Cancer Pain Assessment and Classification
Abstract
1. Introduction
2. Etiology of Pain in Cancer Patients
3. Clinical Presentation and Assessment of Pain
3.1. Pain Intensity
3.2. Pain Site
3.3. Pain Syndromes
3.4. Timing and Temporal Variation
4. Cancer Pain Mechanisms and Pathophysiology
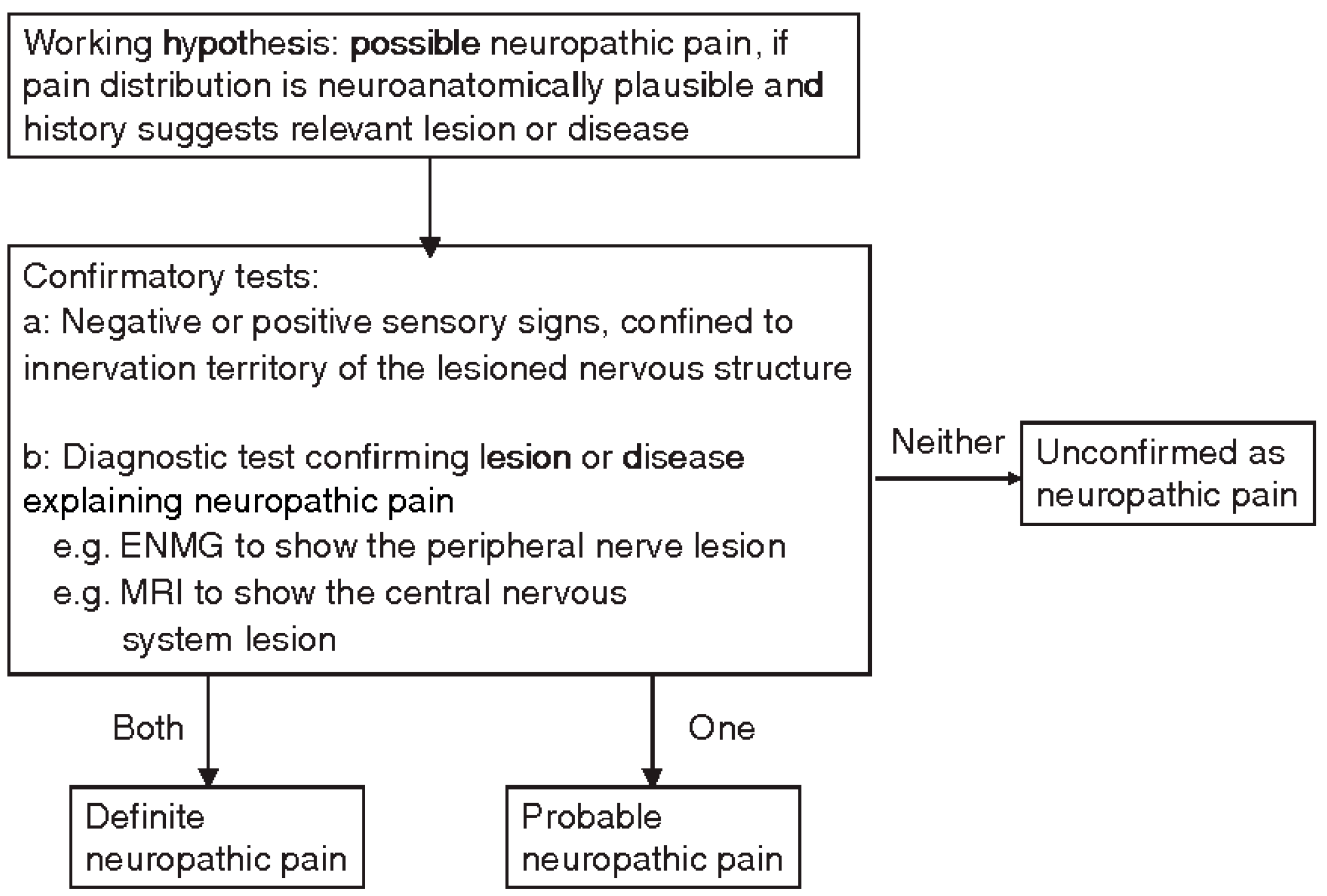
Neuropathic Pain
- history of relevant neurological lesion or disease, including pain descriptors, suggestive of pain being related to a neurological lesion and not other causes;
- pain distribution neuroanatomically plausible;
- pain associated with the presence of sensory signs in the same neuroanatomically plausible;
- diagnostic tests confirming a lesion or disease of the somatosensory systems, explaining the pain perceived by the patient.
