Does Risk-Adapted Proton Beam Therapy Have a Role as a Complementary or Alternative Therapeutic Option for Hepatocellular Carcinoma?
Abstract
:1. Introduction
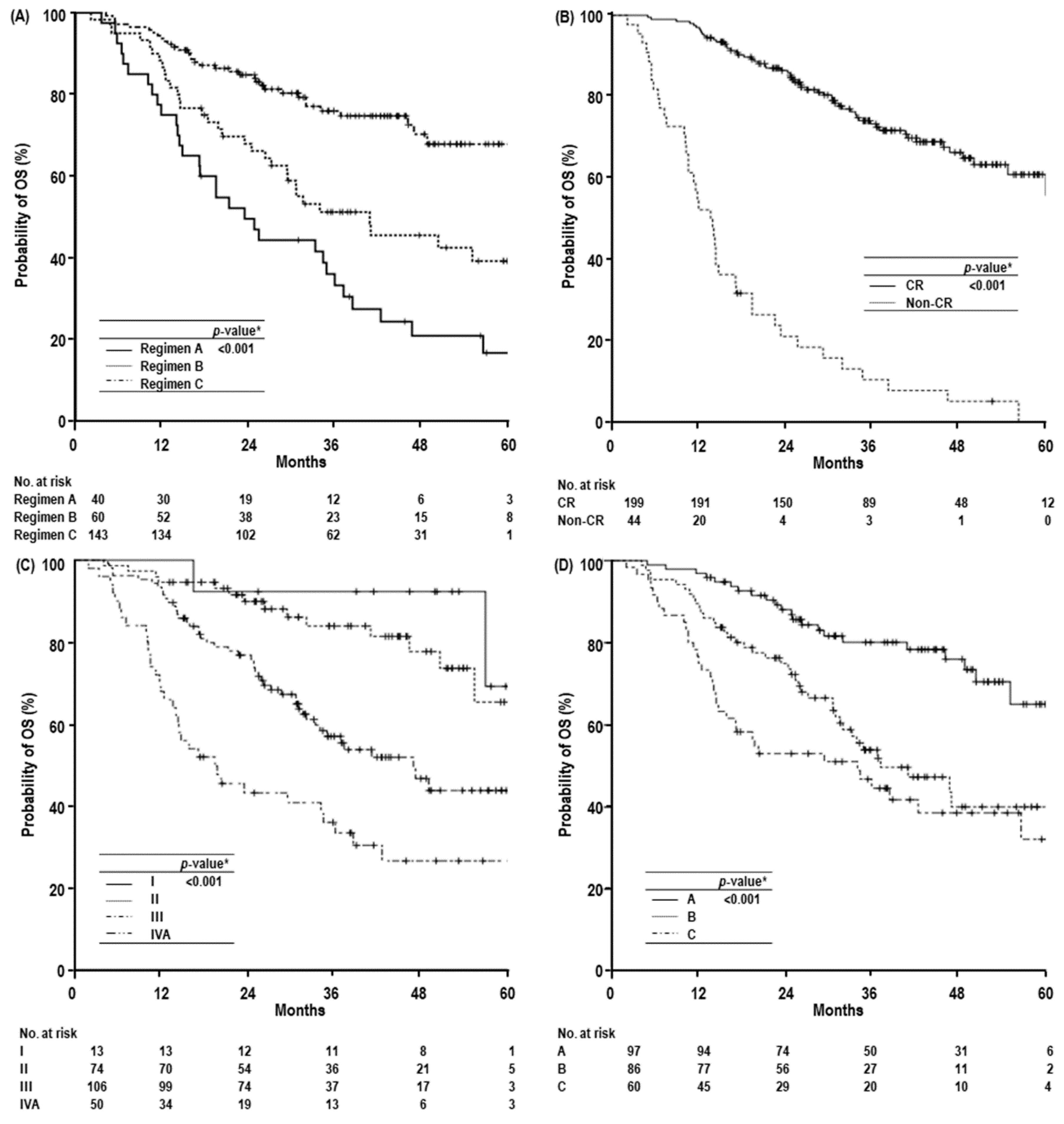
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Treatment
4.3. Evaluation and Statistical Considerations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Korean Liver Cancer Study Group; National Cancer Center. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J. Hepatol. 2009, 15, 391–423. [Google Scholar] [CrossRef] [PubMed]
- Korean Liver Cancer Study Group; National Cancer Center. 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver 2015, 9, 267–317. [Google Scholar]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential phase i and ii trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.A.; Kayali, Z.; Grove, R.; Slater, J.D. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: A phase 2 prospective trial. Cancer 2011, 117, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Okumura, T.; Abei, M.; Fukumitsu, N.; Ishige, K.; Mizumoto, M.; Hasegawa, N.; Numajiri, H.; Ohnishi, K.; Ishikawa, H.; et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 2017, 108, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumitsu, N.; Sugahara, S.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Thono, E.; Tsuboi, K.; Tokuuye, K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-institutional phase ii study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Furuse, J.; Nishio, T.; Konishi, M.; Ishii, H.; Kinoshita, T.; Nagase, M.; Nihei, K.; Ogino, T. Phase ii study of radiotherapy employing proton beam for hepatocellular carcinoma. J. Clin. Oncol. 2005, 23, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, J.W.; Kim, T.H.; Kim, B.H.; Moon, S.H.; Kim, S.S.; Woo, S.M.; Koh, Y.H.; Lee, W.J.; Kim, C.M. Risk-adapted simultaneous integrated boost-proton beam therapy (sib-pbt) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother. Oncol. 2017, 122, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, D.Y.; Park, J.W.; Kim, Y.I.; Kim, S.H.; Park, H.S.; Lee, W.J.; Park, S.J.; Hong, E.K.; Kim, C.M. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am. J. Clin. Oncol. 2006, 29, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, J.W.; Kim, B.H.; Kim, D.Y.; Moon, S.H.; Kim, S.S.; Lee, J.H.; Woo, S.M.; Koh, Y.H.; Lee, W.J.; et al. Optimal time of tumour response evaluation and effectiveness of hypofractionated proton beam therapy for inoperable or recurrent hepatocellular carcinoma. Oncotarget 2018, 9, 4034–4043. