A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique
Abstract
1. Introduction
2. Material and Methods
- -
- Women diagnosed with left-sided breast cancer requiring radiotherapy to the breast, chest wall with/without supraclavicular region, without internal mammary chain irradiation
- -
- Consented to undergo CT simulation scans in DIBH and FB with their data permitted for future use in research
- -
- Planned for treatment between May and September 2015
- -
- Completed treatment at ARC.
- -
- Did not have both CT simulation scans (FB and DIBH) completed.
3. Ethics Approval and Consent to Participate
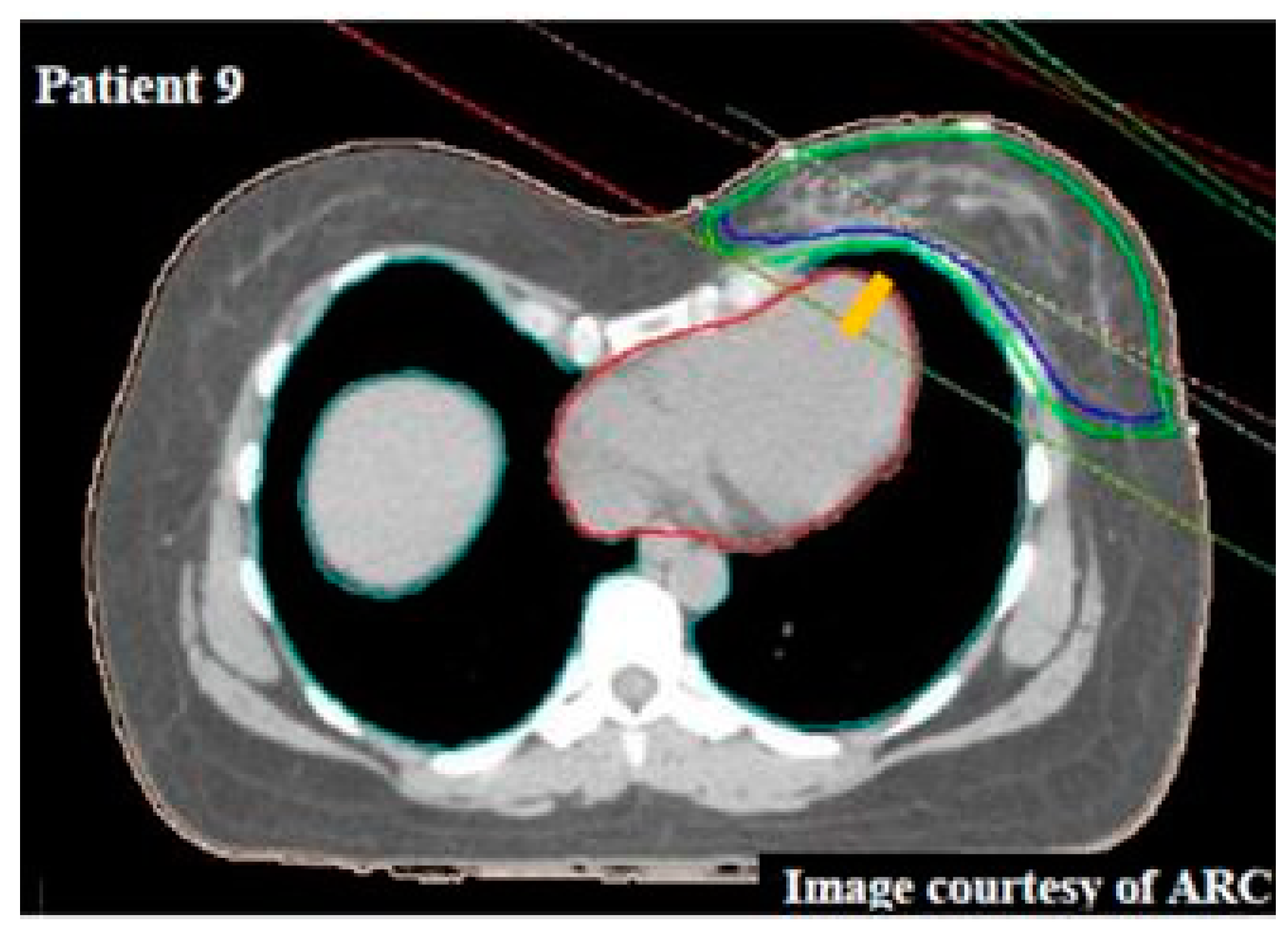
4. Delineation of Regions of Interest
5. Inter- and Intra-Observer Testing of LAD
6. Treatment Planning and Evaluation
7. Patient Data Collection
8. Haller Index
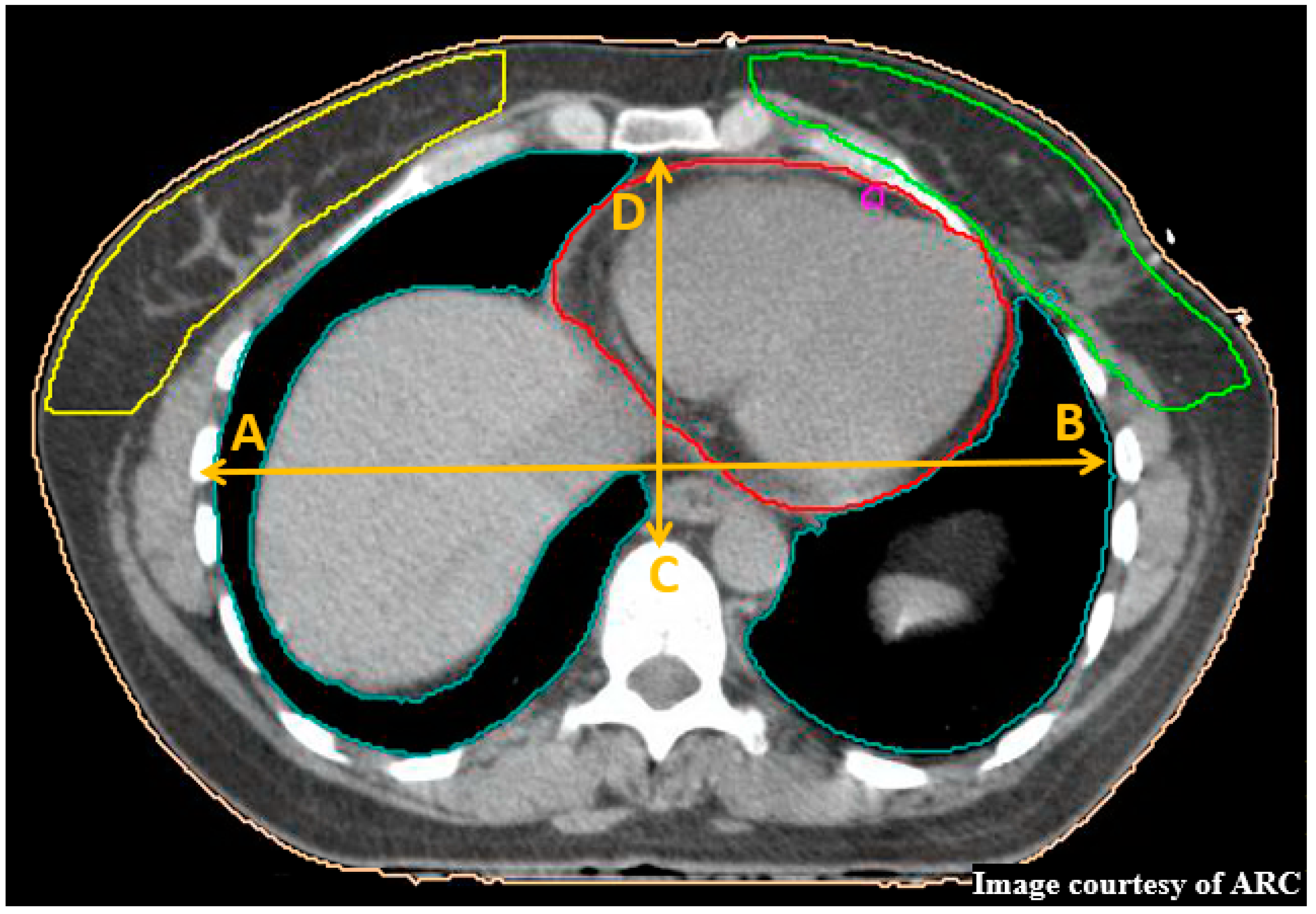
9. Maximum Heart Distance
10. Central Lung Distance and Chest Wall Separation
11. Statistical Analysis
12. Results
12.1. Planning Volumes
12.2. Inter- and Intra-Observer Testing of LAD
12.3. Dosimetric Evaluation of DIBH
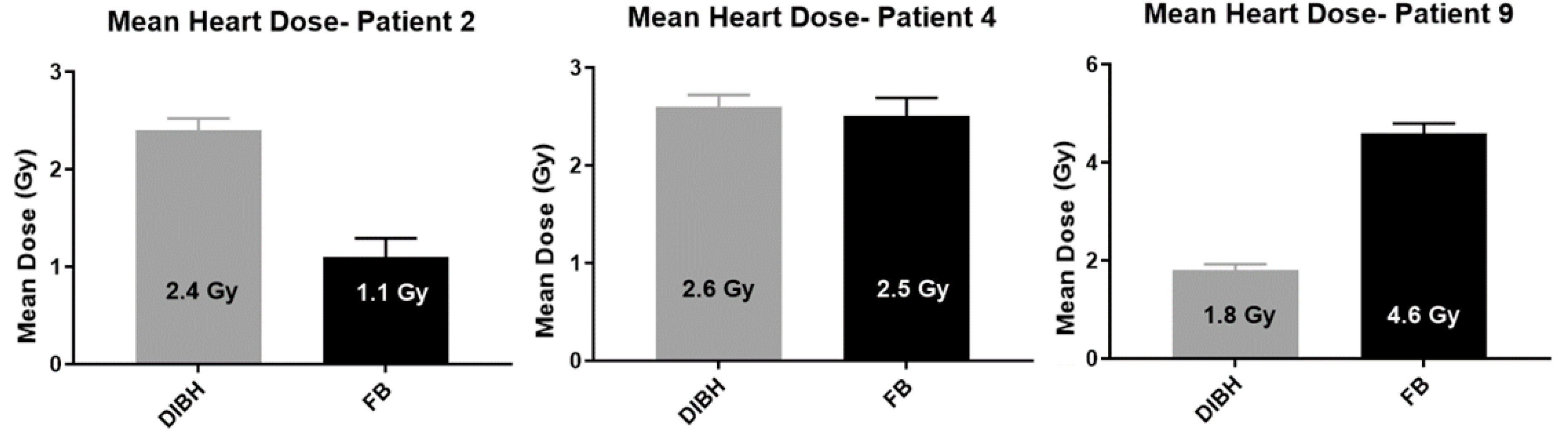
12.4. Heart Dose
12.5. Left Anterior Descending Artery Dose
12.6. Total Lung Volume
12.7. Maximum Heart Distance
12.8. Central Lung Distance
12.9. Chest Wall Separation
12.10. Correlation and Linear Regression of Analysed Dosimetric Data
12.11. Selection Criteria
13. Discussion
14. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Darby, S.; Ewertz, M.; McGale, P.; Bennet, A.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. New Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Darapu, A.; Balakrishnan, R.; Sebastian, P.; Hussain, M.; Ravindran, P.; John, S. Is the Deep Inspiration Breath-Hold Technique Superior to the Free Breathing Technique in Cardiac and Lung Sparing while Treating both Left-Sided Post-Mastectomy Chest Wall and Supraclavicular Regions? Case Rep. Oncol. 