5. Patient Characteristics and Disease Factors
6. Cancer Pain Classification Systems
6.1. International Association for the Study of Pain (IASP) Taxonomy
- anatomical region or pain site,
- system responsible for the pain perceived,
- temporal characteristics and pattern of pain occurrence,
- pain intensity and time since onset,
- etiology.
6.2. ICD-11
6.3. Edmonton Classification System for Cancer Pain (ECS-CP)
6.4. The Cancer Pain Prognostic Scale
7. Clinical Relevance
7.1. Pain Assessment Clinical Relevance
7.2. Classification Systems Clinical Relevance
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van den Beuken-van, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Pain Relief; World Health Organization: Geneva, Switzerland, 1986. [Google Scholar]
- Kwon, J.H. Overcoming Barriers in Cancer Pain Management. J. Clin. Oncol. 2014, 32, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.T.; Roberto, A.; Corli, O.; Deandrea, S.; Bandieri, E.; Cavuto, S.; Apolone, G. Quality of Cancer Pain Management: An Update of a Systematic Review of Undertreatment of Patients with Cancer. J. Clin. Oncol. 2014, 32, 4149–4154. [Google Scholar] [CrossRef]
- Reis-Pina, P.; Lawlor, P.G.; Barbosa, A. Adequacy of Cancer-Related Pain Management and Predictors of Undertreatment at Referral to a Pain Clinic. J. Pain Res. 2017, 10, 2097–2107. [Google Scholar] [CrossRef]
- Foley, K.M. Pain syndromes in patients with cancer. In Cancer Pain; Swerdlow, M., Ventafridda, V., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 45–54. [Google Scholar]
- Bonica, J.J. The Management of Pain. Am. J. Med. Sci. 1954, 227, 593. [Google Scholar] [CrossRef]
- Twycross, R. Cancer Pain Classification. Acta Anaesthesiol. Scand. 1997, 41, 141–145. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fainsinger, R.; Kaasa, S.; European Palliative Care Research Collaborative (EPCRC). Assessment and Classification of Cancer Pain. Curr. Opin. Support. Palliat. Care 2009, 3, 24–30. [Google Scholar] [CrossRef]
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D.; IASP Taskforce for the Classification of Chronic Pain. The IASP Classification of Chronic Pain for ICD-11: Chronic Cancer-Related Pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef]
- Grond, S.; Zech, D.; Diefenbach, C.; Radbruch, L.; Lehmann, K.A. Assessment of Cancer Pain: A Prospective Evaluation in 2266 Cancer Patients Referred to a Pain Service. Pain 1996, 64, 107–114. [Google Scholar] [CrossRef]
- Rayment, C.; Bennett, M.I. Definition and Assessment of Chronic Pain in Advanced Disease. Oxf. Textb. Palliat. Med. 2015, 519–524. [Google Scholar] [CrossRef]
- Knudsen, A.K.; Aass, N.; Fainsinger, R.; Caraceni, A.; Klepstad, P.; Jordhøy, M.; Hjermstad, M.; Kaasa, S. Classification of Pain in Cancer patients—A Systematic Literature Review. Palliat. Med. 2009, 23, 295–308. [Google Scholar] [CrossRef]
- Swarm, R.A.; Abernethy, A.P.; Anghelescu, D.L.; Benedetti, C.; Buga, S.; Cleeland, C.; Deleon-Casasola, O.A.; Eilers, J.G.; Ferrell, B.; Green, M.; et al. Adult Cancer Pain. J. Natl. Compr. Cancer Netw. 2013, 11, 992–1022. [Google Scholar] [CrossRef]
- Serlin, R.C.; Mendoza, T.R.; Nakamura, Y.; Edwards, K.R.; Cleeland, C.S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995, 61, 277–284. [Google Scholar] [CrossRef]
- Jones, K.R.; Vojir, C.P.; Hutt, E.; Fink, R. Determining Mild, Moderate, and Severe Pain Equivalency Across Pain-Intensity Tools in Nursing Home Residents. J. Rehabil. Res. Dev. 2007, 44, 305–315. [Google Scholar] [CrossRef]