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, J.W.; Kim, Y.J.; Kim, B.H.; Woo, S.M.; Moon, S.H.; Kim, S.S.; Koh, Y.H.; Lee, W.J.; Park, S.J.; et al. Phase i dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res. Treat. 2015, 47, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, J.W.; Kim, Y.J.; Kim, B.H.; Woo, S.M.; Moon, S.H.; Kim, S.S.; Lee, W.J.; Kim, D.Y.; Kim, C.M. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther. Onkol. 2014, 190, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Dawson, L.A. Hepatocellular carcinoma radiation therapy: Review of evidence and future opportunities. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Park, J.W.; Kim, T.H.; Kim, Y.J.; Woo, S.M.; Koh, Y.H.; Lee, W.J.; Park, S.J.; Kim, D.Y.; Kim, C.M. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther. Onkol. 2014, 190, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Rim, C.H.; Seong, J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat. Oncol. J. 2016, 34, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dionisi, F.; Widesott, L.; Lorentini, S.; Amichetti, M. Is there a role for proton therapy in the treatment of hepatocellular carcinoma? A systematic review. Radiother. Oncol. 2014, 111, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mizuhata, M.; Takamatsu, S.; Shibata, S.; Bou, S.; Sato, Y.; Kawamura, M.; Asahi, S.; Tameshige, Y.; Maeda, Y.; Sasaki, M.; et al. Respiratory-gated proton beam therapy for hepatocellular carcinoma adjacent to the gastrointestinal tract without fiducial markers. Cancers 2018, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Takamatsu, S.; Yamamoto, K.; Mizuhata, M.; Bou, S.; Sato, Y.; Kawamura, M.; Asahi, S.; Tameshige, Y.; Maeda, Y.; et al. Proton beam therapy without fiducial markers using four-dimensional ct planning for large hepatocellular carcinomas. Cancers 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Sugahara, S.; Fukuda, K.; Abei, M.; Shoda, J.; Sakurai, H.; Tsuboi, K.; Matsuzaki, Y.; Tokuuye, K. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, Y.K.; Kim, T.H.; Cho, K.H.; Choi, S.H.; Jeong, H.; Kim, D.W.; Park, J.H.; Shin, D.H.; Lee, S.B.; et al. Normal liver sparing by proton beam therapy for hepatocellular carcinoma: Comparison with helical intensity modulated radiotherapy and volumetric modulated arc therapy. Acta Oncol. 2015, 54, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.X.; Fu, S.; Zhang, Q.; Guo, X.M. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2015, 114, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The bclc staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Llovet, J.M. Major achievements in hepatocellular carcinoma. Lancet 2009, 373, 614–616. [Google Scholar] [CrossRef]
