CYFRA 21-1 in Lymph Node Fine Needle Aspiration Washout Improves Diagnostic Accuracy for Metastatic Lymph Nodes of Differentiated Thyroid Cancer
Abstract
1. Introduction
2. Results
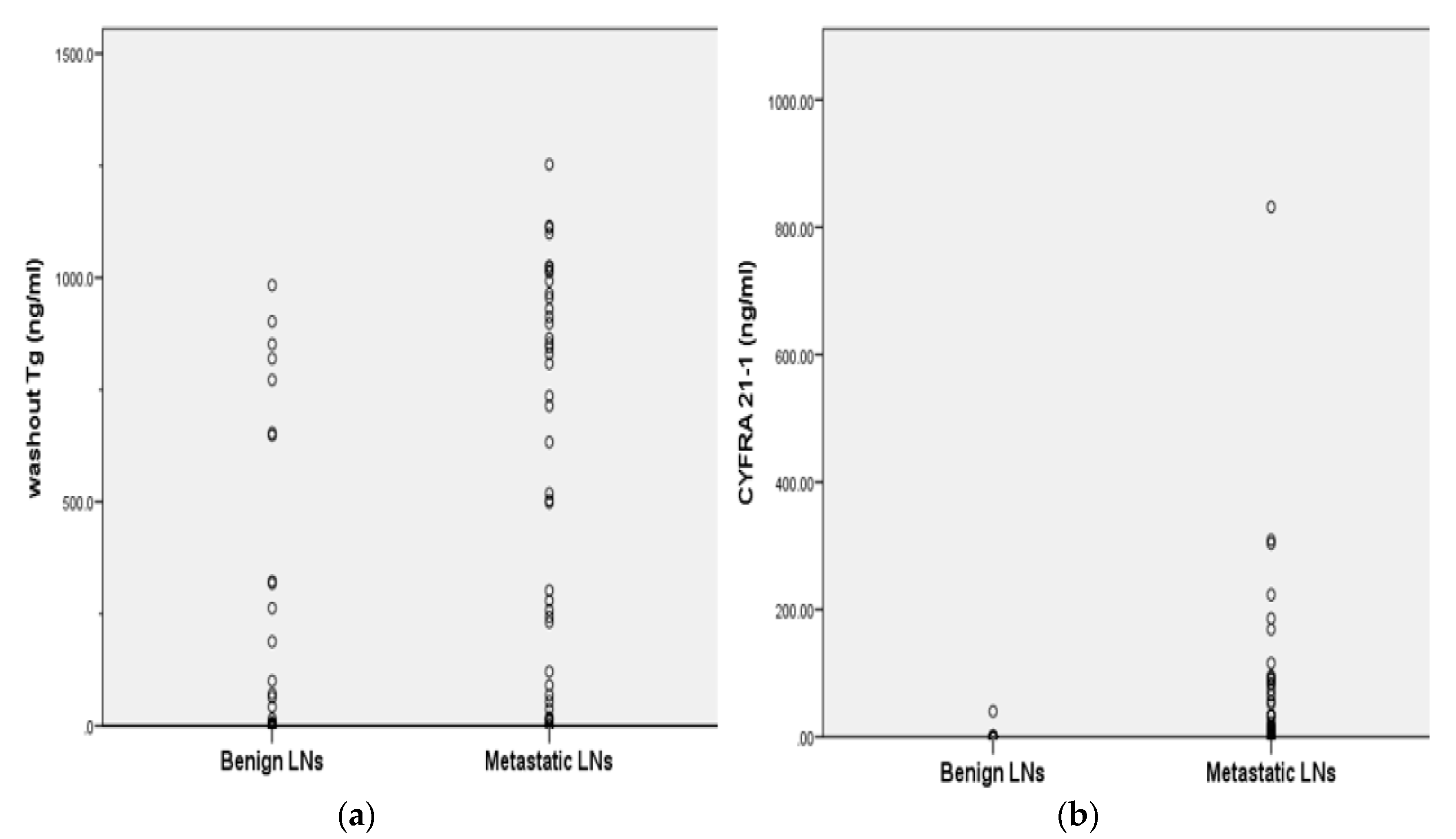
2.1. Clinical Characteristics of Malignant and Benign LNs
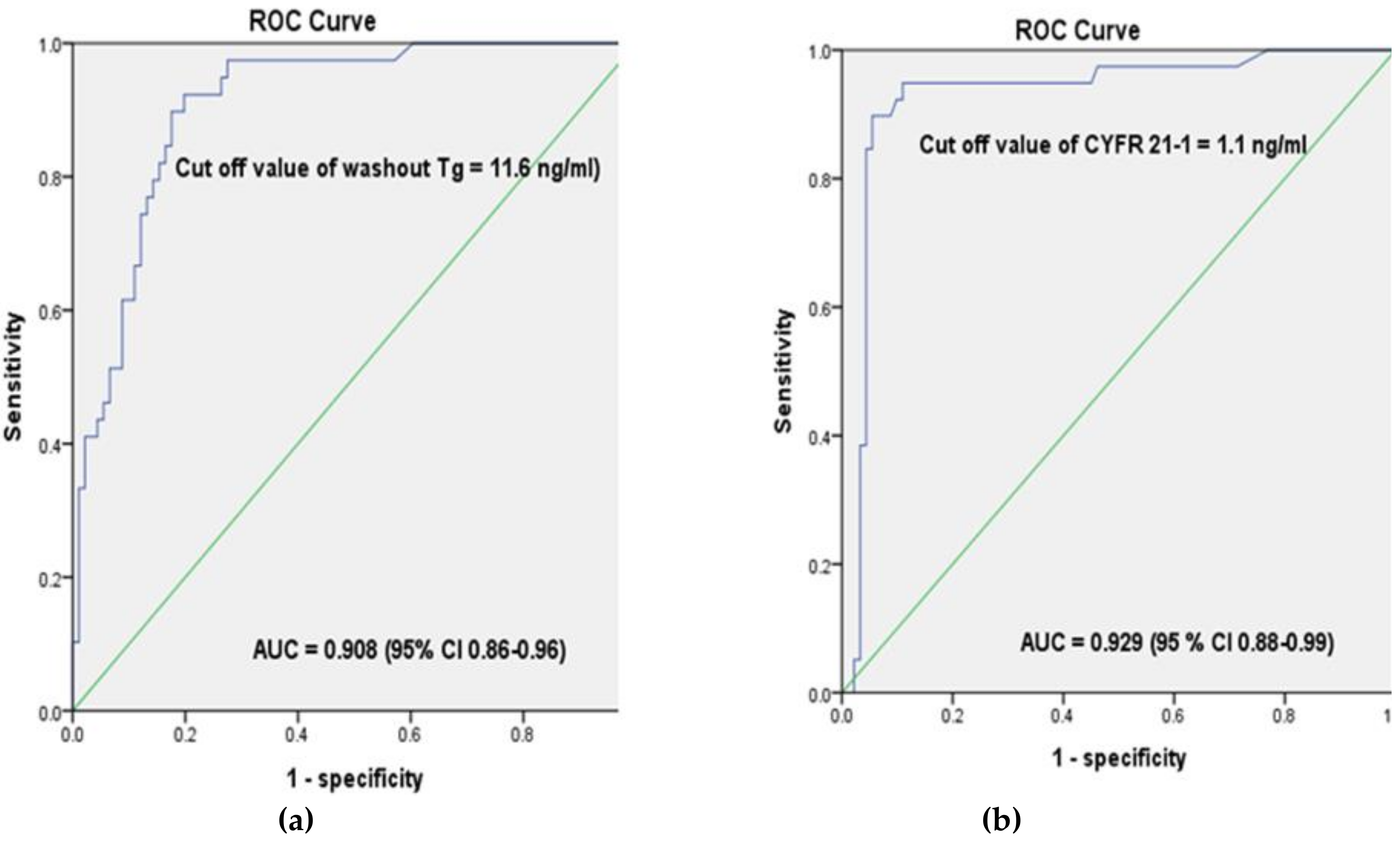
2.2. The Cut-Off Values of Washout Tg and Washout CYFRA 21-1
2.3. Individual Diagnostic Performance of FNAC, Washout Tg, and Washout CYFRA 21-1, and Their Correlations with Final Outcomes
2.4. Comparison of the Diagnostic Performances of FNAC, Washout Tg, Washout CYFRA 21-1, and Their Combinations
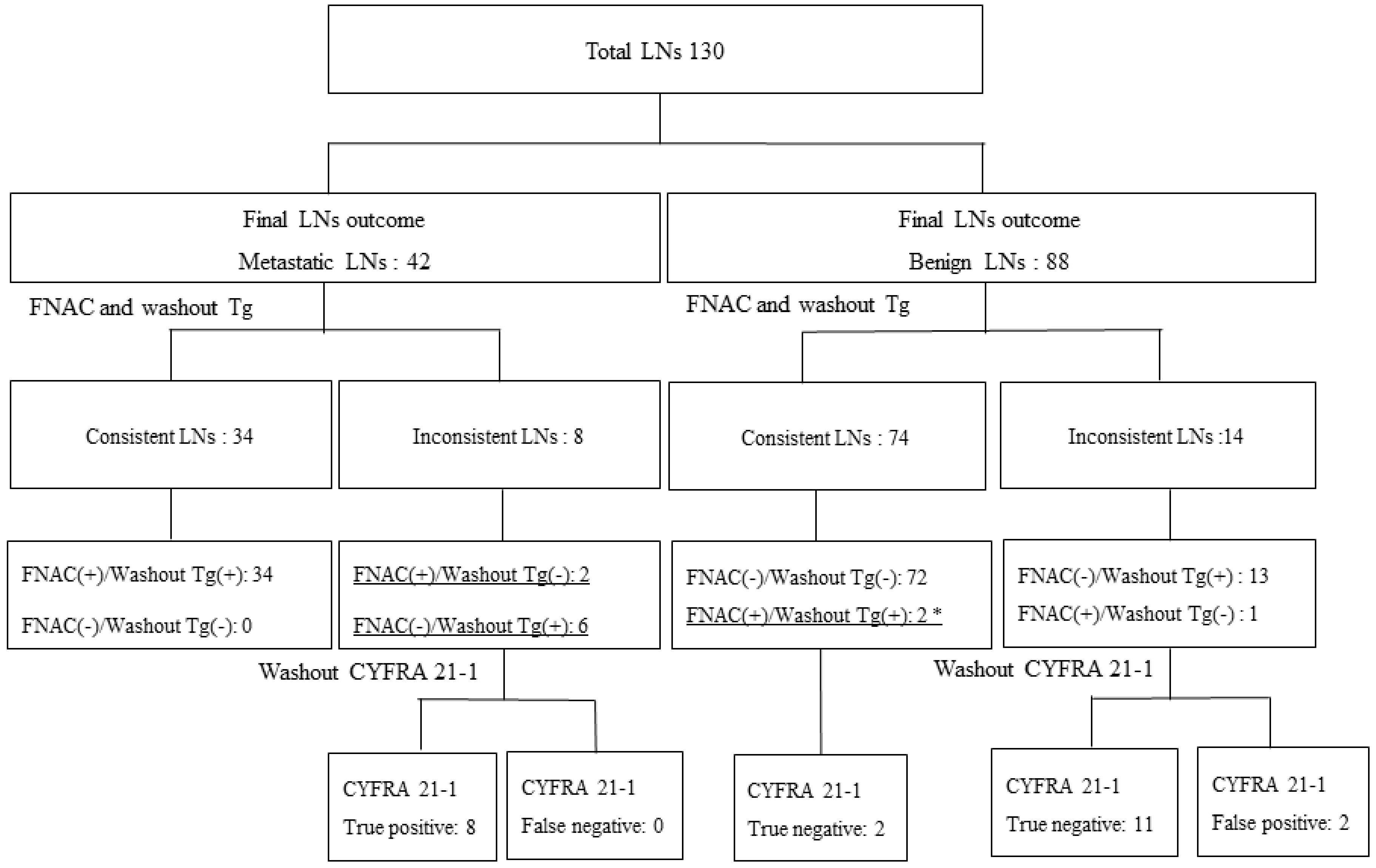
2.5. CYFRA 21-1 Diagnostic Performance in Discordant LNs between FNAC and Washout Tg
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Tg, TgAb, and Washout Tg Measurements
4.3. US and FNAC of Suspicious LNs
4.4. Washout CYFRA 21-1 Measurement
4.5. FNAC and Final LN Outcomes
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Sampanis, D.; Safioleas, M. Cervical lymph node dissection in papillary thyroid cancer: Current trends, persisting controversies, and unclarified uncertainties. Surg. Oncol. 2010, 19, e57–e70. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Grani, G.; Fumarola, A. Thyroglobulin in lymph node fine-needle aspiration washout: A systematic review and meta-analysis of diagnostic accuracy. J. Clin. Endocrinol. Metab. 2014, 99, 1970–1982. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Ceriani, L.; Suriano, S.; Crippa, S. Thyroglobulin measurement on fine-needle washout fluids: Influence of sample collection methods. Diagn. Cytopathol. 2009, 37, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Cunha, N.; Rodrigues, F.; Curado, F.; Ilheu, O.; Cruz, C.; Naidenov, P.; Rascao, M.J.; Ganho, J.; Gomes, I.; Pereira, H.; et al. Thyroglobulin detection in fine-needle aspirates of cervical lymph nodes: A technique for the diagnosis of metastatic differentiated thyroid cancer. Eur. J. Endocrinol. 2007, 157, 101–107. [Google Scholar] [CrossRef]
