, L.; Kim, J.; Esteva, F.J.; Moretti, E.; Buzdar, A.U.; Di Leo, A.; Le, X.-F.; Bast, R.C., Jr.; Park, S.-T.; et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer 2012, 118, 2603–2614. [Google Scholar] [CrossRef]
- Patel, Y.; Shah, N.; Lee, J.S.; Markoutsa, E.; Jie, C.; Liu, S.; Botbyl, R.; Reisman, D.; Xu, P.; Chen, H. A novel double-negative feedback loop between Mir-489 and the HER2-shp2-mapk signaling axis regulates breast cancer cell proliferation and tumor growth. Oncotarget 2016, 7, 18295–18308. [Google Scholar] [CrossRef]
- Kikkawa, N.; Hanazawa, T.; Fujimura, L.; Nohata, N.; Suzuki, H.; Chazono, H.; Sakurai, D.; Horiguchi, S.; Okamoto, Y.; Seki, N. Mir-489 is a tumour-suppressive miRNA target ptpn11 in hypopharyngeal squamous cell carcinoma (hscc). Br. J. Cancer 2010, 103, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, D.; Yang, D.; Chen, Z.; Pan, Q.; Mao, A.; Cai, Y.; Li, X.; Xing, H.; Shi, M.; et al. Mir-489 regulates chemoresistance in breast cancer via epithelial mesenchymal transition pathway. FEBS Lett. 2014, 588, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, S.; Giordano, A.; Gao, H.; Cohen, E.N.; Tin, S.; Wu, Q.; Garza, R.J.; Debeb, B.G.; Alvarez, R.H.; Valero, V.; et al. High serum Mir-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+ inflammatory breast cancer. PLoS ONE 2014, 9, e83113. [Google Scholar] [CrossRef]
- Cheung, T.H.; Quach, N.L.; Charville, G.W.; Liu, L.; Park, L.; Edalati, A.; Yoo, B.; Hoang, P.; Rando, T.A. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 2012, 482, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Filkowski, J.N.; Tryndyak, V.P.; Golubov, A.; Shpyleva, S.I.; Kovalchuk, O. Alterations of microRNAs and their targets are associated with acquired resistance of mcf-7 breast cancer cells to cisplatin. Int. J. Cancer 2010, 127, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Zhang, X.; Xu, J.; Sun, Y.; Zhang, P. MicroRNA-10b expression in breast cancer and its clinical association. PLoS ONE 2018, 13, e0192509. [Google Scholar] [CrossRef]
- Hong, Y.; Liang, H.; Uzair ur, R.; Wang, Y.; Zhang, W.; Zhou, Y.; Chen, S.a.; Yu, M.; Cui, S.; Liu, M.; et al. Mir-96 promotes cell proliferation, migration and invasion by targeting ptpn9 in breast cancer. Sci. Rep. 2016, 6, 37421. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.K.O.; Li, R.; Shin, V.Y.; Siu, J.M.; Ma, E.S.K.; Kwong, A. MicroRNA-143 is downregulated in breast cancer and regulates DNA methyltransferases 3a in breast cancer cells. Tumor Biol. 2014, 35, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y.; Wang, H.; Zheng, X.; Li, C.; Han, Z. Mir-376a inhibits breast cancer cell progression by targeting neuropilin-1 nr. OncoTargets Ther. 2018, 11, 5293–5302. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Wang, J.; Wang, D.; Yao, A.; Li, Q. Prognostic and biological significance of microRNA-127 expression in human breast cancer. Dis. Markers 2014, 2014, 401986. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Chu, P.-Y.; Hou, M.-F.; Hung, W.-C. Mir-182 promotes proliferation and invasion and elevates the hif-1α-vegf-a axis in breast cancer cells by targeting fbxw7. Am. J. Cancer Res. 2016, 6, 1785–1798. [Google Scholar] [PubMed]