- Kwak, H.W.; Park, J.W.; Nam, B.H.; Yu, A.; Woo, S.M.; Kim, T.H.; Kim, S.H.; Koh, Y.H.; Kim, H.B.; Park, S.J.; et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis b virus-endemic area. J. Gastroenterol. Hepatol. 2014, 29, 820–829. [Google Scholar] [CrossRef]
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Kudo, M.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.F.; de Guevara, L.L.; Papandreou, C.; et al. Gideon (global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafenib): Second interim analysis. Int. J. Clin. Pract. 2014, 68, 609–617. [Google Scholar] [CrossRef]
- Ueno, S.; Tanabe, G.; Nuruki, K.; Hamanoue, M.; Komorizono, Y.; Oketani, M.; Hokotate, H.; Inoue, H.; Baba, Y.; Imamura, Y.; et al. Prognostic performance of the new classification of primary liver cancer of japan (4th edition) for patients with hepatocellular carcinoma: A validation analysis. Hepatol. Res. 2002, 24, 395–403. [Google Scholar] [CrossRef]
- Takeda, A.; Oku, Y.; Sanuki, N.; Kunieda, E.; Koike, N.; Aoki, Y.; Ohashi, T.; Iwabuchi, S.; Takatsuka, K.; Takeda, T.; et al. Dose volume histogram analysis of focal liver reaction in follow-up multiphasic ct following stereotactic body radiotherapy for small hepatocellular carcinoma. Radiother. Oncol. 2012, 104, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.C.; Bentzen, S.M. Time-dose relationships: The linear-quadrantic approach. In Basic Clinical Radiobiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2003; pp. 121–133. [Google Scholar]
- Kim, T.H.; Kim, D.Y.; Park, J.W.; Kim, S.H.; Choi, J.I.; Kim, H.B.; Lee, W.J.; Park, S.J.; Hong, E.K.; Kim, C.M. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified recist (mrecist) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total | Dose-Fx Regimen A | Dose-Fx Regimen B | Dose-Fx Regimen C | p Value | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||
| Gender | Male | 211 (86.8) | 34 (85.0) | 53 (88.3) | 124 (86.7) | 0.888 * |
| Female | 32 (13.2) | 6 (15.0) | 7 (11.7) | 19 (13.3) | ||
| Age, years | Median (range) | 61 (24–92) | 59 (24–81) | 62.5 (39–80) | 62 (34–92) | 0.133 † |
| <60 | 100 (41.2) | 22 (55.0) | 24 (40.0) | 54 (37.8) | 0.144 * | |
| ≥60 | 143 (58.8) | 18 (45.0) | 36 (60.0) | 89 (62.2) | ||