- Jeon, S.J.; Kim, E.; Park, J.S.; Son, K.R.; Baek, J.H.; Kim, Y.S.; Park, D.J.; Cho, B.Y.; Na, D.G. Diagnostic benefit of thyroglobulin measurement in fine-needle aspiration for diagnosing metastatic cervical lymph nodes from papillary thyroid cancer: Correlations with US features. Korean J. Radiol. 2009, 10, 106–111. [Google Scholar] [CrossRef]
- Cignarelli, M.; Ambrosi, A.; Marino, A.; Lamacchia, O.; Campo, M.; Picca, G.; Giorgino, F. Diagnostic utility of thyroglobulin detection in fine-needle aspiration of cervical cystic metastatic lymph nodes from papillary thyroid cancer with negative cytology. Thyroid 2003, 13, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Jack, B.-H. Detection of recurrent papillary thyroid carcinoma by thyroglobulin assessment in the needle washout after fine-needle aspiration of suspicious lymph nodes. Thyroid 2004, 14, 959–963. [Google Scholar] [CrossRef]
- Pacini, F.; Fugazzola, L.; Lippi, F.; Ceccarelli, C.; Centoni, R.; Miccoli, P.; Elisei, R.; Pinchera, A. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: A clue to the diagnosis of metastatic differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 1992, 74, 1401–1404. [Google Scholar]
- Boi, F.; Baghino, G.; Atzeni, F.; Lai, M.; Faa, G.; Mariotti, S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J. Clin. Endocrinol. Metab. 2006, 91, 1364–1369. [Google Scholar]
- Kim, M.J.; Kim, E.K.; Kim, B.M.; Kwak, J.Y.; Lee, E.J.; Park, C.S.; Cheong, W.Y.; Nam, K.H. Thyroglobulin measurement in fine-needle aspirate washouts: The criteria for neck node dissection for patients with thyroid cancer. Clin. Endocrinol. 2009, 70, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Snozek, C.L.; Chambers, E.P.; Reading, C.C.; Sebo, T.J.; Sistrunk, J.W.; Singh, R.J.; Grebe, S.K. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J. Clin. Endocrinol. Metab. 2007, 92, 4278–4281. [Google Scholar] [CrossRef]
- Borel, A.-L.; Boizel, R.; Faure, P.; Barbe, G.; Boutonnat, J.; Sturm, N.; Seigneurin, D.; Bricault, I.; Caravel, J.-P.; Chaffanjon, P. Significance of low levels of thyroglobulin in fine needle aspirates from cervical lymph nodes of patients with a history of differentiated thyroid cancer. Eur. J. Endocrinol. 2008, 158, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, M.; Chen, X.; Chen, S.; Liu, L.; Zhu, J.; Wang, J.; Yang, X.; Cai, X. In Vitro Expression of Cytokeratin 19 in Adipose-Derived Stem Cells Is Induced by Epidermal Growth Factor. Med. Sci. Monit. 2018, 24, 4254–4261. [Google Scholar] [CrossRef] [PubMed]