2017, 10, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Latty, D.; Stuart, K.; Wang, W.; Ahern, V. Review of deep inspiration breath-hold techniques for the treatment of breast cancer. J. Med. Radiat. Sci. 2015, 62, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bruzzaniti, V.; Abate, A.; Pinnaro, P.; D’Andrea, M.; Infusino, E.; Landoni, V.; Soriani, A.; Giordano, C.; Ferraro, A.; Strigari, L. Dosimetric and clinical advantages of deep inspiration breath-hold (DIBH) during radiotherapy of breast cancer. J. Exp. Clin. Cancer Res. 2013, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Parasuramar, A.; DeSmit, A.; Borg, M. Deep Inspiration Breath Hold Techniques in the Radiotherapeutic Management of Left-Sided Breast Cancer-Active Breathing Coordinator vs. Voluntary Breath Hold. Int. J. Gynecol. Clin. Pract. 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Lin, A.; Sharieff, W.; Juhasz, J.; Whelan, T.; Kim, D. The benefit of deep inspiration breath hold: Evaluating cardiac radiation exposure in patients after mastectomy and after breast-conserving surgery. Breast Cancer 2017, 24, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Schonecker, S.; Walter, F.; Freislederer, P.; Marisch, C.; Scheithauer, H.; Harbeck, N.; Corradini, S.; Belka, C. Treatment planning and evaluation of gated radiotherapy in left-sided breast cancer patients using the Catalyst/Sentinel system for deep inspiration breath-hold (DIBH). Radiat. Oncol. 2016, 11, 143–153. [Google Scholar] [CrossRef]
- Kim, T.; Reardon, K.; Trifiletti, D.; Geesey, C.; Sukovich, K.; Crandley, E.; Read, P.; Wijesooriya, K. How dose sparing of cardiac structures correlates with in-field heart volume and sternal displacement. J. Appl. Clin. Med. Phys. 2016, 17, 60–68. [Google Scholar] [CrossRef]
- Meyer, P.; Niederst, C.; Scius, M.; Jarnet, D.; Dehaynin, N.; Gantier, M.; Waissi, W.; Poulin, N.; Karamanoukian, D. Is the lack of respiratory gating prejudicial for left breast TomoDirect treatments? Phys. Med. 2016, 32, 644–650. [Google Scholar] [CrossRef]
- Joo, J.; Kim, S.; Ahn, S.; Kwak, J.; Jeong, C.; Ahn, S.; Son, B.; Lee, J. Cardiac dose reduction during tangential breast irradiation using deep inspiration breath hold: A dose comparison study based on deformable image registration. Radiat. Oncol. 2015, 10, 264–273. [Google Scholar] [CrossRef]