- Ripamonti, C.; Santini, D.; Maranzano, E.; Berti, M.; Roila, F.; ESMO Guidelines Working Group. Management of Cancer Pain: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23, vii139–vii154. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. The Short-Form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Cleeland, C.; Ryan, K. Pain Assessment: Global Use of the Brief Pain Inventory; Annals Academy of Medicine: Singapore, 1994. [Google Scholar]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. Pain DETECT: A New Screening Questionnaire to Identify Neuropathic Components in Patients with Back Pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ahmed, E. Cancer Pain Syndromes. Hematol. Oncol. Clin. N. Am. 2018, 32, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Weinstein, S.M. Classification of Cancer Pain Syndromes. Oncology 2001, 15, 1627–1641. [Google Scholar]
- Foley, K. Pain Syndromes in Patients with Cancer. Adv. Pain Res. Ther. 1979, 2, 59–76. [Google Scholar]
- Cherny, N.I.; Portenoy, R.K. Cancer Pain: Principles of Assessment and Syndromes. In Textbook of Pain; Churchill Livingstone: Edinburgh, UK, 1994. [Google Scholar]
- Caraceni, A.; Portenoy, R.K. An International Survey of Cancer Pain Characteristics and Syndromes. Pain 1999, 82, 263–274. [Google Scholar] [CrossRef]
- Niscola, P.; Cartoni, C.; Romani, C.; Brunetti, G.A.; D’Elia, G.M.; Cupelli, L.; Tendas, A.; de Fabritiis, P.; Mandelli, F.; Foà, R. Epidemiology, Features and Outcome of Pain in Patients with Advanced Hematological Malignancies Followed in a Home Care Program: An Italian Survey. Ann. Hematol. 2007, 86, 671–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niscola, P.; Tendas, A.; Scaramucci, L.; Giovaninni, M.; Cupelli, L.; De Sanctis, V.; Brunetti, G.A.; Bondanini, F.; Palumbo, R.; Lamanda, M. Pain in Malignant Hematology. Expert Rev. Hematol. 2011, 4, 81–93. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Hagen, N.A. Breakthrough Pain: Definition, Prevalence and Characteristics. Pain 1990, 41, 273–281. [Google Scholar] [CrossRef]
- Deandrea, S.; Corli, O.; Consonni, D.; Villani, W.; Greco, M.T.; Apolone, G. Prevalence of Breakthrough Cancer Pain: A Systematic Review and a Pooled Analysis of Published Literature. J. Pain Symptom Manag. 2014, 47, 57–76. [Google Scholar] [CrossRef]
- Mercadante, S.; Portenoy, R.K. Breakthrough Cancer Pain: Twenty-Five Years of Study. Pain 2016, 157, 2657–2663. [Google Scholar] [CrossRef]
- Mercadante, S. Breakthrough Pain in Cancer Patients: Prevalence, Mechanisms and Treatment Options. Curr. Opin. Anaesthesiol. 2015, 28, 559–564. [Google Scholar] [CrossRef]
- Davies, A.; Buchanan, A.; Zeppetella, G.; Porta-Sales, J.; Likar, R.; Weismayr, W.; Slama, O.; Korhonen, T.; Filbet, M.; Poulain, P. Breakthrough Cancer Pain: An Observational Study of 1000 European Oncology Patients. J. Pain Symptom Manag. 2013, 46, 619–628. [Google Scholar] [CrossRef]
- Zeppetella, G.; Ribeiro, M.D. Pharmacotherapy of Cancer-Related Episodic Pain. Expert Opin. Pharmacother. 2003, 4, 493–502. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chang, V.T.; Kasimis, B. Cancer Breakthrough Pain Characteristics and Responses to Treatment at a VA Medical Center. Pain 2003, 101, 55–64. [Google Scholar] [CrossRef]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.; Brunelli, C.; Cherny, N.; Dale, O.; De Conno, F.; Fallon, M.; Hanna, M. For the European Palliative Care Research Collaborative (EPCRC) on Behalf of the European Association for Palliative Care (EAPC). Use of Opioid Analgesics in the Treatment of Cancer Pain: Evidence-Based Recommendations from the EAPC. Lancet Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef]
- Swanwick, M.; Haworth, M.; Lennard, R. The Prevalence of Episodic Pain in Cancer: A Survey of Hospice Patients on Admission. Palliat. Med. 2001, 15, 9–18. [Google Scholar] [CrossRef]