- Barillo, J.L.; da Silva Junior, C.T.; Silva, P.S.; de Souza, J.B.S.; Kanaan, S.; Xavier, A.R.; de Araujo, E.G. Increased Cytokeratin 19 Fragment Levels Are Positively Correlated with Adenosine Deaminase Activity in Malignant Pleural Effusions from Adenocarcinomas. Dis. Mark. 2018, 2018, 2609767. [Google Scholar] [CrossRef] [PubMed]
- Han, A.L.; Kim, H.R.; Choi, K.H.; Ryu, J.W.; Hwang, K.E.; So, H.S.; Park, M.C.; Zhu, M.; Huang, Y.; Lee, Y.J. Expression Profile of Three Splicing Factors in Pleural Cells Based on the Underlying Etiology and Its Clinical Values in Patients with Pleural Effusion. Transl. Oncol. 2018, 11, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Han, K.H.; Kim, E.-K.; Moon, H.J.; Kim, M.J.; Suh, Y.J.; Choi, J.S.; Park, B.W. Fine-needle aspirates CYFRA 21-1 is a useful tumor marker for detecting axillary lymph node metastasis in breast cancer patients. PLoS ONE 2013, 8, e57248. [Google Scholar] [CrossRef] [PubMed]
- Dinets, A.; Pernemalm, M.; Kjellin, H.; Sviatoha, V.; Sofiadis, A.; Juhlin, C.C.; Zedenius, J.; Larsson, C.; Lehtiö, J.; Höög, A.J. Differential protein expression profiles of cyst fluid from papillary thyroid carcinoma and benign thyroid lesions. PLoS ONE 2015, 10, e0126472. [Google Scholar] [CrossRef]
- Martins-Costa, M.C.; Maciel, R.; Kasamatsu, T.S.; Nakabashi, C.C.; Camacho, C.P.; Crispim, F.; Ikejiri, E.S.; Mamone, M.C.O.; Andreoni, D.M.; Biscolla, R.P.M. Clinical impact of thyroglobulin (Tg) and Tg autoantibody (TgAb) measurements in needle washouts of neck lymph node biopsies in the management of patients with papillary thyroid carcinoma. Arch. Endocrinol. Metab. 2017, 61, 108–114. [Google Scholar] [CrossRef]