- Hepp, R.; Ammerpohl, M.; Morgenstern, C.; Nielinger, L.; Erichsen, P.; Abdallah, A.; Galalae, R. Deep inspiration breath-hold (DIBH) radiotherapy in left-sided breast cancer: Dosimetrical comparison and clinical feasibility in 20 patients. Strahlenther. Onkol. 2015, 191, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Lee, K.; Lee, S.; Ahn, S.; Lee, S.; Choi, J. Cardiac dose reduction with breathing adapted radiotherapy using self respiration monitoring system for left-sided breast cancer. Radiat. Oncol. 2014, 32, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chang, J.; Lee, I.; Park, K.; Kim, Y.; Suh, C.; Kim, J.; Keum, K. The deep inspiration breath hold technique using Abches reduces cardiac dose in patients undergoing left-sided breast irradiation. Radiat. Oncol. 2013, 31, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Swanson, T.; Grills, I.; Ye, H.; Entwistle, A.; Teahan, M.; Letts, N.; Yan, D.; Duquette, J.; Vicini, F. Six-year experience routinely using moderate deep inspiration breath-hold for the reduction of cardiac dose in left-sided breast irradiation for patients with early-stage or locally advanced breast cancer. Am. J. Clin. Oncol. 2013, 36, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hjelstuen, M.; Mjaaland, I.; Vikstrom, J.; Dybvik, K.I. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol. 2012, 51, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Purdie, T.; Rahman, M.; Marshall, A.; Liu, F.; Fyles, A. Rapid automated treatment planning process to select breast cancer patients for active breathing control to achieve cardiac dose reduction. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Vikstrom, J.; Hjelstuen, M.; Mjaaland, I.; Dybvik, K. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011, 50, 42–50. [Google Scholar] [CrossRef]
- Fokin, A.; Steuerwald, N.; Ahrens, W.; Allen, K. Anatomical, Histologic, and Genetic Characteristics of Congenital Chest Wall Deformities. Semin. Thorac. Cardiovasc. Surg. 2009, 21, 44–57. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Kim, E.; Kim, H.; Kim, J.; Cho, J.; Yang, H. Proper compression landmark and depth for cardiopulmonary resuscitation in patients with pectus excavatum: A study using CT. Emerg. Med. J. 2015, 32, 301–303. [Google Scholar] [CrossRef]
- Hayden, A.J.; Rains, M.; Tiver, K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J. Med. Imaging Radiat. Oncol. 2012, 56, 464–472. [Google Scholar] [CrossRef]
