Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes
Abstract
1. Introduction
2. HPV-Related Tumors
3. Current Anti-HPV Therapeutic Strategies
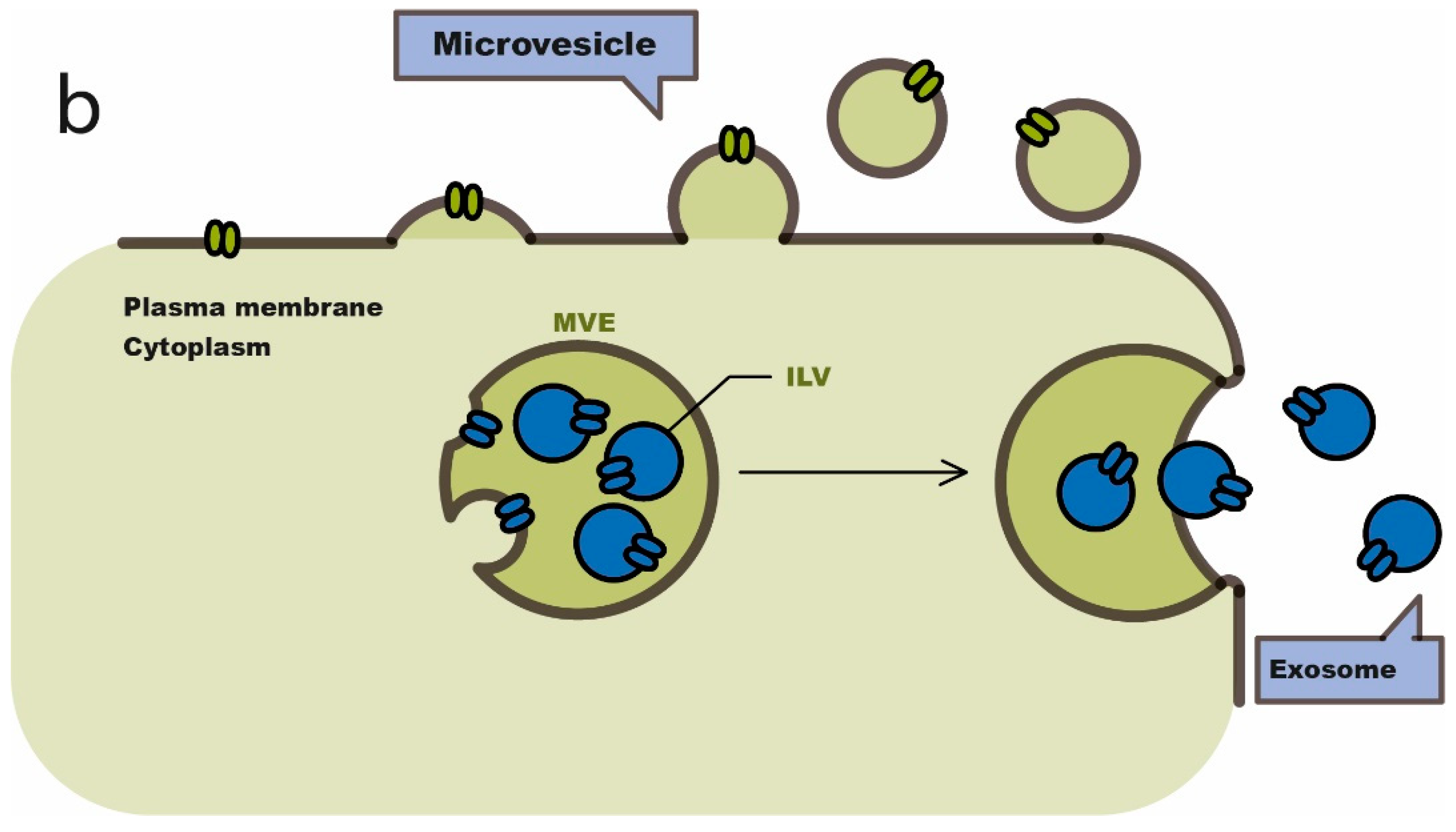
4. Exosomes in Cancer Immunotherapy
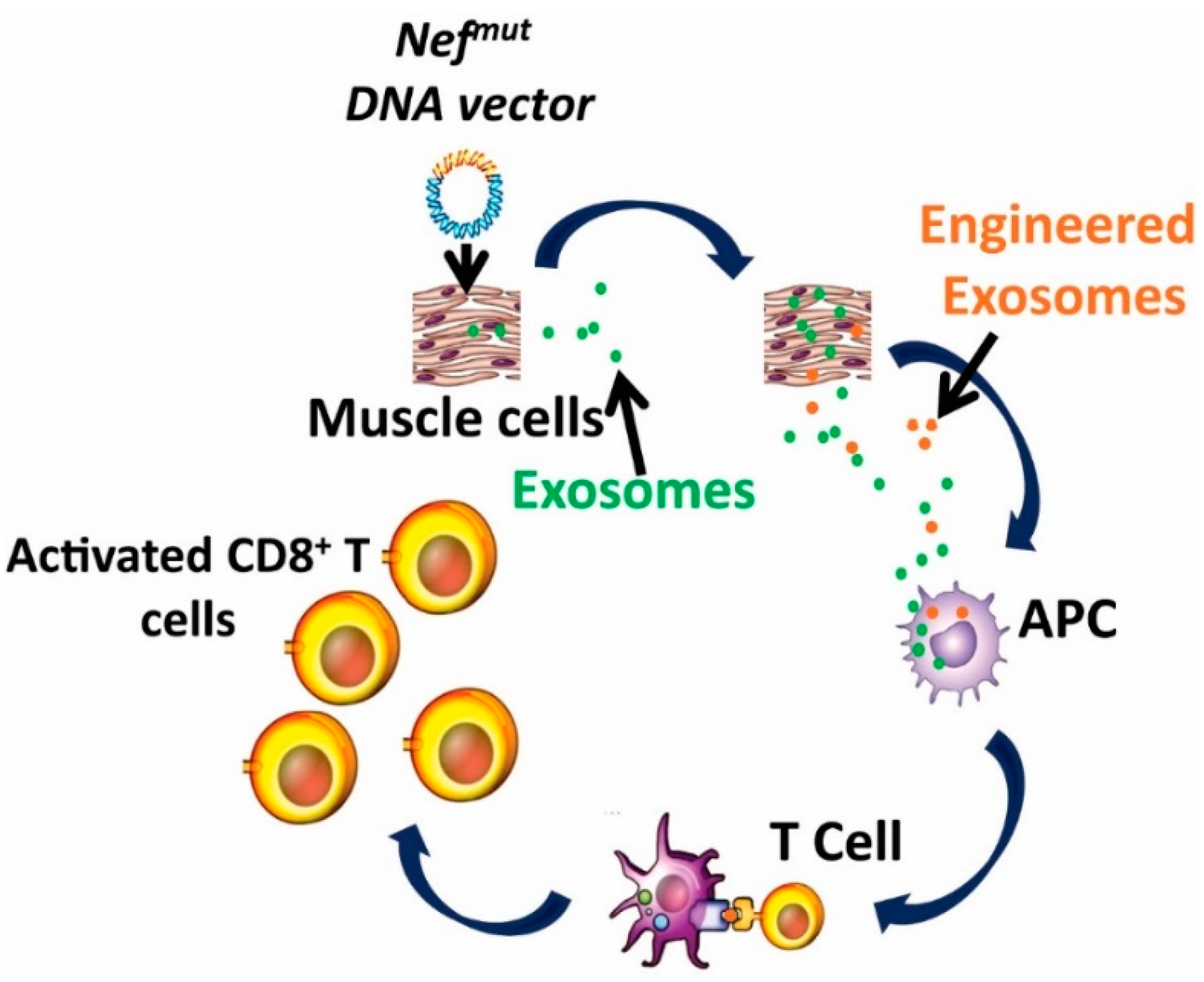
5. The Nefmut-Based Technology for the Induction of Anti-Tumor CTL Immune Response by Endogenous Engineered Exosomes
- The high level of incorporation of the Nefmut protein into EVs [68];
- The Nefmut ability to act as an exosome-anchoring element upon fusion with heterologous proteins [67];
- The experimental evidence that Nefmut-based exosomes loaded with an antigen of choice produced in vitro, induce a strong CTL activity when inoculated in mice [76];
- The possibility to generate recombinant EVs carrying the fusion product of Nefmut—with an antigen of choice in vivo, through intra muscular (i.m.) injection in mice of a DNA vectors coding for the fused genes [76];
- The therapeutic antitumor effect induced by endogenously engineered EVs incorporating HPV-E7 fused with Nefmut [77].