| ECOG PS | 0 | 237 (97.5) | 38 (95.0) | 59 (98.3) | 140 (97.9) | 0.520 * |
| 1 | 6 (2.5) | 2 (5.0) | 1 (1.7) | 3 (2.1) | ||
| Etiology of LC | HBV | 188 (77.4) | 33 (82.5) | 45 (75.0) | 110 (76.9) | 0.799 * |
| HCV | 20 (8.2) | 3 (7.5) | 6 (10.0) | 11 (7.7) | ||
| Alcoholic | 17 (7.0) | 1 (2.5) | 6 (10.0) | 10 (7.0) | ||
| Unknown | 18 (7.4) | 3 (7.5) | 3 (5.0) | 12 (8.4) | ||
| Child-Pugh | A | 228 (93.8) | 36 (90.0) | 54 (90.0) | 138 (96.5) | 0.117 * |
| Classification | B7 | 15 (6.2) | 4 (10.0) | 6 (10.0) | 5 (3.5) | |
| AFP, ng/mL | Median (range) | 10.2 (1.2–38,396.4) | 25.3 (1.9–31,466.3) | 10.9 (2.2–38,396.4) | 9.3 (1.2–16,788.3) | 0.062 † |
| <10 | 120 (49.4) | 14 (35.0) | 30 (50.0) | 76 (53.1) | 0.127 * | |
| ≥10 | 123 (50.6) | 26 (65.0) | 30 (50.0) | 67 (46.9) | ||
| Tumor size, cm | Median (range) | 2.2 (1.0–17) | 6.0 (1.3–17) | 3.6 (1.0–12) | 1.5 (1.0–12.7) | <0.001 † |
| ≤2 | 115 (47.3) | 1 (2.5) | 16 (26.7) | 98 (68.5) | <0.001 * | |
| >2 | 128 (52.7) | 39 (97.5) | 44 (73.3) | 45 (31.5) | ||
| TVT | No | 184(75.7) | 11 (27.5) | 40 (66.7) | 133 (93.0) | <0.001 * |
| Branch | 29 (11.9) | 7 (17.5) | 15 (25.0) | 7 (4.9) | ||
| Main | 30 (12.3) | 22 (55.0) | 5 (8.3) | 3 (2.1) | ||
| mUICC stage | I | 13 (5.3) | 1 (2.5) | 2 (3.3) | 10 (7.0) | <0.001 * |
| II | 74 (30.5) | 1 (2.5) | 13 (21.7) | 60 (42.0) | ||
| III | 106 (43.6) | 12 (30.0) | 31 (51.7) | 63 (44.1) | ||
| IVA | 50 (20.6) | 26 (65.0) | 14 (23.3) | 10 (7.0) | ||
| BCLC stage | A | 97 (39.9) | 0 (0) | 17 (28.3) | 80 (55.9) | <0.001 * |
| B | 86 (35.4) | 11 (27.5) | 22 (36.7) | 53 (37.1) | ||
| C | 60 (24.7) | 29 (72.5) | 21 (35.0) | 10 (7.0) | ||
| Diagnosis at PBT | Primary | 10 (4.1) | 5 (12.5) | 2 (3.3) | 3 (2.1) | 0.021 |
| Recurrence | 233 (95.9) | 35 (87.5) | 58 (96.7) | 140 (95.9) | ||
| Pre-Tx to PBT site | No | 52 (21.4) | 7 (17.5) | 4 (6.7) | 41 (28.7) | 0.002 * |
| Yes | 191 (78.6) | 33 (82.5) | 56 (93.3) | 102 (71.3) | ||
| LRT | 186 (97.4) | 27 (81.8) | 54 (96.4) | 102 (97.4) | ||
| LRT + sorafenib | 5 (2.6) | 3 (9.1) | 2 (3.6) | 0 (0) | ||
| Sorafenib ± chemo | 3 (1.6) | 3 (9.1) | 0 (0) | 0 (0) | ||
| Pre-Tx to other site | No | 70 (28.8) | 22 (55.0) | 23 (38.3) | 25 (17.5) | <0.001 * |
| Yes | 173 (43.9) | 18 (45.0) | 37 (61.7) | 118 (82.5) | ||
| LRT | 171 (98.3) | 18 (100) | 36 (97.3) | 117 (98.3) | ||
| LRT + sorafenib | 3 (1.7) | 0 (0) | 1 (2.7) | 2 (1.7) | ||
| Concurrent Tx | No | 236 (97.1) | 34 (85.0) | 59 (98.3) | 143 (100) | <0.001 * |
| Sorafenib | 7 (2.9) | 6 (15.0) | 1 (1.7) | 0 (0) | ||