- Rochet, N.; Drake, J.; Harrington, K.; Wolfgang, J.; Napolitano, B.; Sadek, B.; Shenouda, M.; Keruakous, A.; Niemierko, A.; Taghian, A. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: Evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Pract. Radiat. Oncol. 2015, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Register, S.; Takita, C.; Reis, I.; Zhao, W.; Amestoy, W.; Wright, J. Deep inspiration breath-hold technique for left-sided breast cancer: An analysis of predictors for organ-at-risk sparing. Med. Dosim. 2015, 40, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Johansen, S.; Vikstrom, J.; Hjelstuen, M.; Mjaaland, I.; Dybvik, K.; Olsen, D. Dose evaluation and risk estimation for secondary cancer in contralateral breast and a study of correlation between thorax shape and dose to organs at risk following tangentially breast irradiation during deep inspiration breath-hold and free breathing. Acta Oncol. 2011, 50, 563–568. [Google Scholar] [CrossRef]
- Chilukuri, S.; Adulkar, D.; Subramaniam, S.; Mohammed, N.; Gandhi, A.; Kathirvel, M.; Swamy, T.; Kumar, K.; Yadala, N. Patient selection for DIBH technique for left sided breast cancers: Impact of chest wall shape. Radiat. Oncol. 2016, 119, S422. [Google Scholar] [CrossRef]
- Nissen, H.; Appelt, A. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother. Oncol. 2013, 106, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power 3.1.0; Universität Kiel: Kiel, Germany, 2009. [Google Scholar]
- International Commission on Radiation Units & Measurements (ICRU). Prescribing, Recording and Reporting Photon Beam Therapy (ICRU Report 62); ICRU: Bethesda, MD, USA, 1999. [Google Scholar]
- Feng, M.; Moran, J.; Koelling, T.; Chughtai, A.; Chan, J.; Freedman, L.; Hayman, J.; Jagsi, R.; Jolly, S.; Larouere, J.; et al. Development and Validation of a Heart Atlas to Study Cardiac Exposure to Radiation Following Treatment for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Andrews, J.; Cao, M.; Johnstone, P. Correlation of 2D parameters to lung and heart dose-volume in radiation treatment of breast cancer. Acta Oncol. 2013, 52, 178–183. [Google Scholar] [CrossRef]