6. The Nefmut-Based Technology for the Therapy of HPV-Associated Tumors
7. Other Applications of the Nefmut-Based Exosome Technology
8. Pros and Cons of the Nefmut/E7 Based DNA Vaccines
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Warshauer, J.T.; Bluestoneet, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 5, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kakimi, K.; Karasaki, T.; Matsushita, H.; Sugie, T. Advances in personalized cancer immunotherapy. Breast Cancer 2017, 24, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Di Tucci, C.; Schiavi, M.C.; Faiano, P.; D’Oria, O.; Prata, G.; Sciuga, V.; Giannini, A.; Palaia, I.; Muzii, L.; Benedetti Panici, P. Therapeutic vaccines and immune checkpoints inhibition options for gynecological cancers. Crit. Rev. Oncol. Hematol. 2018, 128, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Li, W.A.; Mooney, D.J.; Dranoff, G. Advances in Therapeutic Cancer Vaccines. Adv. Immunol. 2016, 130, 191–249. [Google Scholar] [PubMed]
- Tang, J.; Pearce, L.; O’Donnell-Tormey, J.; Hubbard-Lucey, V.M. Trends in the global immune-oncology landscape. Nature Rev. 2018, 17, 783–784. [Google Scholar]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: pathways to transformation. Rev. Cancer. 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 1, 2–23. [Google Scholar] [CrossRef]
- Trottier, H.; Franco, E.L. The epidemiology of genital human papillomavirus infection. Vaccine 2006, 1, S1–S15. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomatarama, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Näsman, A.; Bersani, C.; Lindquist, D.; Du, J.; Ramqvist, T.; Dalianis, T. Human Papillomavirus and Potentially Relevant Biomarkers in Tonsillar and Base of Tongue Squamous Cell Carcinoma. Anticancer Res. 2017, 37, 5319–5328. [Google Scholar] [PubMed]
- Lewis, A.; Kang, R.; Levine, A.; Maghami, E. The New Face of Head and Neck Cancer: The HPV Epidemic. Oncology 2015, 29, 616–626. [Google Scholar] [PubMed]
- Berman, T.A.; Schiller, J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Yan, H.; Che, J.; Huihui, L.; Jun, W.; Liu, B.; Cao, B. A Meta-Analysis and Systematic Review on the Association between Human Papillomavirus (Types 16 and 18) Infection and Esophageal Cancer Worldwide. PLoS ONE 2016, 11, e0159140. [Google Scholar] [CrossRef]
- Zhai, K.; Ding, J.; Shi, H.Z. HPV and lung cancer risk: A meta-analysis. J. Clin. Virol. 2015, 63, 84–90. [Google Scholar] [CrossRef]
- Yang, L.; Xie, S.; Feng, X.; Chen, Y.; Zheng, T.; Dai, M.; Zhou, C.K.; Hu, Z.; Li, N.; Hang, D. Worldwide Prevalence of Human Papillomavirus and Relative Risk of Prostate Cancer: A Meta-analysis. Sci. Rep. 2015, 5, 14667. [Google Scholar] [CrossRef]
- Russo, G.I.; Calogero, A.E.; Condorelli, R.A.; Scalia, G.; Morgia, G.; La Vignera, S. Human papillomavirus and risk of prostate cancer: A systematic review and meta-analysis. Aging Male 2018, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Svahn, M.F.; Faber, M.T.; Duun-Henriksen, A.K.; Junge, J.; Norrild, B.; Kjaer, S.K. Prevalence of Human Papillomavirus in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2014, 134, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Glenn, W.K.; Whitaker, N.J. Human Papilloma Viruses and Breast Cancer—Assessment of Causality. Front. Oncol. 