| Post-Tx to PBT site | No | 195 (80.2) | 12 (30.0) | 48 (80.0) | 135 (94.4) | <0.001 * |
| Yes | 48 (19.8) | 28 (70.0) | 12 (20.0) | 8 (5.6) | ||
| LRT | 16 (33.3) | 7 (25.0) | 7 (58.3) | 2 (25.0) | ||
| LRT ± sorafenib ± chemo | 7 (14.6) | 6 (21.4) | 0 (0) | 1 (12.5) | ||
| Sorafenib ± chemo | 25 (52.1) | 15 (53.6) | 5 (41.7) | 5 (62.5) | ||
| Post-Tx to other site | No | 66 (27.2) | 8 (20.0) | 18 (30.0) | 40 (28.0) | 0.515 * |
| Yes | 177 (72.8) | 32 (80.0) | 42 (70.0) | 103 (72.0) | ||
| LRT | 91 (51.7) | 4 (12.5) | 19 (45.2) | 68 (66.0) | ||
| LRT ± sorafenib ± chemo | 57 (32.4) | 17 (51.1) | 15 (35.7) | 26 (25.2) | ||
| Sorafenib ± chemo | 28 (15.9) | 11 (34.4) | 8 (19.0) | 9 (8.7) | ||
| Response | Dose-Fractionation Regimen, n (%) | p Value * | Pre-Tx to PBT Site, n (%) | p Value * | Post-Tx to PBT Site, n (%) | p Value * | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen A | Regimen B | Regimen C | No | Yes | No | Yes | |||||
| Primary tumor | CR | 16 (40.0) | 51 (85.0) | 132 (92.3) | <0.001 | 47 (90.4) | 152 (79.6) | 0.405 | 174 (89.2) | 25 (53.1) | <0.001 |
| (n = 243) | PR | 18 (45.0) | 6 (10.0) | 6 (4.2) | 4 (7.7) | 26 (13.6) | 16 (8.2) | 14 (29.2) | |||
| SD | 6 (15.02) | 2 (3.3) | 5 (3.5) | 1 (1.9) | 12 (6.3) | 5 (2.6) | 1 (2.1) | ||||
| PD | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (2.1) | ||||
| TVT | CR | 9 (31.0) | 15 (75.0) | 6 (60.0) | 0.021 | 3 (42.9) | 27 (51.9) | 0.877 | 15 (60.0) | 15 (44.1) | 0.610 |
| (n = 59) | PR | 12 (41.4) | 3 (15.0) | 3 (30.0) | 3 (42.9) | 15 (28.9) | 7 (28.0) | 11 (32.4) | |||
| SD | 8 (27.6) | 1 (5.0) | 1 (10.0) | 1 (14.2) | 9 (17.3) | 3 (12.0) | 7 (20.6) | ||||
| PD | 0 (0.0) | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (2.9) | ||||
| Characteristics | Univariate † | Multivariate ‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| 1-yr OS, % (95% CI) | 3-yr OS, % (95% CI) | 5-yr OS, % (95% CI) | Median OS, Months (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | ||
| Gender | Male | 87.7 (83.2–92.2) | 62.2 (55.1–69.3) | 48.3 (38.5–58.1) | 56.5 (45.5–67.2) | 0.964 | - | NS |
| Female | 96.9 (90.8–100) | 60.2 (42.6–77.8) | 49.3 (29.3–69.3) | 46.6 (-) | - | |||
| Age, years | <60 | 88.0 (81.7–94.3) | 63.3 (53.3–73.3) | 45.0 (29.1–60.9) | 56.5 (35.1–77.9) | 0.945 | - | NS |
| ≥60 | 89.5 (84.4–94.6) | 60.8 (52.0–69.6) | 49.5 (38.3–60.7) | 55.0 (44.5–65.5) | - | |||
| ECOG PS | 0 | 88.6 (84.5–92.7) | 62.8 (56.1–69.5) | 48.6 (39.4–57.8) | 56.5 (44.9–68.0) | 0.175 | - | NS |
