Fecal Immunochemical Tests for Colorectal Cancer Screening: Is Fecal Sampling from Multiple Sites Necessary?
Abstract
:1. Introduction
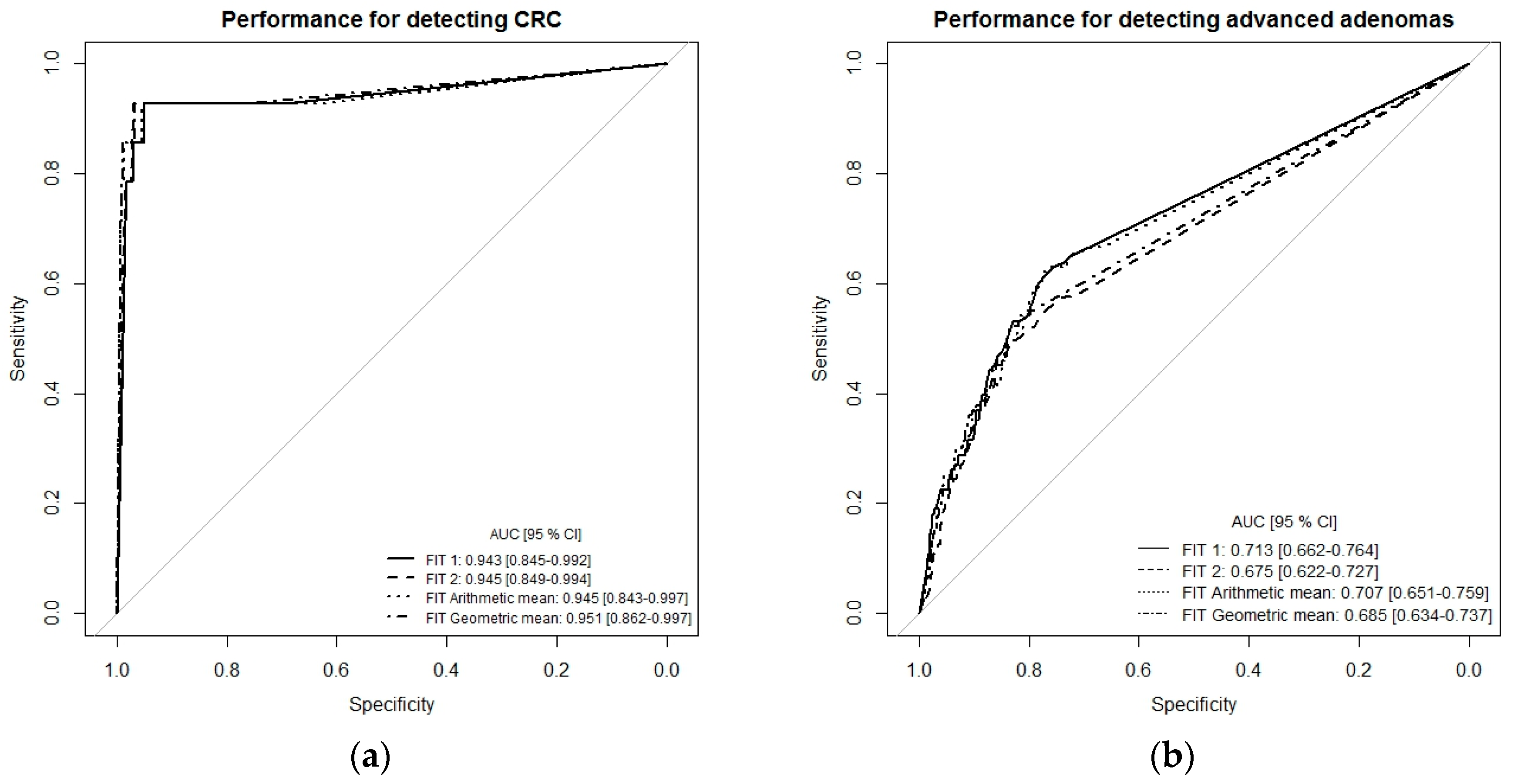
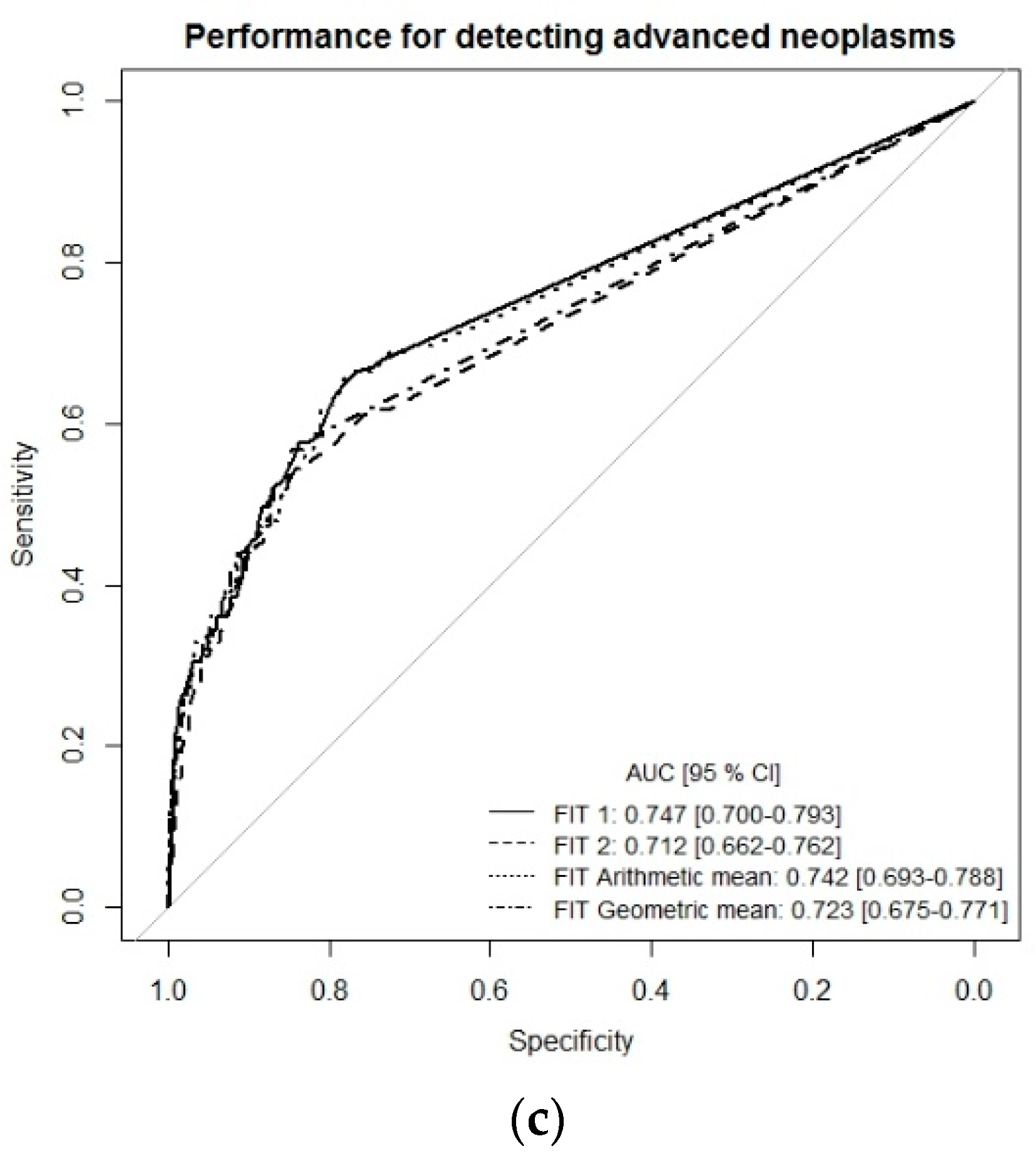
2. Results
Detecting CRC by Single-Site Versus Multiple-Site Fecal Sampling from the Same Bowel Movement
3. Discussion
4. Materials and Methods
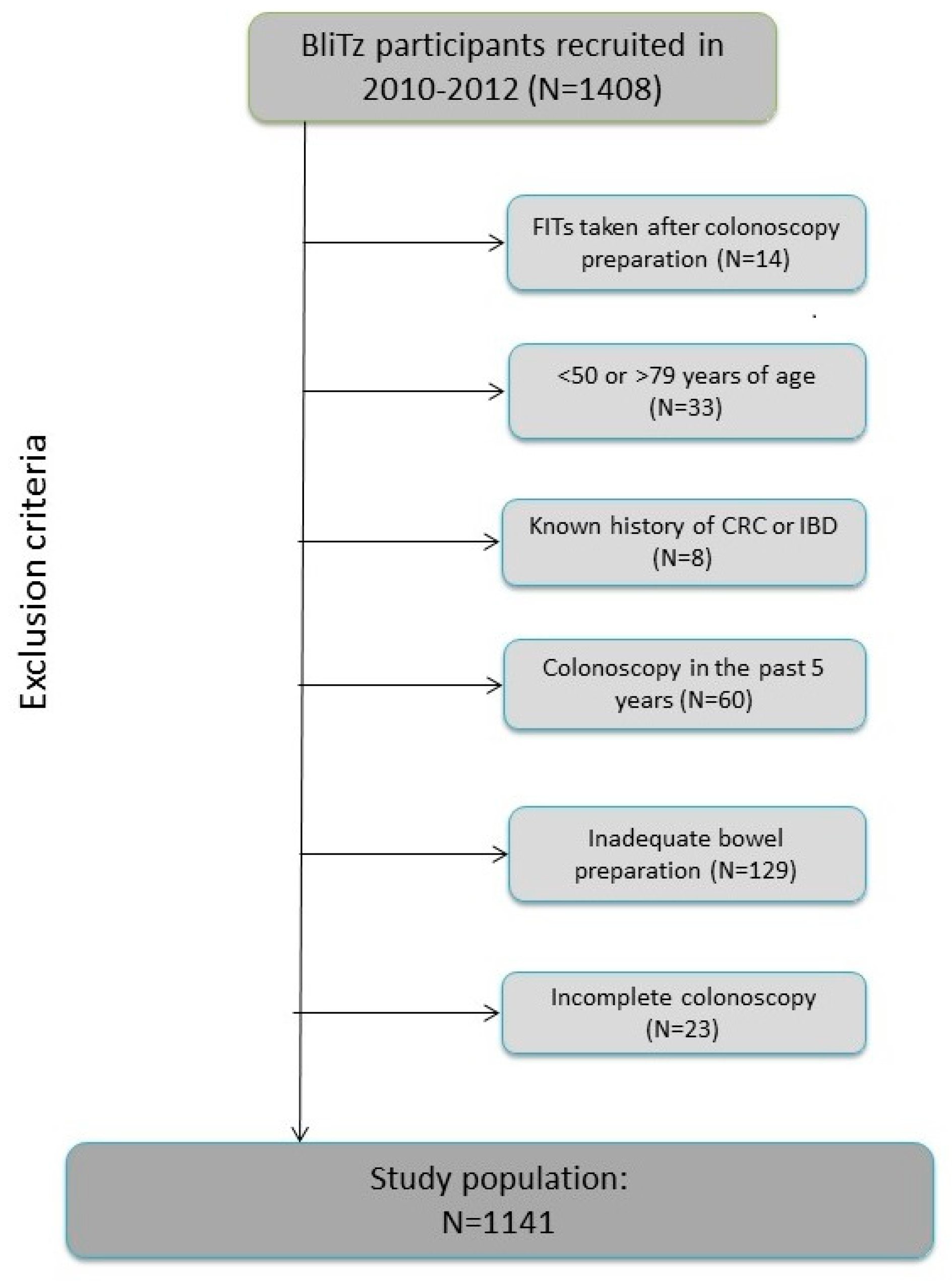
4.1. Study Design and Population
4.2. Data and Sample Collection
4.3. FIT Analyses
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halloran, S.P.; Launoy, G.; Zappa, M. International Agency for Research on C. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Faecal occult blood testing. Endoscopy 2012, 44 (Suppl. 3), SE65–SE87. [Google Scholar] [CrossRef] [PubMed]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- USPSTF; Bibbins-Domingo, K.; Grossman, D.C. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef] [PubMed]
- Gies, A.; Bhardwaj, M.; Stock, C.; Schrotz-King, P.; Brenner, H. Quantitative fecal immunochemical tests for colorectal cancer screening. Int. J. Cancer 2018, 143, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Von Wagner, C.; Good, A.; Smith, S.G.; Wardle, J. Responses to procedural information about colorectal cancer screening using faecal occult blood testing: The role of consideration of future consequences. Health Expect. 2012, 15, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.; Cubiella, J.; Gonzalez-Mao, M.C. Fecal immunochemical test accuracy in average-risk colorectal cancer screening. World J. Gastroenterol. 