- Eng, J. Sample Size Estimation: How Many Individuals Should Be Studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef]
- Shim, J.; Kim, J.; Park, W.; Seo, J.; Hong, C.; Song, K.; Lim, C.; Jung, H.; Kim, C. Dose-Volume Analysis of Lung and Heart according to Respiration in Breast Cancer Patients Treated with Breast Conserving Surgery. Breast Cancer 2012, 15, 105–110. [Google Scholar] [CrossRef]
- Tanguturi, S.; Lyatskaya, Y.; Chen, Y.; Catalano, P.; Truong, L.; Yeh, M.; Yeo, W.; Orlina, L.; Wong, J.; Punglia, R.; et al. Prospective Assessment of Deep Inspiration Breath Hold Using 3-Dimensional Surface Tracking for Irradiation of Left-Sided Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, E4. [Google Scholar] [CrossRef]
| Region of Interest | Target Goals | Variation Accepted |
|---|---|---|
| CTV | D95 ≥ 95% | D95 ≥ 90% |
| PTV | D90 ≥ 98% | D90 ≥ 95% |
| Heart | V10 < 10% | V10 < 15% |
| V25 < 3% | V25 < 4% | |
| Dmean < 3 Gy | Dmean < 4 Gy | |
| Ipsilateral lung | V30 < 12% | V30 < 15% |
| V20 < 15% | V20 < 20% | |
| V10 < 255 | V10 < 30% | |
| V5 < 30% | V5 < 50% | |
| Total lung | V20 < 15% | V20 < 20% |
| V10 < 20% | V10 < 25% | |
| V5 < 20% | V5 < 30% | |
| Contralateral Breast | V3 < 3% |
| Parameters | Dosimetric Parameters | |
|---|---|---|
| Dosimetric | CTV | D95 |
| PTV | D90 | |
| Ipsilateral lung | V5, V10, V20, V30 and volume | |
| Total lung | V5, V10, V20 and volume | |
| Heart | Dmean, Dmax, V10, V20, V25 and V30 | |
| LAD | Dmean, Dmax, V20 and D0.2cm³ | |
| Contralateral Breast | V3 | |
| Anatomical | Chest Wall Separation (CWS) Maximum Heart Distance (MHD) Central Lung Distance (CLD) | |
| Treatment | Type of deep inspiration breath hold Chemotherapy details Prescription Histological diagnosis Age Height Weight Comorbidities | |
| Target Coverage | CTDIBH | CTFB | p-value | ||
|---|---|---|---|---|---|
| Mean Value (Range) | SD | Mean Value (Range) | SD | ||
| PTV D90 (%) | 88.5 (6.1–98.3) | 23.0 | 89.8 (9.5–98.3) | 19.3 | >0.05 * |
| CTV D95 (%) | 89.4 (4.5–98.7) | 21.0 | 89.6 (5.7–98.9) | 20.1 | >0.05 * |