- Sohn, Y.M.; Kim, M.J.; Kim, E.-K.; Kwak, J.Y. Diagnostic performance of thyroglobulin value in indeterminate range in fine needle aspiration washout fluid from lymph nodes of thyroid cancer. Yonsei Med. J. 2012, 53, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Paeng, J.C.; Kim, S.E.; Kim, Y.I.; Chung, J.-K.; Choi, H.S.; Lim, J.A.; Cho, S.W.; Park, Y.J.; Park, D.J.; et al. Thyroglobulin in Washout Fluid From Lymph Node Fine-needle Aspiration Biopsy in Papillary Thyroid Cancer: Large-scale Validation of the Cutoff Value to Determine Malignancy and Evaluation of Discrepant Results. J. Clin. Endocrinol. Metab. 2013, 98, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Jeon, S.J.; Kim, C.G. Usefulness of thyroglobulin measurement in needle washouts of fine-needle aspiration biopsy for the diagnosis of cervical lymph node metastases from papillary thyroid cancer before thyroidectomy. Endocrine 2012, 42, 399–403. [Google Scholar] [CrossRef]
- Jo, K.; Kim, M.H.; Lim, Y.; Jung, S.L.; Bae, J.S.; Jung, C.K.; Kang, M.I.; Cha, B.Y.; Lim, D.J. Lowered cutoff of lymph node fine-needle aspiration thyroglobulin in thyroid cancer patients with serum anti-thyroglobulin antibody. Eur. J. Endocrinol. 2015, 173, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Kristjansdottir, B.; Partheen, K.; Fung, E.T.; Marcickiewicz, J.; Yip, C.; Brannstrom, M.; Sundfeldt, K. Ovarian cyst fluid is a rich proteome resource for detection of new tumor biomarkers. Clin. Proteom. 2012, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kim, Y.; Han, K.; Kang, C.S.; Jeon, H.M.; Shim, S.I. Detection of tumor markers including carcinoembryonic antigen, APC, and cyclin D2 in fine-needle aspiration fluid of breast. Arch. Pathol. Lab. Med. 2004, 128, 1251–1256. [Google Scholar] [PubMed]
- Singh, S.K.; Bhargava, R.; Gupta, V. High levels of tumor markers in pleural fluid correlate with poor survival in patients with adenocarcinomatous or squamous malignant effusions. Eur. J. Intern. Med. 2009, 20, e147. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Rahimi, A. Correlation of serum and salivary CA15-3 levels in patients with breast cancer. Med. Oral Patol. Oral Cir. Bucal 2009, 14, e521–e524. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Wu, H.H.; Wang, P.H.; Yeh, J.Y.; Chen, Y.J.; Yen, M.S.; Huang, R.L.; Tsai, Y.J.; Yuan, C. Serum cytokeratin-19 fragment (Cyfra 21-1) is a prognostic indicator for epithelial ovarian cancer. Taiwan J. Obstet. Gynecol. 2014, 53, 30–34. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Chang, J.H. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, neuron-specific enolase, and cytokeratin 19 fragments in patients with effusions from primary lung cancer. Chest 2005, 128, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Nakata, B.; Ogawa, Y.; Ishikawa, T.; Ikeda, K.; Kato, Y.; Nishino, H.; Hirakawa, K. Serum CYFRA 21-1 is one of the most reliable tumor markers for breast carcinoma. Cancer 2000, 89, 1285–1290. [Google Scholar] [CrossRef]
- Liscia, D.S.; Detoma, P.; Zanchetta, M.; Anro, P.; Molinar, D.; Favettini, E.; Paduos, A. The Use of CYFRA 21-1 for the Detection of Breast Cancer Axillary Lymph Node Metastases in Needle Washouts of Fine-Needle Aspiration Biopsies. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Ezzat, S.; Freeman, J.L.; Rosen, I.B.; Asa, S.L. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod. Pathol. 2001, 14, 338–342. [Google Scholar] [CrossRef]
- Giovanella, L.; Ceriani, L.; Ghelfo, A.; Maffioli, M. Circulating cytokeratin 19 fragments in patients with benign nodules and carcinomas of the thyroid gland. Int. J. Biol. Mark. 2008, 23, 54–57. [Google Scholar] [CrossRef]
- Appetecchia, M.; Meçule, A.; Ducci, M.; Palma, L.; Castelli, M. Serum Cytokeratins Determination in Differentiated Thyroid. J. Exp. Clin. Cancer Res. 2001, 20, 2. [Google Scholar]
- Giovanella, L.; Imperiali, M.; Trimboli, P. Role of serum cytokeratin 19 fragment (Cyfra 21.1) as a prognostic biomarker in patients with differentiated thyroid cancer. Sci. Rep. 2017, 7, 7359. [Google Scholar] [CrossRef] [PubMed]
- Indrasena, B.S.H. Use of thyroglobulin as a tumour marker. World J. Biol. Chem. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Park, J.W.; Han, J.M.; Yim, J.H.; Song, D.E.; Gong, G.; Kim, T.Y.; Baek, J.H.; Lee, J.H.; Shong, Y.K. Serum antithyroglobulin antibodies interfere with thyroglobulin detection in fine-needle aspirates of metastatic neck nodes in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 153–160. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Benign LN (n = 88) | Metastatic LN (n = 42) | p-Value |