2014, 20, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Park, D.I.; Ryu, S.; Kim, Y.H. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am. J. Gastroenterol. 2010, 105, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Werner, S.; Brenner, H. Fresh vs Frozen Samples and Ambient Temperature Have Little Effect on Detection of Colorectal Cancer or Adenomas by a Fecal Immunochemical Test in a Colorectal Cancer Screening Cohort in Germany. Clin. Gastroenterol. Hepatol. 2017, 15, 1547–1556e5. [Google Scholar] [CrossRef] [PubMed]
- Hundt, S.; Haug, U.; Brenner, H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann. Intern. Med. 2009, 150, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Tao, S.; Haug, U. Low-dose aspirin use and performance of immunochemical fecal occult blood tests. JAMA 2010, 304, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Tao, S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur. J. Cancer 2013, 49, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krause, F.; Rolny, V. Evaluation of a 5-Marker Blood Test for Colorectal Cancer Early Detection in a Colorectal Cancer Screening Setting. Clin. Cancer Res. 2016, 22, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.G.; Allison, J.E.; Young, G.P.; Halloran, S.P.; Seaman, H. A standard for Faecal Immunochemical TesTs for haemoglobin evaluation reporting (FITTER). Ann. Clin. Biochem. 2014, 51 Pt 2, 301–302. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
| FIT Brand | Manufacturer | Stool Collection Instructions as Given by the Manufacturer | Number of Sites |
|---|---|---|---|