| Patient No. | DIBH Device | Heart | LAD | Total Lung | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔDmean (Gy) | ΔDmax (Gy) | ΔV10 (%) | ΔV30 (%) | ΔDmean (Gy) | ΔDmax (Gy) | ΔV20 (%) | ΔD0.2cm3 (Gy) | ΔV10 (%) | ΔV20 (%) | ||
| 1 | Voluntary BH § | 1.9 | 8.4 | 5.8 | 3.0 | 15.1 | 33.7 | 38.0 | 42.5 | 1.0 | 1.4 |
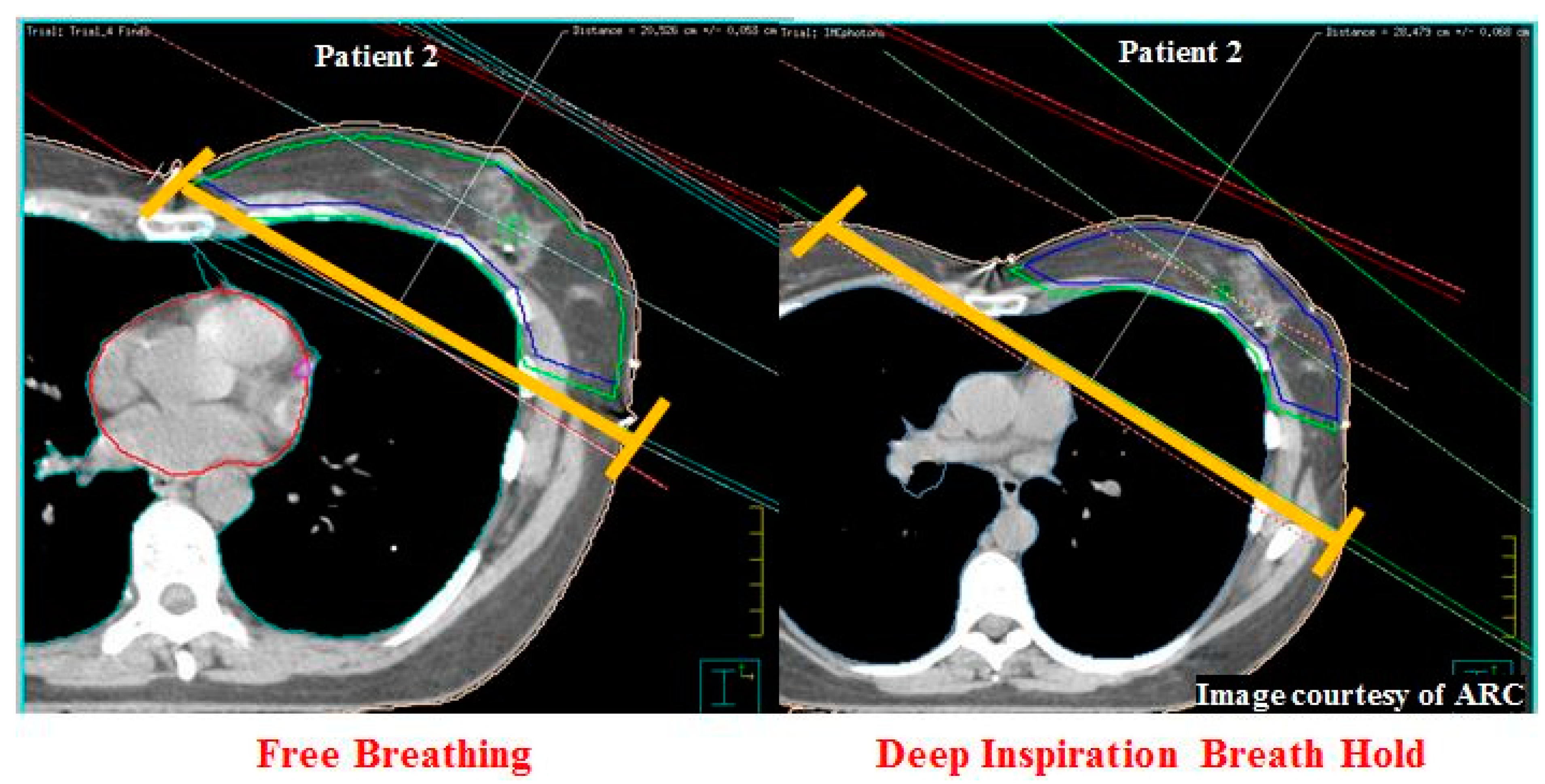
| 2 | Voluntary BH § | −1.3 | −25.4 | −2.6 | 0.0 | −3.9 | −19.6 | −8.0 | −12.4 | −5.9 | −5.8 |
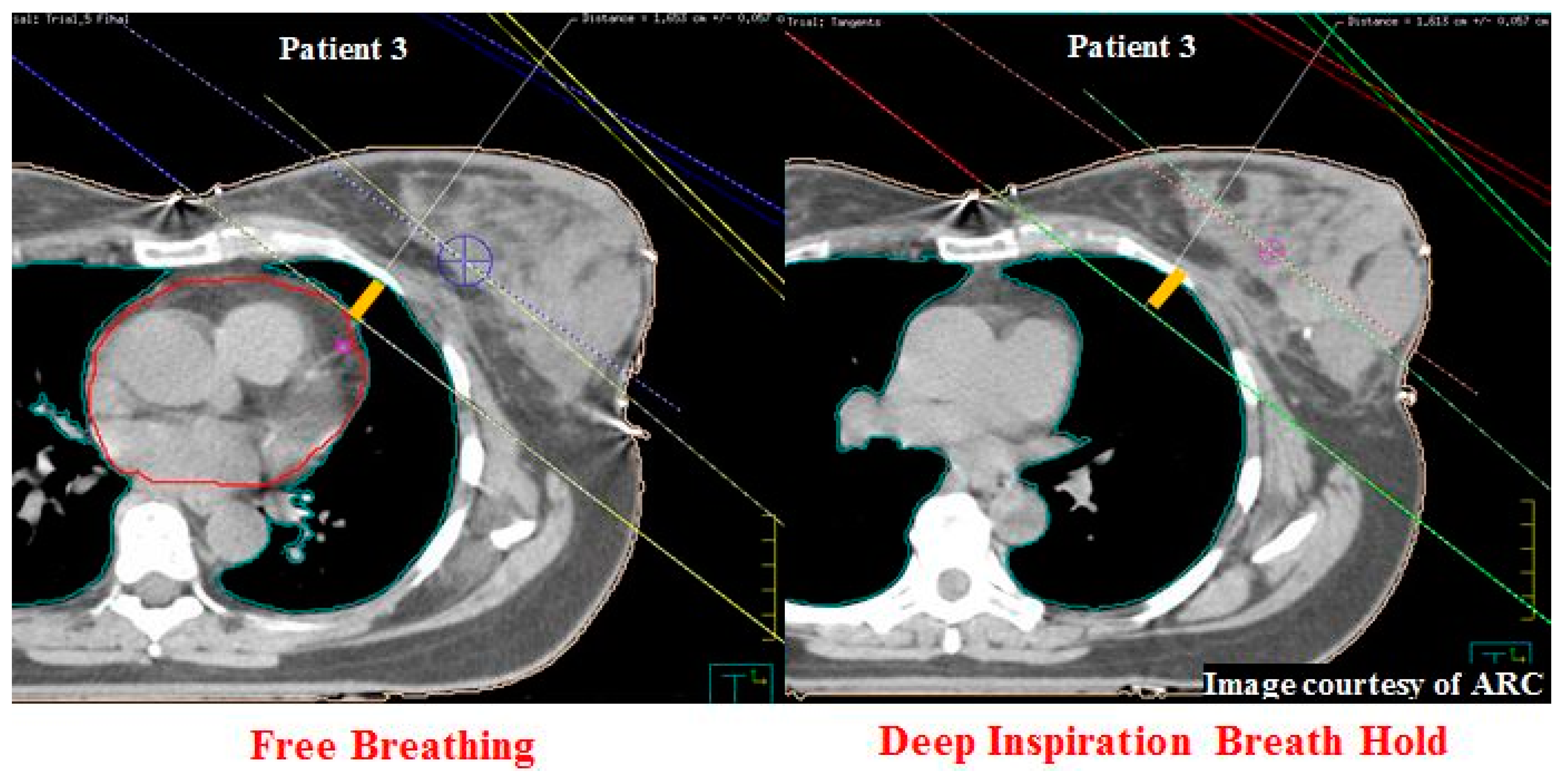
| 3 | ABC § | 1.1 | 11.5 | 3 | 2.0 | 8.7 | 11.7 | 22.0 | 24.1 | 0.3 | 0.6 |
| 4 | Voluntary BH † | −0.1 | 2.7 | 2.8 | 1.0 | 11.7 | 4.9 | 44.0 | 19.5 | 13.4 | 2.7 |
| 5 | ABC † | 1.5 | −1.1 | 3.9 | 3.0 | −3.5 | −22.1 | −10.0 | −2.3 | 0.1 | 0.2 |
| 6 | Voluntary BH † | 1.2 | −4.1 | 0.1 | 0.0 | 0.6 | −3.3 | −5.0 | −4.0 | 0.9 | 0.9 |
| 7 | ABC § | 0.6 | 14.7 | 1.5 | 1.0 | 0 | −5.5 | 0 | −1.0 | 2.8 | 2.9 |
| 8 | Voluntary BH § | 1.5 | 2.5 | 5.1 | 1.0 | 12.0 | 4.6 | 52.0 | 16.9 | −1.9 | −1.6 |
| 9 | Voluntary BH § | 2.8 | 7.8 | 5.3 | 3.0 | 9.6 | 34.6 | 25.0 | 37.9 | −1.1 | −0.9 |
| 10 | ABC § | 1.9 | 10.5 | 3.5 | 2.0 | 12.2 | 38.1 | 31.0 | 39.6 | 4.1 | 4 |
| 11 | ABC § | 1.6 | 2.3 | −15.7 | 3.0 | 11.0 | 3.2 | 25.0 | 11.7 | 3.9 | 4.1 |
| 12 | ABC § | 1.7 | 33.0 | 4.5 | 3.0 | 11.0 | 42.6 | 29.0 | 39.6 | 3.6 | 3.3 |
| 13 | Voluntary BH † | 0.2 | 13.8 | 1.4 | 0.0 | 2.8 | 19.9 | 2.0 | 9.4 | 3.9 | 3.5 |
| 14 | Voluntary BH § | 1.3 | 4.8 | 3.1 | 2.0 | 1.0 | 6.6 | 0 | 1.6 | 2.1 | 2.2 |
| 15 | ABC § | 1.0 | 17.4 | 2.9 | 1.0 | 4.5 | 27.4 | 10.0 | 18.0 | −2.2 | −1.7 |
| 16 | Voluntary BH † | 2.1 | 6.6 | 6.4 | 4.0 | 17.1 | 22.8 | 53.0 | 30.1 | 1.1 | 1.6 |
| 17 | Voluntary BH § | 1.2 | −1.6 | 4.9 | 3.0 | 9.7 | 28.1 | 34.0 | 35.5 | −3.2 | −2 |
| 18 | ABC † | 1.7 | 0.7 | 6.1 | 4.0 | 5.4 | −0.6 | 17.0 | 5.1 | 5.0 | 4.8 |
| 19 | ABC § | 1.5 | 0.0 | 3.9 | 2.0 | 12.5 | 19.1 | 34.0 | 34.3 | −2.3 | −1.5 |
| 20 | ABC § | 1.8 | −2.6 | 5.9 | 3.0 | 12.3 | 9.2 | 41.0 | 22.1 | 1.3 | 1.1 |
| Mean | 1.3 (1.3 §, 1.1 †) | 5.1 (5.9 §, 3.1 †) | 2.6 (2.2 §, 3.5 †) | 2.0 (2.1 §, 2.0 †) | 7.5 (8.2 §, 5.7 †) | 12.8 (16.7 §, 3.6 †) | 21.7 (23.8 §, 16.8 †) | 18.4 (22.2 §, 9.6 †) | 1.3 (0.2 §, 4.1 †) | 1 (0.4 §, 2.3 †) | |
| Patient No. | CLD (cm) | TLV (cm3) | MHD (cm) | CWS (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTFB | CTDIBH | Difference Δ | CTFB | CTDIBH | Difference Δ | CTFB | CTDIBH | Difference Δ | CTFB | CTDIBH | Difference Δ | |