2016, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Wang, W.; Wang, Y.Q.; Jia, D.F.; Zhu, L. Human papillomavirus infection and colorectal cancer in the Chinese population: A meta-analysis. Colorectal Dis. 2018, 20, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, M.K.; Tsyganov, M.M.; Litviakov, N.V. Human papillomavirus and colorectal cancer. Med. Oncol. 2018, 35, 140. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, M.A.; Gordon, N.S.; Abbotts, B.; James, N.D.; Zeegers, M.P.; Cheng, K.K.; Macdonald, A.; Roberts, S.; Parish, J.L.; Ward, D.G.; et al. Defining the frequency of human papillomavirus and polyomavirus infection in urothelial bladder tumours. Sci. Rep. 2018, 8, 11290. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Accardi, L.; D’Oro, G.; Della Libera, S.; Muscillo, M.; Di Bonito, P. Mucosal and Cutaneous Human Papillomaviruses Detected in Raw Sewages. PLoS ONE 2013, 8, 75–77. [Google Scholar] [CrossRef]
- Di Bonito, P.; Iaconelli, M.; Gheit, T.; Tommasino, M.; Della Libera, S.; Bonadonna, L.; La Rosa, G. Detection of oncogenic viruses in water environments by a Luminex-based multiplex platform for high throughput screening of infectious agents. Water Res. 2017, 123, 549–555. [Google Scholar] [CrossRef]
- La Rosa, G. Papillomavirus. Available online: http://www.waterpathogens.org/book/papillomavirus (accessed on 24 January 2019).
- Yang, A.; Jeang, J.; Cheng, K.; Cheng, T.; Yang, B.; Wu, T.C.; Hung, C.F. Current State in the Development of Candidate Therapeutic HPV Vaccines. Expert Rev. Vaccines 2016, 15, 989–1007. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 23 January 2019).
- Cheng, M.A.; Farmer, E.; Huang, C.; Lin, J.; Hung, C.F.; Wu, T.C. Therapeutic DNA Vaccines for Human Papillomavirus and Associated Diseases. Hum. Gene Ther. 2018, 29, 971–996. [Google Scholar] [CrossRef]
- Peng, S.; Trimble, C.; Ji, H.; He, L.; Tsai, Y.C.; Macaes, B.; Hung, C.F.; Wu, T.C. Characterization of HPV-16 E6 DNA vaccines employing intracellular targeting and intercellular spreading strategies. J. Biomed. Sci. 2005, 12, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Tomson, T.T.; Trimble, C.; He, L.; Hung, C.F.; Wu, T.C. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Ther. 2006, 13, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Tsai, Y.C.; He, L.; Calizo, R.; Chou, H.H.; Chang, T.C.; Soong, Y.K.; Hung, C.F.; Lai, C.H. A DNA vaccine encoding a codon-optimized human papillomavirus type 16 E6 gene enhances CTL response and anti-tumor activity. J. Biomed. Sci. 2006, 13, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Harris, K.; Khan, A.S.; Draghia-Akli, R.; Sewell, D.; Weiner, D.B. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine 2008, 26, 5210–5215. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Reichenbach, D.K.; Corbitt, N.; Hokey, D.A.; Ramanathan, M.P.; McKinney, K.A.; Weiner, D.B.; Sewell, D. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine 2009, 27, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Jin, H.T.; Park, S.H.; Youn, J.I.; Sung, Y.C. Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation. Vaccine 2009, 27, 5906–5912. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.M.; Stern, P.L.; Rankin, E.M.; Sommer, H.; Nuessler, V.; Schneider, A.; Adams, M.; Onon, T.S.; Bauknecht, T.; Wagner, U.; et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin. Cancer Res. 2002, 8, 3676–3685. [Google Scholar] [PubMed]