| OC-Sensor | Eiken Chemical co., Tokyo, Japan | Collect fecal samples by scraping the surface of the stools in different areas | Scrape the surface |
| MagStream HemSp | Fujirebio, Tokyo, Japan | Put the tip of the blue stick into your bowel motion and drag back and forth | Scrape the surface |
| HM-JACKarc | Kyowa Medex, Tokyo, Japan | Scrape the end of the stick along the stool sample | Scrape the surface |
| RIDASCREEN hemoglobin | R-Biopharm, Darmstadt, Germany | Swab the stool with the sampling rod | Swab the surface |
| SENTiFIT FOB Gold | Sentinel Diagnostics, Milan, Italy | Insert the stick into 4 different places, scraping the surface in a crosswise motion | 4 + scrape the surface |
| Eurolyser FOB test | Eurolyser Diagnostica, Salzburg, Austria | Insert the stick into the stool sample at 5 to 6 different positions to ensure a homogenous sampling | 5–6 |
| FIT NS-Prime | Alfesa Pharma, Osaka, Japan | Collect sample feces onto the gutters of the collector stick by scraping 4–5 areas on the surface of fecal specimens | 4–5 |
| QuantOn Hem | Immundiagnostik, Bensheim, Germany | Prick the tip of the sample collection stick in at least 3 different locations in the stool sample in one go | 3+ |
| QuikRead go iFOBT | Orion Diagnostica, Espoo, Finland | Collect the sample by poking the sample collecting stick… in three (3) different places on the faeces sample | 3 |
| IDK Hemoglobin ELISA | Immundiagnostik, Bensheim, Germany | “Insert the dosing stick 3-times into different areas of the stool specimen | 3 |
| ImmoCARE-C | CARE diagnostic, Voerde, Germany | Insert the applicator stick upright into the stool at 3 different areas in one go | 3 |