- Ye, X.; Bai, W.; Zhu, H.; Zhang, X.; Chen, Y.; Wang, L.; Yang, A.; Zhao, J.; Jia, L. Mir-221 promotes trastuzumab-resistance and metastasis in HER2-positive breast cancers by targeting pten. BMB Rep. 2014, 47, 268–273. [Google Scholar] [CrossRef]
- Yang, F.; Li, Y.; Xu, L.; Zhu, Y.; Gao, H.; Zhen, L.; Fang, L. Mir-17 as a diagnostic biomarker regulates cell proliferation in breast cancer. OncoTargets Ther. 2017, 10, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Cheng, J.; Yang, R.; Zhao, S.; Wang, T.; Chen, Y.; Chen, D.; Zhang, T.; Hong, S.; Wang, K. Decreased Mir-320 expression is associated with breast cancer progression, cell migration, and invasiveness via targeting aquaporin 1. Acta Biochim. Biophys. Sin. (Shanghai). 2018, 50, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Barbano, R.; Pasculli, B.; Rendina, M.; Fontana, A.; Fusilli, C.; Copetti, M.; Castellana, S.; Valori, V.M.; Morritti, M.; Graziano, P.; et al. Stepwise analysis of Mir9 loci identifies Mir-9-5p to be involved in oestrogen regulated pathways in breast cancer patients. Sci. Rep. 2017, 7, 45283. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; He, T.; Yang, L.; Yang, G.; Chen, Y.; Zhang, X. The role of Mir-100 in regulating apoptosis of breast cancer cells. Sci. Rep. 2015, 5, 11650. [Google Scholar] [CrossRef]
- Wang, S.E.; Lin, R.-J. MicroRNA and HER2-overexpressing cancer. MicroRNA 2013, 2, 137–147. [Google Scholar] [CrossRef]
- Wee, E.J.H.; Peters, K.; Nair, S.S.; Hulf, T.; Stein, S.; Wagner, S.; Bailey, P.; Lee, S.Y.; Qu, W.J.; Brewster, B.; et al. Mapping the regulatory sequences controlling 93 breast cancer-associated miRNA genes leads to the identification of two functional promoters of the HSA-Mir-200b cluster, methylation of which is associated with metastasis or hormone receptor status in advanced breast cancer. Oncogene 2012, 31, 4182–4195. [Google Scholar]
- Matamala, N.; Vargas, M.T.; González-Cámpora, R.; Miñambres, R.; Arias, J.I.; Menéndez, P.; Andrés-León, E.; Gómez-López, G.; Yanowsky, K.; Calvete-Candenas, J.; et al. Tumor microRNA expression profiling identifies circulating microRNAs for early breast cancer detection. Clin. Chem. 2015, 61, 1098–1106. [Google Scholar] [CrossRef]
- Bertoli, G.; Cava, C.; Castiglioni, I. The potential of miRNAs for diagnosis, treatment and monitoring of breast cancer. Scand. J. Clin. Lab. Invest. Suppl. 2016, 245, S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Cuk, K.; Zucknick, M.; Madhavan, D.; Schott, S.; Golatta, M.; Heil, J.; Marmé, F.; Turchinovich, A.; Sinn, P.; Sohn, C.; et al. Plasma microRNA panel for minimally invasive detection of breast cancer. PLoS ONE 2013, 8, e76729. [Google Scholar] [CrossRef] [PubMed]