- Løhre, E.T.; Klepstad, P.; Bennett, M.I.; Brunelli, C.; Caraceni, A.; Fainsinger, R.L.; Knudsen, A.K.; Mercadante, S.; Sjøgren, P.; Kaasa, S. From “breakthrough” to “episodic” Cancer Pain? A European Association for Palliative Care Research Network Expert Delphi Survey Toward a Common Terminology and Classification of Transient Cancer Pain Exacerbations. J. Pain Symptom Manag. 2016, 51, 1013–1019. [Google Scholar] [CrossRef]
- Gatti, A.; Gentili, M.; Baciarello, M.; Lazzari, M.; Marzi, R.; Palombo, E.; Sabato, A.F.; Fanelli, G. Breakthrough Pain in Patients with Controlled or Uncontrolled Pain: An Observational Study. Pain Res. Manag. 2014, 19, e168–e171. [Google Scholar] [CrossRef][Green Version]
- Hjermstad, M.J.; Kaasa, S.; Caraceni, A.; Loge, J.H.; Pedersen, T.; Haugen, D.F.; Aass, N.; European Palliative Care Research Collaborative (EPCRC). Characteristics of Breakthrough Cancer Pain and its Influence on Quality of Life in an International Cohort of Patients with Cancer. BMJ Support. Palliat. Care 2016, 6, 344–352. [Google Scholar] [CrossRef]
- Caraceni, A.; Davies, A.; Poulain, P.; Cortes-Funes, H.; Panchal, S.J.; Fanelli, G. Guidelines for the Management of Breakthrough Pain in Patients with Cancer. J. Natl. Compr. Cancer Netw. 2013, 11, S29–S36. [Google Scholar] [CrossRef][Green Version]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.; ESMO Guidelines Committee. Management of Cancer Pain in Adult Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- IASP. IASP Taxonomy. 2012. Available online: http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576 (accessed on 5 January 2019).
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T. Neuropathic Pain: An Updated Grading System for Research and Clinical Practice. Pain 2016, 157, 1599. [Google Scholar] [CrossRef]
- Bennett, M.I.; Rayment, C.; Hjermstad, M.; Aass, N.; Caraceni, A.; Kaasa, S. Prevalence and Aetiology of Neuropathic Pain in Cancer Patients: A Systematic Review. Pain 2012, 153, 359–365. [Google Scholar] [CrossRef]
- Bennett, M. The LANSS Pain Scale: The Leeds Assessment of Neuropathic Symptoms and Signs. Pain 2001, 92, 147–157. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A. Comparison of Pain Syndromes Associated with Nervous or Somatic Lesions and Development of a New Neuropathic Pain Diagnostic Questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.I.; Attal, N.; Backonja, M.M.; Baron, R.; Bouhassira, D.; Freynhagen, R.; Scholz, J.; Tölle, T.R.; Wittchen, H.; Jensen, T.S. Using Screening Tools to Identify Neuropathic Pain. Pain 2007, 127, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.; Boland, E.; Bouhassira, D.; Freynhagen, R.; Hardy, J.; Hjermstad, M.; Mercadante, S.; Pérez, C.; Bennett, M. Neuropathic Pain in Cancer: Systematic Review, Performance of Screening Tools and Analysis of Symptom Profiles. Br. J. Anaesth. 2017, 119, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Tölle, T.; Baron, R.; de Bock, E.; Junor, R.; Dias Barbosa, C.; Marshall, S.; Arnould, B.; Freynhagen, R. PainPREDICT: First Interim Data from the Development of a New Patient-Reported Pain Questionnaire to Predict Treatment Response using Sensory Symptom Profiles. Curr. Med. Res. Opin. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic Pain: Redefinition and a Grading System for Clinical and Research Purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Brunelli, C.; Bennett, M.I.; Kaasa, S.; Fainsinger, R.; Sjøgren, P.; Mercadante, S.; Løhre, E.T.; Caraceni, A.; European Association for Palliative Care (EAPC) Research Network. Classification of Neuropathic Pain in Cancer Patients: A Delphi Expert Survey Report and EAPC/IASP Proposal of an Algorithm for Diagnostic Criteria. Pain 2014, 155, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Baron, R.; Bouhassira, D.; Drangholt, M.; Dyck, P.J.; Edwards, R.R.; Freeman, R.; Gracely, R.; Haanpaa, M.H.; Hansson, P. Value of Quantitative Sensory Testing in Neurological and Pain Disorders: NeuPSIG Consensus. Pain 2013, 154, 1807–1819. [Google Scholar]