- Liao, C.W.; Chen, C.A.; Lee, C.N.; Su, Y.N.; Chang, M.C.; Syu, M.H.; Hsieh, C.Y.; Cheng, W.F. Protein Vaccine by Domains of Bacterial Exotoxin Linked with a Tumor Antigen Generates Potent Immunologic Responses and Antitumor Effects. Cancer Res. 2005, 65, 9089–9098. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Berry, J.M.; Jay, N.; Krogstad, M.; Da Costa, M.; Darragh, T.M.; Lee, J.Y. A trial of SGN-00101 (HspE7) to treat high-grade anal intraepithelial neoplasia in HIV-positive individuals. AIDS 2006, 20, 1151–1155. [Google Scholar] [CrossRef]
- Riezebos-Brilman, A.; Walczak, M.; Regts, J.; Rots, M.G.; Kamps, G.; Dontje, B.; Haisma, H.Y.; Wilschut, J.; Daemen, T. A comparative study on the immunotherapeutic efficacy of recombinant Semliki Forest virus and adenovirus vector systems in a murine model for cervical cancer. Gene Ther. 2007, 14, 1695–1704. [Google Scholar] [CrossRef]
- Bagarazzi, M.L.; Yan, J.; Morrow, M.P.; Shen, X.; Parker, R.L.; Lee, J.C.; Giffear, M.; Pankhong, P.; Khan, A.S.; Broderick, K.E. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012, 4, 155ra138. [Google Scholar] [CrossRef] [PubMed]
- Diehl, M.C.; Lee, J.C.; Daniels, S.E.; Tebas, P.; Khan, A.S.; Giffear, M.; Sardesai, N.Y.; Bagarazzi, M.L. Tolerability of intramuscular and intradermal delivery by CELLECTRA(®) adaptive constant current electroporation device in healthy volunteers. Hum. Vaccin Immunother. 2013, 9, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- van Poelgeest, M.E.; Welters, M.J.P.; van Esch, E.M.G.; Stynenbosch, L.F.M.; Kerpershoek, G.; van Persijn van Meerten, E.; van den Hende, M.; Löwik, M.J.G.; Berends-van der Meer, D.M.A.; Fathers, L.M. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Karkada, M.; Quinton, T.; Blackman, R.; Mansour, M. Tumor Inhibition by DepoVax-Based Cancer Vaccine Is Accompanied by Reduced Regulatory/Suppressor Cell Proliferation and Tumor Infiltration. ISRN Oncol. 2013, 753427. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, W.W.; Stratton, S.L.; Myrick, R.S.; Vaughn, R.; Donnalley, L.M.; Coleman, H.N.; Mercado, M.; Moerman-Herzog, A.M.; Spencer, H.J.; Andrews-Collins, N.R.; et al. A phase I dose-escalation clinical trial of a peptide-based human papillomavirus therapeutic vaccine with Candida skin test reagent as a novel vaccine adjuvant for treating women with biopsy-proven cervical intraepithelial neoplasia 2/3. Oncoimmunology 2015, 4, e1031439. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.N.; Greenfield, W.W.; Stratton, S.L.; Vaughn, R.; Kieber, A.; Moerman-Herzog, A.M.; Spencer, H.J.; Hitt, W.C.; Quick, C.M.; Hutchins, L.F.; et al. Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol. Immunother. 2016, 65, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.G.; Basu, P. ADXS11-001 immunotherapy targeting HPV-E7: Updated survival and safety data from a phase 2 study in Indian women with recurrent/refractory cervical cancer. J. Immunother. Cancer 2013, 1, 231. [Google Scholar] [CrossRef]
- The Vaccibody Vaccine Technology Platform. Available online: http://www.vaccibody.com/technology/ (accessed on 23 January 2019).