| 1 | 2.4 | 2.3 | −0.1 | 3207 | 5076 | 1869 | 1.8 | 0.6 | 1.2 | 22.5 | 20.5 | 2 |
| 2 | 3.3 | 5.6 | 2.3 | 4788 | 6608 | 1820 | 0.0 | 0.0 | 0.0 | 20.4 | 28.4 | −8 |
| 3 | 1.6 | 1.6 | 0 | 1712 | 3443 | 1731 | 1.2 | 0.6 | 0.6 | 21.3 | 21.0 | 0.3 |
| 4 | 2.4 | 2.5 | 0.1 | 2769 | 4714 | 1945 | 1.0 | 0.5 | 0.5 | 20.4 | 19.4 | 1 |
| 5 | 2.5 | 2.7 | 0.2 | 3330 | 4922 | 1592 | 1.7 | 1.4 | 0.3 | 22.0 | 22.1 | −0.1 |
| 6 | 2.2 | 2.6 | 0.4 | 2840 | 4579 | 1739 | 2.3 | 1.0 | 1.3 | 22.1 | 19.1 | 3 |
| 7 | 2.9 | 3.0 | 0.1 | 3186 | 5765 | 2579 | 1.1 | 0.0 | 1.1 | 21.6 | 22.4 | −0.8 |
| 8 | 2.1 | 2.4 | 0.3 | 2275 | 3755 | 1480 | 1.5 | 1.3 | 0.2 | 29.1 | 28.8 | 0.3 |
| 9 | 2.1 | 3.1 | 1 | 2336 | 4277 | 1941 | 1.9 | 1.2 | 0.7 | 21.7 | 21.8 | −0.1 |
| 10 | 3.9 | 4.1 | 0.2 | 2783 | 4354 | 1571 | 3.1 | 2.2 | 0.9 | 23.5 | 21.3 | 2.2 |
| 11 | 2.0 | 3.3 | 1.3 | 2520 | 4461 | 1941 | 1.7 | 1.4 | 0.3 | 26.2 | 27.6 | −1.4 |
| 12 | 2.2 | 1.8 | −0.4 | 2335 | 4714 | 2379 | 1.6 | 0.1 | 1.5 | 22.8 | 22.2 | 0.6 |
| 13 | 2.9 | 2.4 | −0.5 | 3341 | 5320 | 1979 | 1.6 | 0.8 | 0.8 | 22.6 | 21.4 | 1.2 |
| 14 | 2.4 | 2.2 | −0.2 | 2561 | 4166 | 1605 | 1.3 | 0.8 | 0.5 | 22.3 | 21.8 | 0.5 |
| 15 | 2.1 | 2.2 | 0.1 | 2271 | 3893 | 1622 | 2.1 | 0.8 | 1.3 | 22.6 | 22.6 | 0 |
| 16 | 2.6 | 2.9 | 0.3 | 2730 | 5214 | 2484 | 2 | 0.7 | 1.3 | 25.7 | 25.8 | −0.1 |
| 17 | 1.6 | 2.2 | 0.6 | 2698 | 4863 | 2165 | 1.2 | 1.0 | 0.2 | 21.9 | 22.8 | −0.9 |
| 18 | 3.4 | 3.0 | −0.4 | 2600 | 4787 | 2187 | 1.9 | 0.6 | 1.3 | 21.1 | 19.7 | 1.4 |
| 19 | 1.6 | 1.8 | 0.2 | 2767 | 5005 | 2238 | 1.6 | 0.5 | 1.1 | 26.9 | 26.6 | 0.3 |
| 20 | 2.5 | 2.5 | 0 | 2556 | 5181 | 2625 | 3.8 | 3.7 | 0.1 | 26.3 | 27.0 | −0.7 |
| Mean | 2.4 | 2.7 | 0.3 | 2780 | 4755 | 1975 | 1.7 | 1.0 | 0.8 | 23.1 | 23.1 | 0 |
| Benefit Group | Patient | Δ TLV (cm3) | Heart Relative Reduction | LAD Relative Reduction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ Dmean (Gy) | Δ V10 (%) | Δ V20 (%) | Δ V25 (%) | Δ V30 (%) | Δ Dmean (Gy) | Δ D0.2cm3 (Gy) | Δ V20 (%) | |||
| Minimum | 8 | 1480 | 1.5 | 5.1 | 4 | 2.1 | 1 | 12.0 | 16.9 | 52 |
