Preoperative Imaging Evaluation after Downstaging of Pancreatic Ductal Adenocarcinoma: A Multi-Center Study
Abstract
1. Introduction
2. Methods
2.1. Patients Population
2.2. Imaging Technique
2.3. Imaging and Anatomopathological Evaluation
2.4. Statistical Analysis
3. Results
3.1. Population and Tumour Characteristics
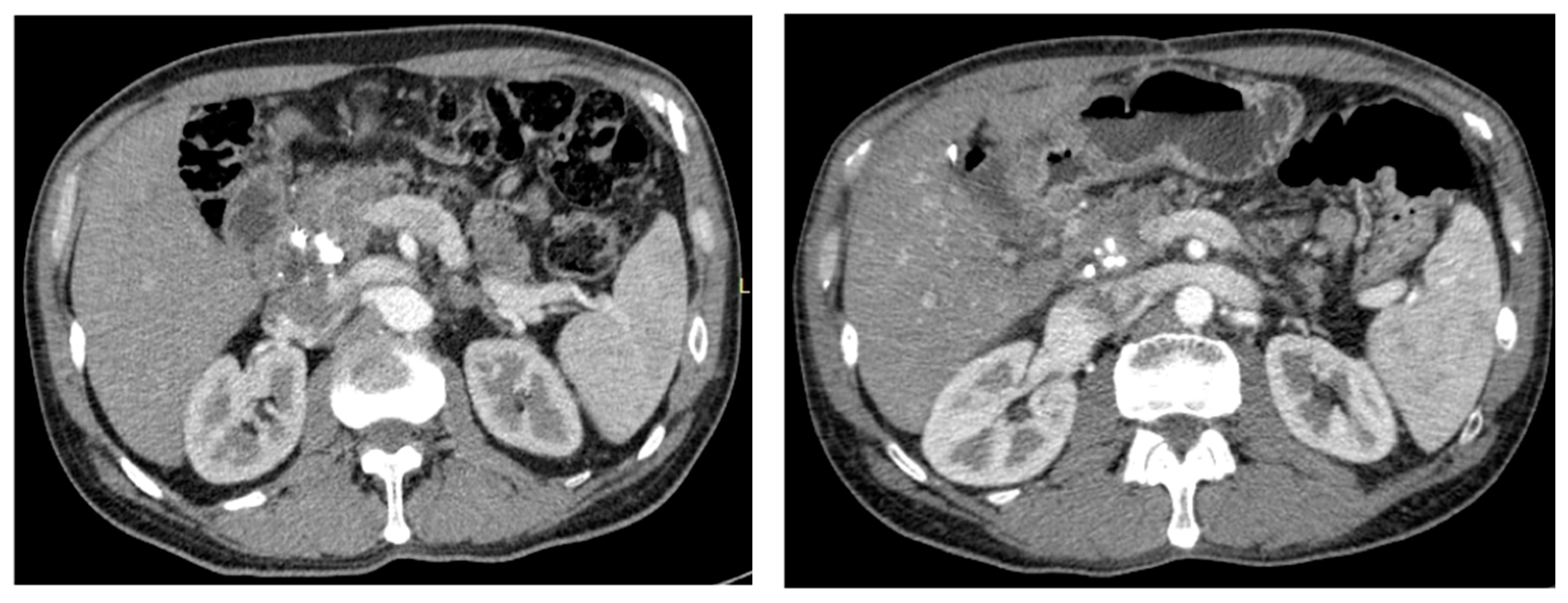
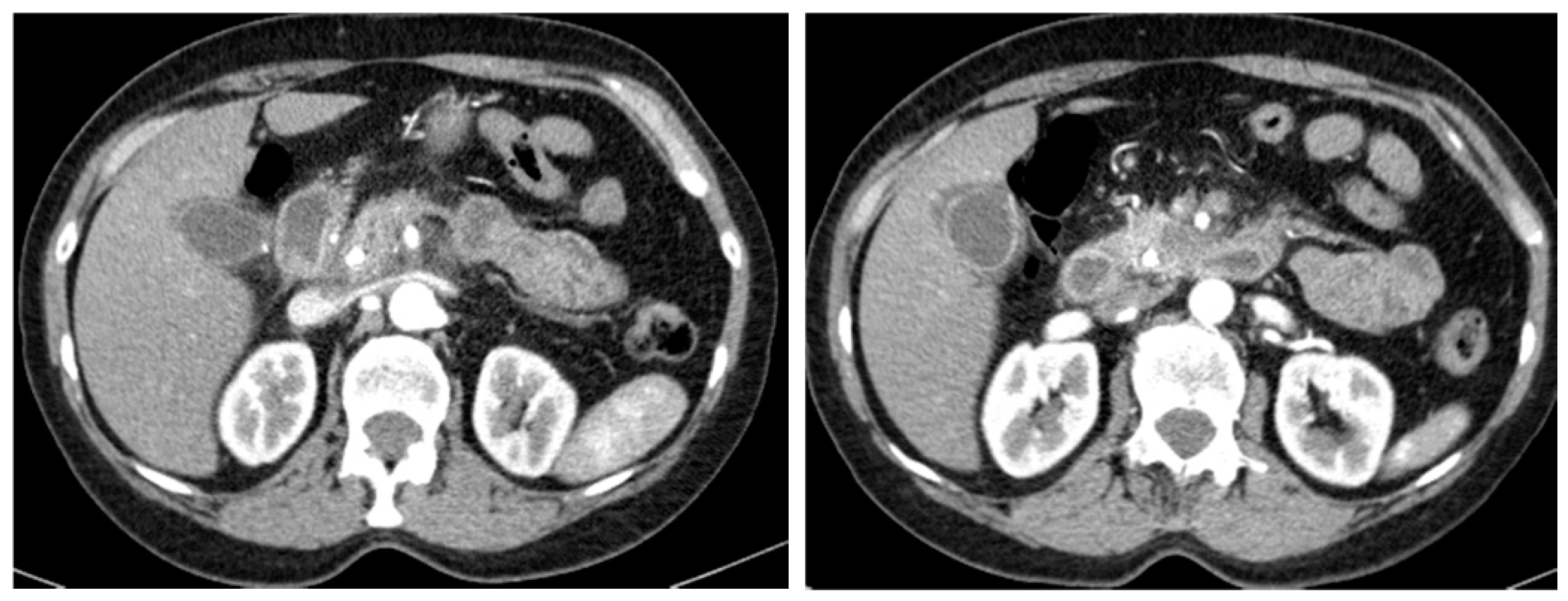
3.2. Imaging and Anatomopathological Correlation
3.3. Interobserver Agreement
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| CRT | Chemoradiotherapy |
| HASTE | Half-Fourier Acquisition Single-shot Turbo spin-Echo |
| IOA | Inter-Observer Agreement |
| MR | Magnetic Resonance |
| OR | Odds Ratio |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| R0 | No Surgical Margin Infiltration |
| R+ | Positive Surgical Margin Infiltration |
References
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goere, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Asbun, H.; Bain, A.; Behrman, S.W.; Benson, A.B., III; Binder, E.; Cardin, D.B.; Cha, C.; et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 1028–1061. [Google Scholar] [CrossRef] [PubMed]
- Laeseke, P.F.; Chen, R.; Jeffrey, R.B.; Brentnall, T.A.; Willmann, J.K. Combining in Vitro Diagnostics with in Vivo Imaging for Earlier Detection of Pancreatic Ductal Adenocarcinoma: Challenges and Solutions. Radiology 2015, 277, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Antunes, C.; Pietrasz, D.; Cassinotto, C.; Zappa, M.; Sa Cunha, A.; Lucidarme, O.; Bachet, J.B. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur. Radiol. 2017, 27, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.P.; Gatta, G.; Verdecchia, A.; Esteve, J.; Sant, M.; Storm, H.; Allemani, C.; Ciccolallo, L.; Santaquilani, M.; Berrino, F. EUROCARE-3 summary: Cancer survival in Europe at the end of the 20th century. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2003, 14 (Suppl. 5), v128–v149. [Google Scholar] [CrossRef]
- Puleo, F.; Marechal, R.; Demetter, P.; Bali, M.A.; Calomme, A.; Closset, J.; Bachet, J.B.; Deviere, J.; Van Laethem, J.L. New challenges in perioperative management of pancreatic cancer. World J. Gastroenterol. 2015, 21, 2281–2293. [Google Scholar] [CrossRef] [PubMed]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Meyer Zum Buschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [PubMed]