| 1 | 100 (-) | 25.0 (0–65.0) | - (-) | 21.2 (4.1–38.4) | - | |||
| Etiology of LC | HBV | 88.8 (84.3–93.3) | 62.4 (55.0–69.8) | 49.4 (39.2–59.6) | 56.5 (-) | 0.784 | - | NS |
| Others | 89.1 (80.9–97.3) | 60.0 (45.5–74.5) | 44.9 (26.1–63.7) | 55.0 (32.0–78.0) | - | |||
| Child-Pugh Classification | A | 90.4 (86.5–94.3) | 65.3 (58.6–72.0) | 50.5 (41.1–59.9) | 60.3 (-) | <0.001 | 1.000 | 0.016 |
| B7 | 66.7 (42.8–90.6) | 0.91 (0–26.0) | 0.91 (0–26.0) | 17.1 (1.6–32.6) | 2.221 (1.162–4.246) | |||
| AFP, ng/mL | <10 | 92.5 (87.8–97.2) | 74.3 (65.7–82.9) | 56.3 (41.6–71.0) | NR | <0.001 | 1.000 | 0.008 |
| ≥10 | 85.4 (79.1–91.7) | 49.9 (40.5–59.3) | 39.6 (28.6–50.6) | 34.3 (24.4–44.2) | 1.773 (1.158–2.713) | |||
| Tumor size, cm | ≤2 | 94.8 (90.7–98.9) | 79.5 (71.5–87.5) | 64.4 (49.3–7.5) | NR | <0.001 | - | NS |
| >2 | 83.6 (77.1–90.1) | 46.5 (37.1–55.9) | 34.0 (23.6–44.4) | 33.9 (28.4–39.5) | - | |||
| TVT | No | 93.5 (90.0–97.0) | 67.8 (60.4–75.3) | 54.1 (43.9–64.3) | 60.3 (-) | <0.001 | – | NS |
| Branch | 79.3 (60.4–91.4) | 49.0 (29.8–68.2) | 49.0 (29.8–68.2) | 34.3 (-) | ||||
| Main | 73.3 (57.4–89.2) | 38.8 (21.0–56.6) | 18.9 (0–38.1) | 19.4 (6.1–32.8) | - | |||
| mUICC stage | I | 100 (-) | 92.3 (77.8–100) | 69.2 (28.6–100) | NR | <0.001 | 1.000 | |
| II | 94.6 (89.5–99.7) | 83.9 (74.5–93.3) | 65.4 (45.8–85) | NR | 3.186 (0.699–14.525) | 0.134 | ||
| III | 93.4 (88.7–98.1) | 57.0 (46.8–67.2) | 43.8 (31.3–56.3) | 46.6 (34.9–58.4) | 6.563 (1.557–27.669) | 0.010 | ||
| IVA | 68.0 (55.1–80.9) | 33.4 (19.7–47.1) | 26.6 (12.7–40.5) | 19.4 (10.0–28.8) | 7.119 (1.673–30.288) | 0.008 | ||
| BCLC stage | A | 96.9 (93.4–100) | 80.1 (71.5–88.7) | 65.1 (50.2–80.0) | NR | <0.001 | - | NS |
| B | 89.5 (83.0–96.0) | 53.9 (42.3–65.5) | 40.0 (26.1–53.9) | 37.1 (24.2–50.1) | - | |||
| C | 75.0 (64.0–86.0) | 44.6 (31.5–57.7) | 32.2 (16.0–48.6) | 33.9 (16.2–51.7) | - | |||
| Diagnosis at PBT | Primary | 90.0 (71.4–100) | 56.0 (22.5–89.5) | 42.0 (7.5–76.5) | 38.4 (8.5–68.2) | 0.578 | - | NS |
| Recurrence | 88.8 (83.8–92.1) | 62.0 (55.3–68.7) | 48.2 (38.8–57.6) | 56.5 (45.3–67.7) | - | |||
| Pre-Tx to PBT site | No | 98.1 (94.4–100) | 82.3 (71.1–93.5) | 78.4 (65.3–91.5) | NR | <0.001 | - | NS |
| Yes | 86.4 (81.5–91.3) | 56.8 (49.4–64.2) | 41.7 (32.1–51.3) | 46.9 (33.7–60.1) | - | |||
| Pre-Tx to other site | No | 87.1 (79.3–94.9) | 54.0 (41.3–66.7) | 41.4 (26.3–56.5) | 38.4 (19.7–57.1) | 0.130 | - | NS |
| Yes | 89.6 (85.1–94.1) | 64.9 (57.3–72.5) | 51.2 (39.8–62.5) | NR | - | |||