- Kim, T.J.; Jin, H.T.; Hur, S.Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.W.; Kim, S.; Woo, J.W.; Park, K.S. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Peng, S.; Han, L.; Qiu, J.; Song, L.; Tsai, Y.; Yang, B.; Roden, R.B.; Trimble, C.L.; Hung, C.F.; et al. Local HPV Recombinant Vaccinia Boost Following Priming with an HPV DNA Vaccine Enhances Local HPV-Specific CD8+ T-cell-Mediated Tumor Control in the Genital Tract. Clin. Cancer Res. 2016, 22, 657–669. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kang, M.C.; Seo, Y.B.; Namkoong, H.; Park, Y.; Choi, D.H.; Suh, Y.S.; Lee, S.W.; Sung, Y.C.; Jin, H.T. Intravaginal Administration of Fc-Fused IL7 Suppresses the Cervicovaginal Tumor by Recruiting HPV DNA Vaccine-Induced CD8 T Cells. Clin. Cancer Res. 2016, 22, 5898–5908. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.D.; Huh, W.K.; Bae, S.; Lamb, L.S., Jr.; Conner, M.G.; Boyer, J.; Wang, C.; Hung, C.F.; Sauter, E.; Paradis, M.; et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16 + cervical intraepithelial neoplasia 2/3 (CIN2/3). Ginecol. Oncol. 2016, 140, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bates, T.M.; Kim, E.; Concha-Benavente, F.; Trivedi, S.; Mailliard, R.B.; Gambotto, A.; Ferris, R.L. Enhanced Cytotoxic CD8 T Cell Priming Using Dendritic Cell-Expressing Human Papillomavirus-16 E6/E7-p16INK4 Fusion Protein with Sequenced Anti-Programmed Death-1. J. Immunol. 2016, 196, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.J.; Stephenson, K.B.; Nikota, J.K.; Hu, Q.N.; Nguyen, A.; Wan, Y.; Lichty, B.D. Preclinical development of peptide vaccination combined with oncolytic MG1-E6E7 for HPV-associated cancer. Vaccine 2018, 36, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors using a Novel Cell-Free Vaccine: Dendritic Cell-Derived Exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Thery, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-Derived Exosomes are a Source of Shared Tumor Rejection Antigens for CTL Cross-Priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Tan, A.; De La Pena, H.; Seifalian, A.M. The Application of Exosomes as a Nanoscale Cancer Vaccine. Int. J. Nanomed. 2010, 5, 889–900. [Google Scholar]
- Chaput, N.; Théry, C. Exosomes: immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef]
- Syn, N.L.; Wang, L.; Chow, E.K.; Lim, C.T.; Goh, B.C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of Metastatic Melanoma Patients with Autologous Dendritic Cell (DC) Derived-Exosomes: Results of Thefirst Phase I Clinical Trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A Phase I Study of Dexosome Immunotherapy in Patients with Advanced Non-Small Cell Lung Cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-Derived Exosomes Combined with GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, F.; Di Bonito, P.; Arenaccio, C.; Anticoli, S.; Federico, M. Incorporation of Heterologous Proteins in Engineered Exosomes. Methods Mol. Biol. 2016, 1448, 249–260. [Google Scholar]