- Sa Cunha, A.; Rault, A.; Laurent, C.; Adhoute, X.; Vendrely, V.; Bellannee, G.; Brunet, R.; Collet, D.; Masson, B. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J. Am. Coll. Surg. 2005, 201, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Marthey, L.; Sa-Cunha, A.; Blanc, J.F.; Gauthier, M.; Cueff, A.; Francois, E.; Trouilloud, I.; Malka, D.; Bachet, J.B.; Coriat, R.; et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: Results of an AGEO multicenter prospective observational cohort. Ann. Surg. Oncol. 2015, 22, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Zins, M.; Matos, C.; Cassinotto, C. Pancreatic Adenocarcinoma Staging in the Era of Preoperative Chemotherapy and Radiation Therapy. Radiology 2018, 287, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Cortade, J.; Belleannee, G.; Lapuyade, B.; Terrebonne, E.; Vendrely, V.; Laurent, C.; Sa-Cunha, A. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur. J. Radiol. 2013, 82, 589–593. [Google Scholar] [CrossRef] [PubMed]
- White, R.R.; Paulson, E.K.; Freed, K.S.; Keogan, M.T.; Hurwitz, H.I.; Lee, C.; Morse, M.A.; Gottfried, M.R.; Baillie, J.; Branch, M.S.; et al. Staging of pancreatic cancer before and after neoadjuvant chemoradiation. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2001, 5, 626–633. [Google Scholar] [CrossRef]
- Xia, B.T.; Fu, B.; Wang, J.; Kim, Y.; Ahmad, S.A.; Dhar, V.K.; Levinsky, N.C.; Hanseman, D.J.; Habib, D.A.; Wilson, G.C.; et al. Does radiologic response correlate to pathologic response in patients undergoing neoadjuvant therapy for borderline resectable pancreatic malignancy? J. Surg. Oncol. 2017, 115, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Sa-Cunha, A.; Trillaud, H. Radiological evaluation of response to neoadjuvant treatment in pancreatic cancer. Diagn. Interv. Imaging 2016, 97, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Al-Hawary, M.M.; Francis, I.R.; Chari, S.T.; Fishman, E.K.; Hough, D.M.; Lu, D.S.; Macari, M.; Megibow, A.J.; Miller, F.H.; Mortele, K.J.; et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014, 270, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Park, M.S.; Hong, H.S.; Kang, C.M.; Choi, J.Y.; Lim, J.S.; Lee, W.J.; Kim, M.J.; Kim, K.W. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology 2009, 250, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.E.; Waggoner, C.N.; Canon, C.L.; Lockhart, M.E.; Fineberg, N.S.; Posey, J.A., III; Vickers, S.M. Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: A challenge for MDCT. AJR Am. J. Roentgenol. 2010, 194, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Mouries, A.; Lafourcade, J.P.; Terrebonne, E.; Belleannee, G.; Blanc, J.F.; Lapuyade, B.; Vendrely, V.; Laurent, C.; Chiche, L.; et al. Locally advanced pancreatic adenocarcinoma: Reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology 2014, 273, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.; Fleming, J.B.; Bhosale, P.; Varadhachary, G.; Lee, J.E.; Wolff, R.; Wang, H.; Abbruzzese, J.; Pisters, P.W.; Vauthey, J.N.; et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012, 118, 5749–5756. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
| Study Population (N = 71) | Values |
|---|---|
| Age (years) | 63.8 ± 8.4 |
| Male (%) | 45.1 |
| CT evaluation (%) | 78.9 |
| MR evaluation (%) | 21.1 |
| FOLFIRINOX (%) | 45.1 |
| Gemcitabine-Abraxane (%) | 32.4 |
| Other CRT protocol (%) | 22.5 |
| Dudoenocephalopancreatectomy (%) | 78.9 |
| Lesion Parameters (N = 71) | PER | Consensus | AP | |
|---|---|---|---|---|
| Major Axis (mm) | 25 (20–30) | 22.3 (18.7–27) | 23.5 (15–30) | |
| Homogeneous Pattern (%) | Arterial | 28.5 | 65.1 | − |
| Venous | 88.3 | 50 | − | |
| Equilibrium | 26.5 | 58.8 | − | |
| Enhancement (%) | Venous | 4.8 | 1.59 | − |
| Equilibrium | 21.9 | 9.4 | − | |
| Perivascular Cuff (%) | 36.6 | 26.8 | − | |
| Tumor Persistence (%) | 49.3 | 47.9 | 78.9 | |
| Retroperitoneal Infiltration (%) | 43.7 | 29.6 | 23.9 | |
| Fibrosis (%) | 64.1 | 71.8 | 57.8 | |
| R+ Resection (%) | − | − | 63.4 | |
| Lesion Parameters (N = 71) | SE (%) | SP (%) | AC (%) | |
|---|---|---|---|---|
| Major Axis | >20 mm | 93 | 46 | 79 |
| >25 mm | 67 | 90 | 77 | |
| >30 mm | 31 | 86 | 66 | |
| Retroperitoneal infiltration | 53 | 68 | 64 | |
| Fibrosis | 50 | 65 | 64 | |
| Tumor Persistence | 64 | 57 | 62 | |
| 25 mm vs. | SE (%) | SP (%) | AC (%) |