| Characteristic | n = 1141 | % | |
|---|---|---|---|
| Sex | Female | 574 | 50.3 |
| Male | 567 | 49.7 | |
| Age (years) | 50–54 | 32 | 2.8 |
| 55–59 | 514 | 45.0 | |
| 60–64 | 240 | 21.0 | |
| 65–69 | 170 | 14.9 | |
| 70–74 | 131 | 11.5 | |
| 75–79 | 54 | 4.7 | |
| median | 60 | ||
| Most advanced finding at screening colonoscopy | Any advanced neoplasm | 125 | 11.0 |
| Colorectal cancer | 14 | 1.2 | |
| Advanced adenoma | 111 | 9.7 | |
| No advanced neoplasm | 1016 | 89.0 | |
| Non-advanced adenoma | 236 | 20.7 | |
| None of above * | 780 | 68.4 | |
| Indicator of Test Performance at Cutoff of 17 µg Hb/g Stool | FIT | ||||||
|---|---|---|---|---|---|---|---|
| Single-Site | Multiple-Site | ||||||
| FIT1 | FIT2 | PP | PN | A_Mean | G_Mean | ||
| Sensitivity for CRC (n = 14) | n | 13/14 | 13/14 | 13/14 | 13/14 | 13/14 | 13/14 |
| % | 92.9 | 92.9 | 92.9 | 92.9 | 92.9 | 92.9 | |
| 95% CI | 66.1–99.8 | 66.1–99.8 | 66.1–99.8 | 66.1–99.8 | 66.1–99.8 | 66.1–99.8 | |
| p-value + | Reference | 1 | 1 | 1 | 1 | 1 | |
| 1 | Reference | 1 | 1 | 1 | 1 | ||
| Sensitivity for advanced adenomas (n = 111) | N | 43/111 | 42/111 | 35/111 | 50/111 | 43/111 | 40/111 |
| % | 38.7 | 37.8 | 31.5 | 45.0 | 38.7 | 36.0 | |
| 95% CI | 29.6–48.5 | 28.8–47.5 | 23.0–41.0 | 35.6–54.8 | 29.6–48.5 | 27.1–45.7 | |
| p-value + | Reference | 1 | 0.008 | 0.016 | 1 | 0.453 | |
| 1 | Reference | 0.016 | 0.008 | 1 | 0.727 | ||
| Sensitivity for any advanced neoplasms (n = 125) | N | 56/125 | 55/125 | 48/125 | 63/125 | 56/125 | 53/125 |