|---|---|---|---|
| FNAC according to operation | 0.268 | ||
| FNAC before surgery (%) | 37 (42.0) | 22 (52.4) | |
| FNAC at postoperative follow-up | 51 (58.0) | 20 (47.6) | |
| Diagnostic methods for final LNs outcomes | 0.000 | ||
| Histological confirm (%) | 42 (47.7) | 38 (90.5) | |
| Confirmed by image or repeat FNAC (%) | 46 (52.3) | 4 (9.5) | |
| Serum Tg (ng/mL) | 15.5 (0.0–205.0) | 18.6 (0.1–126.4) | 0.614 |
| Serum TgAb (IU/mL) | 53.12 (1.6–895.3) | 25.8 (0.1–270.7) | 0.319 |
| Washout Tg (ng/mL) | 80.1 (0.0–983.2) | 568.0 (0.1–1252.4) | 0.000 |
| Washout CYFRA 21-1(ng/mL) | 0.9 (0.1–40.1) | 74.2 (0.2–931.6) | 0.000 |
| Diagnosis | FNAC | Washout Tg | Washout CYFRA 21-1 | |||
|---|---|---|---|---|---|---|
| Final diagnosis | Positive (n = 39) | Negative (n = 91) | Positive (n = 54) | Negative (n = 76) | Positive (n = 41) | Negative (n = 89) |
| Benign (n = 88) | 3 | 85 | 15 | 73 | 2 | 86 |
| Metastatic (n = 42) | 36 | 6 | 39 | 3 | 39 | 3 |
| Diagnostic Tool | Diagnostic Value | ||||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| FNAC | 85.7 | 96.5 | 93.4 | 91.2 | 93.1 |
| Washout Tg | 92.9 | 83.0 | 72.2 | 96.1 | 86.2 |
| FNAC+ washout Tg | 88.1 | 83.0 | 92.5 | 81.1 | 82.3 |
| Washout CYFRA 21-1 | 92.9 | 97.7 | 95.1 | 96.7 | 96.2 |
| FNAC+ washout CYFRA 21-1 | 91.9 | 96.5 | 81.6 | 98.8 | 94.2 |
| FNAC+ washout Tg + washout CYFRA 21-1 | 98.8 | 93.1 | 98.8 | 88.2 | 90.0 |
| No. | Gender | Age | Time of FNAC | Operation | Serum Tg | Serum TgAb | FNAC | Washout Tg | Washout CYFRA 21-1 | Final LN Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Metastatic LNs | ||||||||||
| 1 | F | 82 | During follow-up post-thyroidectomy | Total thyroidectomy | 0.17 | 0.10 | Normal thyroid remnant | 16.67 | 831.60 | Anaplastic carcinoma |
| 2 | M | 63 | During follow-up post-thyroidectomy | Total thyroidectomy | 40.67 | 0.37 | Metastatic LN | 2.45 | 1.33 | Metastatic PTC |