| 10 | 1571 | 1.9 | 3.5 | 3 | 2.5 | 2 | 12.2 | 39.6 | 31 | |
| 5 | 1592 | 1.5 | 3.9 | 3 | 3.1 | 3 | −3.5 | −2.3 | −10 | |
| 14 | 1605 | 1.3 | 3.1 | 3 | 2.1 | 2 | 1.0 | 1.6 | 0 | |
| 15 | 1622 | 1 | 2.9 | 2 | 1.8 | 1 | 4.5 | 18.0 | 10 | |
| 3 | 1731 | 1.1 | 3 | 3 | 1.8 | 2 | 8.7 | 24.1 | 22 | |
| 6 | 1739 | 1.2 | 0.1 | −1 | −0.3 | 0 | 0.6 | −4.0 | −5 | |
| Mean | 1620 | 1.3 | 3.0 | 2.4 | 1.8 | 1.5 | 5.1 | 13.4 | 14.2 | |
| Medium | 2 | 1820 | −1.3 | −2.6 | −1 | −0.4 | 0 | −3.9 | −12.4 | −8 |
| 1 | 1869 | 1.2 | 5.8 | 5 | 3.9 | 3 | 15.1 | 42.5 | 38 | |
| 9 | 1941 | 1.2 | 5.3 | 4 | 3.8 | 3 | 9.6 | 37.9 | 25 | |
| 11 | 1941 | 1.2 | −15.7 | 3 | 2.7 | 3 | 11 | 11.7 | 25 | |
| 4 | 1945 | 1.2 | 2.8 | 2 | 1.5 | 1 | 11.7 | 19.5 | 44 | |
| 13 | 1979 | 1.2 | 1.4 | 1 | 0.3 | 0 | 2.87 | 9.41 | 2 | |
| Mean | 1915 | 0.7 | −0.5 | 2.3 | 1.9 | 1.6 | 7.7 | 18.1 | 21 | |
| Maximum | 17 | 2165 | 1.2 | 4.9 | 3 | 3.3 | 3 | 9.7 | 35.5 | 34 |
| 18 | 2187 | 1.2 | 6.1 | 4 | 4.2 | 4 | 5.4 | 5.11 | 17 | |
| 19 | 2238 | 1.2 | 3.9 | 3 | 2.7 | 2 | 12.5 | 34.3 | 34 | |
| 12 | 2379 | 1.2 | 4.5 | 3 | 2.9 | 3 | 11.0 | 39.6 | 29 | |
| 16 | 2484 | 1.2 | 6.4 | 5 | 4.5 | 4 | 17.1 | 30.1 | 53 | |
| 7 | 2579 | 1.2 | 1.5 | 1 | 0.8 | 1 | 0 | −1 | 0 | |
| 20 | 2625 | 1.2 | 5.9 | 5 | 3.4 | 3 | 12.3 | 22.1 | 41 | |
| Mean | 2379 | 1.2 | 4.7 | 3.4 | 3.1 | 2.8 | 9.7 | 23.7 | 29.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Oro, M.; Giles, E.; Sharkey, A.; Borg, M.; Connell, C.; Bezak, E. A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique. Cancers 2019, 11, 259. https://doi.org/10.3390/cancers11020259
Dell’Oro M, Giles E, Sharkey A, Borg M, Connell C, Bezak E. A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique. Cancers. 2019; 11(2):259. https://doi.org/10.3390/cancers11020259
Chicago/Turabian StyleDell’Oro, Mikaela, Eileen Giles, Amy Sharkey, Martin Borg, Caroline Connell, and Eva Bezak. 2019. "A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique" Cancers 11, no. 2: 259. https://doi.org/10.3390/cancers11020259
APA StyleDell’Oro, M., Giles, E., Sharkey, A., Borg, M., Connell, C., & Bezak, E. (2019). A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique. Cancers, 11(2), 259. https://doi.org/10.3390/cancers11020259