| % | 44.8 | 44.0 | 38.4 | 50.4 | 44.8 | 42.4 | |
| 95% CI | 35.9–54.0 | 35.1–53.2 | 29.8–47.5 | 41.3–59.5 | 35.9–54.0 | 33.6–51.6 | |
| p-value + | Reference | 1 | 0.008 | 0.016 | 1 | 0.453 | |
| 1 | Reference | 0.016 | 0.008 | 1 | 0.727 | ||
| Specificity for no advanced neoplasms (n = 1016) | N | 914/1016 | 909/1016 | 948/1016 | 875/1016 | 904/1016 | 931/1016 |
| % | 90.0 | 89.5 | 93.3 | 86.1 | 89 | 91.6 | |
| 95% CI | 87.9–91.7 | 87.4–91.3 | 91.6–94.8 | 83.8–88.2 | 86.9–90.8 | 89.8–93.3 | |
| p-value + | Reference | 0.640 | <0.001 | <0.001 | 0.110 | 0.008 | |
| 0.640 | Reference | <0.001 | <0.001 | 0.533 | <0.001 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amitay, E.L.; Gies, A.; Weigl, K.; Brenner, H. Fecal Immunochemical Tests for Colorectal Cancer Screening: Is Fecal Sampling from Multiple Sites Necessary? Cancers 2019, 11, 400. https://doi.org/10.3390/cancers11030400
Amitay EL, Gies A, Weigl K, Brenner H. Fecal Immunochemical Tests for Colorectal Cancer Screening: Is Fecal Sampling from Multiple Sites Necessary? Cancers. 2019; 11(3):400. https://doi.org/10.3390/cancers11030400
Chicago/Turabian StyleAmitay, Efrat L., Anton Gies, Korbinian Weigl, and Hermann Brenner. 2019. "Fecal Immunochemical Tests for Colorectal Cancer Screening: Is Fecal Sampling from Multiple Sites Necessary?" Cancers 11, no. 3: 400. https://doi.org/10.3390/cancers11030400
APA StyleAmitay, E. L., Gies, A., Weigl, K., & Brenner, H. (2019). Fecal Immunochemical Tests for Colorectal Cancer Screening: Is Fecal Sampling from Multiple Sites Necessary? Cancers, 11(3), 400. https://doi.org/10.3390/cancers11030400