| 3 | M | 57 | Initial diagnosis | Total thyroidectomy | 29.56 | 3.24 | Cystic fluid | 633.10 | 73.30 | Metastatic PTC |
| 4 | F | 40 | Initial diagnosis | Total thyroidectomy | 0.62 | 67.57 | Negative for malignancy | 120.88 | 33.45 | Metastatic PTC |
| 5 | M | 27 | Initial diagnosis | Total thyroidectomy | 5.95 | 6.34 | Negative for malignancy | 18.37 | 14.95 | Metastatic PTC |
| 6 | F | 41 | Initial diagnosis | Total thyroidectomy | 83.12 | 6.00 | Negative for malignancy | 993.03 | 3.02 | Metastatic PTC |
| 7 | M | 38 | Initial diagnosis | Total thyroidectomy | 11.23 | 9.72 | Negative for malignancy | 14.4 | 1.24 | Metastatic PTC |
| 8 | F | 20 | Initial diagnosis | Rt. Lobectomy | 7.68 | 7.41 | Metastatic LN | 1.33 | 4.00 | Metastatic PTC |
| Benign LNs | ||||||||||
| 1 | F | 50 | During follow-up post-thyroidectomy | Total thyroidectomy | 41.93 | 6.00 | Negative for malignancy | 317.78 | 1.39 | No lesion on follow-up neck CT |
| 2 | F | 69 | During follow-up post-thyroidectomy | Total thyroidectomy | 0.21 | 8.62 | Metastatic cancer | 0.16 | 1.20 | Blood only Decreased in size on neck CT |
| 3 | M | 32 | During follow-up post-thyroidectomy | Total thyroidectomy | 0.20 | 9.02 | Negative for malignancy | 771.83 | 0.96 | Negative for malignancy |
| 4 | F | 51 | During follow-up post-thyroidectomy | Total thyroidectomy | 0.09 | 8.83 | Cystic fluid only | 902.17 | 0.75 | Negative for malignancy |
| 5 | F | 82 | During follow-up post-thyroidectomy | Total thyroidectomy | 0.17 | 0.10 | Negative for malignancy | 819.23 | 0.66 | Negative for malignancy |
| 6 | M | 39 | During follow-up post-thyroidectomy | Total thyroidectomy | 0.20 | 14.00 | Negative for malignancy | 42.28 | 0.43 | Negative for malignancy |
| 7 | F | 57 | During follow-up post-thyroidectomy | Total thyroidectomy | 1.74 | 8.03 | Negative for malignancy | 100.22 | 0.29 | Negative for malignancy |
| 8 | M | 47 | During follow-up post-thyroidectomy | Rt. Lobectomy | 0.19 | 91.76 | Negative for malignancy | 188.14 | 0.34 | Negative for malignancy |
| 9 | F | 37 | During follow-up post-thyroidectomy | Rt. Lobectomy | 7.57 | 52.31 | Negative for malignancy | 15.70 | 0.19 | Negative for malignancy |