- Antolín, S.; Calvo, L.; Blanco-Calvo, M.; Santiago, M.P.; Lorenzo-Patiño, M.J.; Haz-Conde, M.; Santamarina, I.; Figueroa, A.; Antón-Aparicio, L.M.; Valladares-Ayerbes, M. Circulating Mir-200c and Mir-141 and outcomes in patients with breast cancer. BMC Cancer 2015, 15, 297. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Gong, H.-T.; Li, B.-F.; Lv, C.-L.; Wang, H.-T.; Zhou, H.-H.; Li, X.-X.; Xie, S.-Y.; Jiang, B.-F. Higher expression of circulating Mir-182 as a novel biomarker for breast cancer. Oncol. Lett. 2013, 6, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Mangolini, A.; Ferracin, M.; Zanzi, M.V.; Saccenti, E.; Ebnaof, S.O.; Poma, V.V.; Sanz, J.M.; Passaro, A.; Pedriali, M.; Frassoldati, A.; et al. Diagnostic and prognostic microRNAs in the serum of breast cancer patients measured by droplet digital PCR. Biomarker Res. 2015, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Balwierz, A.; Zhang, J.D.; Küblbeck, M.; Pawitan, Y.; Hielscher, T.; Wiemann, S.; Sahin, Ö. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying emt-like properties in breast cancer. Oncogene 2012, 32, 1173–1182. [Google Scholar] [CrossRef]
- Young, J.; Kawaguchi, T.; Yan, L.; Qi, Q.; Liu, S.; Takabe, K. Tamoxifen sensitivity-related microRNA-342 is a useful biomarker for breast cancer survival. Oncotarget 2017, 8, 99978–99989. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, M.; Jin, J.; Lu, X.; Xu, T.; Jin, S. Mir-449a suppresses tamoxifen resistance in human breast cancer cells by targeting adam22. Cell. Physiol. Biochem. 2018, 50, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. The Erbb/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Noyan, S.; Gurdal, H.; Gur Dedeoglu, B. Involvement of Mir-770-5p in trastuzumab response in HER2 positive breast cancer cells. PLoS ONE 2019, 14, e0215894. [Google Scholar] [CrossRef]
- Chen, G.; He, M.; Yin, Y.; Yan, T.; Cheng, W.; Huang, Z.; Zhang, L.; Zhang, H.; Liu, P.; Zhu, W.; et al. Mir-1296-5p decreases erbb2 expression to inhibit the cell proliferation in erbb2-positive breast cancer. Cancer Cell Int. 2017, 17, 95. [Google Scholar] [CrossRef]
- Yang, F.; Fu, Z.; Yang, M.; Sun, C.; Li, Y.; Chu, J.; Zhang, Y.; Li, W.; Huang, X.; Li, J.; et al. Expression pattern of microRNAs related with response to trastuzumab in breast cancer. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Qiu, N.; Luo, K.; Liang, H.; Li, H. Downregulation of miroRNA-141 mediates acquired resistance to trastuzumab and is associated with poor outcome in breast cancer by upregulating the expression of ERBB4. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Choi, H.H.; Han, C.; Fang, Y.; Li, Y.; Van der Jeught, K.; Xu, H.; Zhang, L.; Frieden, M.; et al. Targeting 17q23 amplicon to overcome the resistance to anti-HER2 therapy in HER2+ breast cancer. Nat. Commun. 2018, 9, 4718. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal miRNAs as biomarkers for prostate cancer. Front. Genet. 2013, 4, 36. [Google Scholar] [CrossRef]