- Fallon, M. Neuropathic Pain in Cancer. Br. J. Anaesth. 2013, 111, 105–111. [Google Scholar] [CrossRef]
- Fainsinger, R.L.; Nekolaichuk, C.; Lawlor, P.; Hagen, N.; Bercovitch, M.; Fisch, M.; Galloway, L.; Kaye, G.; Landman, W.; Spruyt, O. An International Multicentre Validation Study of a Pain Classification System for Cancer Patients. Eur. J. Cancer 2010, 46, 2896–2904. [Google Scholar] [CrossRef]
- Knudsen, A.K.; Brunelli, C.; Klepstad, P.; Aass, N.; Apolone, G.; Corli, O.; Montanari, M.; Caraceni, A.; Kaasa, S. Which Domains should be Included in a Cancer Pain Classification System? Analyses of Longitudinal Data. Pain 2012, 153, 696–703. [Google Scholar] [CrossRef]
- Knudsenl, A.K.; Brunellil, C.; Kaasal, S.; Apolonel, G.; Corlil, O.; Montanaril, M.; Fainsingerl, R.; Aassl, N.; Fayersl, P.; Caracenil, A. Which Variables are Associated with Pain Intensity and Treatment Response in Advanced Cancer Patients?—Implications for a Future Classification System for Cancer Pain. Eur. J. Pain 2011, 15, 320–327. [Google Scholar] [CrossRef]
- Merskey, H. Development of a Universal Language of Pain Syndromes. Adv. Pain Res. Ther. 1983, 5, 37–52. [Google Scholar]
- Bruera, E.; Schoeller, T.; Wenk, R.; MacEachern, T.; Marcelino, S.; Hanson, J.; Suarez-Almazor, M. A Prospective Multicenter Assessment of the Edmonton Staging System for Cancer Pain. J. Pain Symptom Manag. 1995, 10, 348–355. [Google Scholar] [CrossRef]
- Fainsinger, R.L.; Nekolaichuk, C.L.; Lawlor, P.G.; Neumann, C.M.; Hanson, J.; Vigano, A. A Multicenter Study of the Revised Edmonton Staging System for Classifying Cancer Pain in Advanced Cancer Patients. J. Pain Symptom Manag. 2005, 29, 224–237. [Google Scholar] [CrossRef]
- Fainsinger, R.L.; Nekolaichuk, C.L. A “TNM” Classification System for Cancer Pain: The Edmonton Classification System for Cancer Pain (ECS-CP). Support. Care Cancer 2008, 16, 547–555. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chang, V.T.; Fairclough, D.L.; Kasimis, B. Development of a Cancer Pain Prognostic Scale. J. Pain Symptom Manag. 2002, 24, 366–378. [Google Scholar] [CrossRef]
- Turk, D.C.; Monarch, E.S.; Williams, A.D. Cancer Patients in Pain: Considerations for Assessing the Whole Person. Hematol. Oncol. Clin. N. Am. 2002, 16, 511–525. [Google Scholar] [CrossRef]
- Falk, S.; Dickenson, A.H. Pain and Nociception: Mechanisms of Cancer-Induced Bone Pain. J. Clin. Oncol. 2014, 32, 1647–1654. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. A Personalized Approach to Assessing and Managing Pain in Patients with Cancer. J. Clin. Oncol. 2014, 32, 1640–1646. [Google Scholar] [CrossRef]
- Miser, A.W.; McCalla, J.; Dothage, J.A.; Wesley, M.; Miser, J.S. Pain as a Presenting Symptom in Children and Young Adults with Newly Diagnosed Malignancy. Pain 1987, 29, 85–90. [Google Scholar] [CrossRef]
- Bankhead, C.R.; Kehoe, S.T.; Austoker, J. Symptoms Associated with Diagnosis of Ovarian Cancer: A Systematic Review. BJOG 2005, 112, 857–865. [Google Scholar] [CrossRef]
- Spiro, S.G.; Gould, M.K.; Colice, G.L. Initial Evaluation of the Patient with Lung Cancer: Symptoms, Signs, Laboratory Tests, and Paraneoplastic Syndromes: ACCP Evidenced-Based Clinical Practice Guidelines. Chest 2007, 132, 149S–160S. [Google Scholar] [CrossRef]