| 10 | M | 44 | During follow-up post-thyroidectomy | Lt. Lobectomy | 2.27 | 183.79 | Negative for malignancy | 653.36 | 0.96 | Negative for malignancy |
| 11 | M | 44 | During follow-up post-thyroidectomy | Lt. Lobectomy | 2.27 | 183.79 | Negative for malignancy | 262.53 | 0.71 | Negative for malignancy |
| 12 | M | 44 | During follow-up post-thyroidectomy | Lt. Lobectomy | 2.27 | 183.79 | Negative for malignancy | 648.03 | 0.35 | Negative for malignancy |
| 13 | F | 46 | Initial diagnosis | Total thyroidectomy | 41.93 | 7.74 | Blood only | 63.21 | 0.52 | Negative for malignancy |
| 14 | M | 71 | Initial diagnosis | Total thyroidectomy | 37.37 | 4.16 | Negative for malignancy | 71.78 | 0.29 | Negative for malignancy |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, H.L.; Jeong, C.-W.; Ha, J.; Jo, K.; Kim, M.-H.; Han, J.-S.; Lee, S.; Bae, J.; Jung, C.K.; et al. CYFRA 21-1 in Lymph Node Fine Needle Aspiration Washout Improves Diagnostic Accuracy for Metastatic Lymph Nodes of Differentiated Thyroid Cancer. Cancers 2019, 11, 487. https://doi.org/10.3390/cancers11040487
Lee J, Park HL, Jeong C-W, Ha J, Jo K, Kim M-H, Han J-S, Lee S, Bae J, Jung CK, et al. CYFRA 21-1 in Lymph Node Fine Needle Aspiration Washout Improves Diagnostic Accuracy for Metastatic Lymph Nodes of Differentiated Thyroid Cancer. Cancers. 2019; 11(4):487. https://doi.org/10.3390/cancers11040487
Chicago/Turabian StyleLee, Jeongmin, Hye Lim Park, Chan-Wook Jeong, Jeonghoon Ha, Kwanhoon Jo, Min-Hee Kim, Jeong-Sun Han, Sohee Lee, Jaseong Bae, Chan Kwon Jung, and et al. 2019. "CYFRA 21-1 in Lymph Node Fine Needle Aspiration Washout Improves Diagnostic Accuracy for Metastatic Lymph Nodes of Differentiated Thyroid Cancer" Cancers 11, no. 4: 487. https://doi.org/10.3390/cancers11040487
APA StyleLee, J., Park, H. L., Jeong, C.-W., Ha, J., Jo, K., Kim, M.-H., Han, J.-S., Lee, S., Bae, J., Jung, C. K., Jung, S. L., Kang, M. I., & Lim, D.-J. (2019). CYFRA 21-1 in Lymph Node Fine Needle Aspiration Washout Improves Diagnostic Accuracy for Metastatic Lymph Nodes of Differentiated Thyroid Cancer. Cancers, 11(4), 487. https://doi.org/10.3390/cancers11040487