- Ogata-Kawata, H.; Izumiya, M.; Kurioka, D.; Honma, Y.; Yamada, Y.; Furuta, K.; Gunji, T.; Ohta, H.; Okamoto, H.; Sonoda, H.; et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS ONE 2014, 9, e92921. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Sueta, A.; Yamamoto, Y.; Tomiguchi, M.; Takeshita, T.; Yamamoto-Ibusuki, M.; Iwase, H. Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget 2017, 8, 69934–69944. [Google Scholar] [CrossRef]
- Eichelser, C.; Stückrath, I.; Müller, V.; Milde-Langosch, K.; Wikman, H.; Pantel, K.; Schwarzenbach, H. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget 2014, 5, 9650–9663. [Google Scholar] [CrossRef]
- Sachdeva, M.; Wu, H.; Ru, P.; Hwang, L.; Trieu, V.; Mo, Y.Y. MicroRNA-101-mediated akt activation and estrogen-independent growth. Oncogene 2010, 30, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Amancio, C.; Carmen, B.-A.; Oliver, R.; Wolfgang, L.; Juan, F.M.L. The pten/pi3k/akt signalling pathway in cancer, therapeutic implications. Current Cancer Drug Targets 2008, 8, 187–198. [Google Scholar]
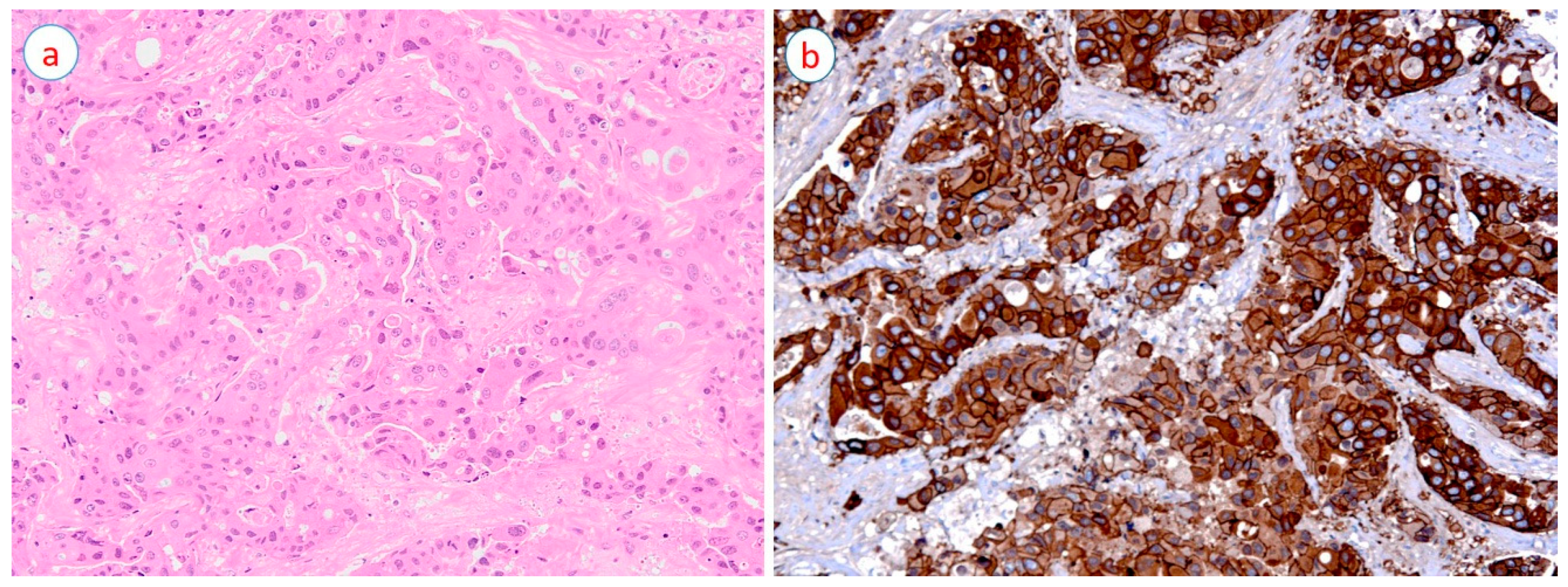
| Gene | Role in HER2-Positive Breast Cancer Progression | References |
|---|---|---|
| DKK1 | High levels correlate with poor prognosis, contribute to lymph nodes metastasis and bone metastasis | [64,65,66] |
| MMP15 | Highly expressed in HER2-positive breast cancer cells and mediate tumor progression | [64,67] |
| BIRC5, CCND1, ORC6L, MKi67, CCNE1 | Highly expressed in HER2-positive cancer cells and promote proliferative activity of cancer and contribute to tumor aggressive | [64,68,69] |
| TP53 | Highly expressed (mutated) in HER2-positive tumor cells and contribute to early onset and progression of HER2-positive breast cancer cells. | [70,71] |
| BCL2 | Upregulated in HER2-positive breast cancer, considered as a diagnostic marker since it inhibits apoptosis and promotes colony growth. | [70,72] |
| HER1/EGFR1 | Overexpressed and contribute to increased tumor size and poor progression-free prognosis. | [70,73] |
| PIK3CA, PTEN, INPP4B | Downregulated (mutated) in HER2-positive breast cancer cells, contribute to tumor growth, cell proliferation, and poor survival outcomes | [70,74] |
| PIK3R1 | Underexpressed (mutated) in HER2-positive breast cancer cells promoting metastasis and contributing to poor metastasis free survival | [70,75] |
| ERBB2 | It is the target of trastuzumab in HER2-positive breast cancer cells. It is amplified in 15–20% of all breast cancers; activating mutations are present in ~3% of breast cancers | [76,77] |