|---|---|---|---|
| Surgical margin infiltration (R+) | 64 | 78 | 69 |
| Retroperitoneal infiltration | 23 | 94 | 58 |
| Tumor Persistence | 51 | 67 | 53 |
| Parameters | PER vs. GR (k) | PER vs. YR (k) | GR vs. YR (k) | |
|---|---|---|---|---|
| Major Axis | >15 mm | −0.03 | 0.31 | −0.07 |
| >20 mm | 0.18 | 0.39 | 0.32 | |
| >25 mm | 0.33 | 0.55 | 0.46 | |
| >30 mm | 0.48 | 0.53 | 0.54 | |
| Homogeneous pattern | Arterial | −0.03 | 0.17 | 0.24 |
| Venous | 0.08 | 0.08 | 0.02 | |
| Equilibrium | 0.09 | 0.24 | 0.18 | |
| Enhancement | Venous | −0.03 | −0.04 | −0.04 |
| Equilibrium | −0.09 | −0.29 | 0.21 | |
| Retroperitoneal infiltration | −0.03 | 0.28 | 0.39 | |
| Fibrosis | 0.28 | −0.11 | 0.27 | |
| Perivascular Cuff | 0.29 | 0.59 | 0.46 | |
| Tumor Persistence | 0.28 | 0.48 | 0.38 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beleù, A.; Calabrese, A.; Rizzo, G.; Capelli, P.; Bellini, N.; Caloggero, S.; Calbi, R.; Tinazzi Martini, P.; De Robertis, R.; Carbognin, G.; et al. Preoperative Imaging Evaluation after Downstaging of Pancreatic Ductal Adenocarcinoma: A Multi-Center Study. Cancers 2019, 11, 267. https://doi.org/10.3390/cancers11020267
Beleù A, Calabrese A, Rizzo G, Capelli P, Bellini N, Caloggero S, Calbi R, Tinazzi Martini P, De Robertis R, Carbognin G, et al. Preoperative Imaging Evaluation after Downstaging of Pancreatic Ductal Adenocarcinoma: A Multi-Center Study. Cancers. 2019; 11(2):267. https://doi.org/10.3390/cancers11020267
Chicago/Turabian StyleBeleù, Alessandro, Angela Calabrese, Giulio Rizzo, Paola Capelli, Nicolò Bellini, Simona Caloggero, Roberto Calbi, Paolo Tinazzi Martini, Riccardo De Robertis, Giovanni Carbognin, and et al. 2019. "Preoperative Imaging Evaluation after Downstaging of Pancreatic Ductal Adenocarcinoma: A Multi-Center Study" Cancers 11, no. 2: 267. https://doi.org/10.3390/cancers11020267
APA StyleBeleù, A., Calabrese, A., Rizzo, G., Capelli, P., Bellini, N., Caloggero, S., Calbi, R., Tinazzi Martini, P., De Robertis, R., Carbognin, G., Marchegiani, G., Scarpa, A., Salvia, R., Bassi, C., & D’Onofrio, M. (2019). Preoperative Imaging Evaluation after Downstaging of Pancreatic Ductal Adenocarcinoma: A Multi-Center Study. Cancers, 11(2), 267. https://doi.org/10.3390/cancers11020267