| Concurrent Tx | No | 89.8 (85.9–93.7) | 62.8 (56.1–69.5) | 49.4 (38.0–60.8) | 56.5 (-) | 0.001 | - | NS |
| Sorafenib | 57.1 (20.4–93.8) | 28.6 (0–62.1) | 0 (-) | 19.4 (0–40.3) | - | |||
| Post-Tx to PBT site | No | 93.8 (90.5–97.1) | 67.5 (60.2–74.8) | 53.8 (42.6–65.0) | 60.3 (-) | <0.001 | - | NS |
| Yes | 68.7 (55.6–81.8) | 40.2 (25.9–54.5) | 26.2 (12.5–39.9) | 25.4 (7.1–43.7) | - | |||
| Post-Tx to other sites | No | 81.8 (72.6–91.0) | 68.8 (56.8–80.8) | 61.9 (45.2–78.6) | NR | 0.294 | - | NS |
| Yes | 91.5 (87.3–95.6) | 59.8 (52.2–67.4) | 44.3 (34.1–54.5) | 48.7 (36.3–61.1) | - | |||
| Dose-fractionation | Regimen A | 75.0 (61.7–88.3) | 33.3 (18.4–48.2) | 16.7 (3.6–29.8) | 23.4 (15.3–31.5) | <0.001 | 2.045 (1.244–3.362) | 0.005 |
| Regimen B | 86.7 (78.1–95.3) | 51.2 (38.1–64.3) | 39.2 (24.9–53.5) | 40.8 (19.7–61.9) | 1.298 (0.740–2.277) | 0.363 | ||
| Regimen C | 93.7 (89.8–97.6) | 76.0 (68.4–83.6) | 67.9 (57.5–78.3) | NR | 1.000 | |||
| Primary tumor response | CR | 97.0 (94.6–99.4) | 73.3 (66.6–80.0) | 60.9 (51.5–70.3) | NR | <0.001 | 1.000 | <0.001 |
| Non-CR | 52.3 (37.6–67.0) | 10.6 (1.0–20.2) | 0 (-) | 13.7 (10.8–16.6) | 7.012 (4.324–11.370) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.H.; Park, J.-W.; Kim, B.H.; Kim, H.; Moon, S.H.; Kim, S.S.; Woo, S.M.; Koh, Y.-H.; Lee, W.J.; Kim, D.Y.; et al. Does Risk-Adapted Proton Beam Therapy Have a Role as a Complementary or Alternative Therapeutic Option for Hepatocellular Carcinoma? Cancers 2019, 11, 230. https://doi.org/10.3390/cancers11020230
Kim TH, Park J-W, Kim BH, Kim H, Moon SH, Kim SS, Woo SM, Koh Y-H, Lee WJ, Kim DY, et al. Does Risk-Adapted Proton Beam Therapy Have a Role as a Complementary or Alternative Therapeutic Option for Hepatocellular Carcinoma? Cancers. 2019; 11(2):230. https://doi.org/10.3390/cancers11020230
Chicago/Turabian StyleKim, Tae Hyun, Joong-Won Park, Bo Hyun Kim, Hyunjung Kim, Sung Ho Moon, Sang Soo Kim, Sang Myung Woo, Young-Hwan Koh, Woo Jin Lee, Dae Yong Kim, and et al. 2019. "Does Risk-Adapted Proton Beam Therapy Have a Role as a Complementary or Alternative Therapeutic Option for Hepatocellular Carcinoma?" Cancers 11, no. 2: 230. https://doi.org/10.3390/cancers11020230
APA StyleKim, T. H., Park, J.-W., Kim, B. H., Kim, H., Moon, S. H., Kim, S. S., Woo, S. M., Koh, Y.-H., Lee, W. J., Kim, D. Y., & Kim, C.-M. (2019). Does Risk-Adapted Proton Beam Therapy Have a Role as a Complementary or Alternative Therapeutic Option for Hepatocellular Carcinoma? Cancers, 11(2), 230. https://doi.org/10.3390/cancers11020230