| ATP6V0A4, PREP, RTN4IP1, KIF18A | Upregulated in HER2-positive breast cancer cells, contribute to visceral metastasis and poor overall survival | [78] |
| TP63 | Downregulated in HER2-positive rich breast cancer cells, inhibits brain metastasis | [79] |
| Biological Functions | miRNAs | References | |
|---|---|---|---|
| Stimulate | Inhibit | ||
| Cell proliferation | miR-96, miR-96-5p, miR-10b, miR-143, miR-127-3p, miR-19a, miR-222-3p | miR-335-5p, miR-376a-3p, miR-452, miR-182, miR-377-3p | [109,141,142,147,150,151,152,153,154,155] |
| Tumor metastases and progression | miR-96, miR-96-5pm miR-10b, miR-127-3p, miR-320, miR-19a, miR-221, miR-221-3p, miR-17, miR-222-3p, miR-9-5p | miR-148a, miR-148a-3p, miR-148b-3p, miR-335-5p, miR-376a-3p, miR-452, miR-182, miR-377-3p | [109,141,142,147,150,151,153,154,155,156,157,158,159] |
| Cell Apoptosis | miR-148a, miR-148a-3p, miR-148b-3p, miR-376a-3p, miR-452, miR-468 | miR-221, miR-221-3p | [109,141,142,153,156,157] |
| Resistance to therapy | miR-200, miR-200c, miR-221, miR-100, miR-222-3p, miR-9-5p | [142,156,159,160] | |
| miRNAs | Intracellular/Extracellular | References |
|---|---|---|
| miR-96 | Extracellular | [161,162] |
| miR-96-5p | Extracellular and Intracellular | [87,163] |
| miR10-b | Extracellular and Intracellular | [164] |
| miR-143 | Extracellular and Intracellular | [164] |
| miR-127-3p | Extracellular | [87,165] |
| miR-19-a | Extracellular and Intracellular | [164] |
| miR-7 | Extracellular | [161,165] |
| miR-148-a | Extracellular | [164] |
| miR-200c | Intracellular | [166] |
| miR-100 | Intracellular | [164] |
| miR-452 | Extracellular and Intracellular | [164] |
| miR-182 | Extracellular and Intracellular | [87,167] |
| miR-148a | Extracellular | [164] |
| miR-148b-3p | Extracellular | [87,168] |
| miR-221 | Intracellular | [110,161] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sareyeldin, R.M.; Gupta, I.; Al-Hashimi, I.; Al-Thawadi, H.A.; Al Farsi, H.F.; Vranic, S.; Al Moustafa, A.-E. Gene Expression and miRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer. Cancers 2019, 11, 646. https://doi.org/10.3390/cancers11050646
Sareyeldin RM, Gupta I, Al-Hashimi I, Al-Thawadi HA, Al Farsi HF, Vranic S, Al Moustafa A-E. Gene Expression and miRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer. Cancers. 2019; 11(5):646. https://doi.org/10.3390/cancers11050646
Chicago/Turabian StyleSareyeldin, Rasha M., Ishita Gupta, Israa Al-Hashimi, Hamda A. Al-Thawadi, Halema F. Al Farsi, Semir Vranic, and Ala-Eddin Al Moustafa. 2019. "Gene Expression and miRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer" Cancers 11, no. 5: 646. https://doi.org/10.3390/cancers11050646
APA StyleSareyeldin, R. M., Gupta, I., Al-Hashimi, I., Al-Thawadi, H. A., Al Farsi, H. F., Vranic, S., & Al Moustafa, A.-E. (2019). Gene Expression and miRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer. Cancers, 11(5), 646. https://doi.org/10.3390/cancers11050646