- Sauter, M.; Keilholz, G.; Kranzbühler, H.; Lombriser, N.; Prakash, M.; Vavricka, S.R.; Misselwitz, B. Presenting Symptoms Predict Local Staging of Anal Cancer: A Retrospective Analysis of 86 Patients. BMC Gastroenterol. 2016, 16, 46. [Google Scholar] [CrossRef]
- Kaasa, S.; Apolone, G.; Klepstad, P.; Loge, J.H.; Hjermstad, M.J.; Corli, O.; Strasser, F.; Heiskanen, T.; Costantini, M.; Zagonel, V.; et al. Expert Conference on Cancer Pain Assessment and Classification—The Need for International Consensus: Working Proposals on International Standards. BMJ Support. Palliat. Care 2011, 1, 281–287. [Google Scholar] [CrossRef]

| Neoplastic damage to bone and joints | 1. Base of the skull syndrome. Headache due to calvarial, maxillary, or mandibular lesion |
| 2. Vertebral syndromes, including sacrum | |
| 3. Pelvic, long bones, direct infiltration of a joint | |
4. Generalized bone pain:
| |
| 5. Chest wall pain from rib lesion | |
6. Pathologic fracture of:
| |
| Neoplastic damage to viscera | 7. Esophageal mediastinal pain. |
8. Shoulder pain from diaphragmatic infiltration
| |
| 9. Epigastric pain from pancreas or other upper abdominal neoplasm “Midline rostral retroperitoneal syndrome” | |
10. Diffuse abdominal pain from abdominal or peritoneal disease:
| |
| 11. Suprapubic pain from infiltration of bladder. Perineal pain from infiltration of rectum or perirectal tissue (including vagina) | |
| 12. Obstruction of ureter | |
| Neoplastic damage to soft tissue and miscellaneous | 13. Damage to oral mucous membranes. Infiltration of skin and subcutaneous tissue |
| 14. Infiltration of muscle and fascia of in the chest or abdominal wall. Infiltration of muscle and fascia in the limbs | |
| 15. Infiltration of muscle and fascia in the head and neck | |
| 16. Retroperitoneal tissue infiltration excluding rostral retroperitoneal syndrome | |
| 17. Pleural infiltration | |
| Lesions of Nervous Tissue | 18. Peripheral nerve syndromes
|
19. Radiculopathy or cauda equina syndrome
| |
20. Plexopathy
| |
21. Cranial neuropathy
| |
22. Pain due to central nervous system lesion
| |
| 23. Headache due to intracranial hypertension | |
| 24. Neck, back pain or headache due to leptomeningeal disease |
| Pain Type | Pain Origin and Syndromes | |
|---|---|---|
| Nociceptive | Deep somatic | Bone marrow expansion and osteolysis. Spleen and liver capsulae distension by tumor infiltration and organ enlargement; intracranial hypertension (meningeal and/or brain tumor involvement) |
| Superficial somatic | Mucositis, cutaneous lesions | |
| Visceral | Infiltration and/or compression of viscera cava by abdominal nodes, spleen, and liver enlargement | |
| Neuropathic | Peripheral neuropathic | Neuropathies due to para-proteins. Amyloidosis. Plexopathy by tumor invasion and/or node enlargement compression (lymphomas) |
| Central Neuropathic | CNS damage and/or tumor involvement | |
| Mixed | Neuropathic + somatic | Meningosis, peripheral nerve damage, and/or tumor involvement |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caraceni, A.; Shkodra, M. Cancer Pain Assessment and Classification. Cancers 2019, 11, 510. https://doi.org/10.3390/cancers11040510
Caraceni A, Shkodra M. Cancer Pain Assessment and Classification. Cancers. 2019; 11(4):510. https://doi.org/10.3390/cancers11040510
Chicago/Turabian StyleCaraceni, Augusto, and Morena Shkodra. 2019. "Cancer Pain Assessment and Classification" Cancers 11, no. 4: 510. https://doi.org/10.3390/cancers11040510
APA StyleCaraceni, A., & Shkodra, M. (2019). Cancer Pain Assessment and Classification. Cancers, 11(4), 510. https://doi.org/10.3390/cancers11040510