- Lattanzi, L.; Federico, M. A Strategy of Antigen Incorporation into Exosomes: Comparing Cross-Presentation Levels of Antigens Delivered by Engineered Exosomes and by Lentiviral Virus-Like Particles. Vaccine 2012, 30, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- D’Aloja, P.; Santarcangelo, A.C.; Arold, S.; Baur, A.; Federico, M. Genetic and functional analysis of the human immunodeficiency virus (HIV) type 1-inhibiting F12-HIVnef allele. J. Gen. Virol. 2001, 82, 2735–2745. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.L.; Denial, S.J.; Temple, B.R.; Garcia, J.V. Mechanisms of HIV-1 Nef Function and Intracellular Signaling. J. Neuroimmune Pharmacol. 2011, 6, 230–246. [Google Scholar] [CrossRef]
- Ren, X.; Park, S.Y.; Bonifacino, J.S.; Hurley, J.H. How HIV-1 Nef hijacks the AP-2 clathrin adaptor to downregulate CD4. Elife 2014, 3, e01754. [Google Scholar] [CrossRef]
- Kestler, H.W., 3rd; Ringler, D.J.; Mori, K.; Panicali, D.L.; Sehgal, P.K.; Daniel, M.D.; Desrosiers, R.C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 1991, 65, 651–662. [Google Scholar] [CrossRef]
- Piguet, V.; Schwartz, O.; Le Gall, S.; Trono, D. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 1999, 168, 51–63. [Google Scholar] [CrossRef]
- Saksela, K. Interactions of the HIV/SIV pathogenetic factor Nef with SH3 domain-containing host cell proteins. Curr. HIV Res. 2011, 9, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Lenassi, M.; Cagney, G.; Liao, M.F.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4(+) T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Ridolfi, B.; Columba-Cabezas, S.; Giovannelli, A.; Chiozzini, C.; Manfredi, F.; Anticoli, S.; Arenaccio, C.; Federico, M. HPV-E7 Delivered by Engineered Exosomes Elicits a Protective CD8(+) T Cell-Mediated Immune Response. Viruses 2015, 7, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Chiozzini, C.; Arenaccio, C.; Anticoli, S.; Manfredi, F.; Olivetta, E.; Ferrantelli, F.; Falcone, E.; Ruggieri, A.; Federico, M. Antitumor HPV E7-Specific CTL Activity Elicited by in Vivo Engineered Exosomes Produced through DNA Inoculation. Int. J. Nanomed. 2017, 12, 4579–4591. [Google Scholar] [CrossRef] [PubMed]
- Anticoli, S.; Manfredi, F.; Chiozzini, C.; Arenaccio, C.; Olivetta, E.; Ferrantelli, F.; Capocefalo, A.; Falcone, E.; Ruggieri, A.; Federico, M. An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity Against Viral Antigens. Biotechnol. J. 2018, 13, e1700443. [Google Scholar] [CrossRef] [PubMed]
- Anticoli, S.; Arico, E.; Arenaccio, C.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Ferrantelli, F.; Lattanzi, L.; D’Urso, M.T.; Proietti, E.; et al. Engineered Exosomes Emerging from Muscle Cells Break Immune Tolerance to HER2 in Transgenic Mice and Induce Antigen-Specific CTLs upon Challenge by Human Dendritic Cells. J. Mol. Med. 2018, 96, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Iezzi, M.; Mastini, C.; Amici, A.; Pericle, F.; Di Carlo, E.; Pupa, S.M.; De Giovanni, C.; Spadaro, M.; Curcio, C.; et al. Electroporated DNA Vaccine Clears Away Multifocal Mammary Carcinomas in Her-2/neu Transgenic Mice. Cancer Res. 2004, 64, 2858–2864. [Google Scholar] [CrossRef]
- Shin, T.; Pankhong, P.; Yan, J.; Khan, A.S.; Sardesai, N.Y.; Weiner, D.B. Induction of robust cellular immunity against HPV6 and HPV11 in mice by DNA vaccine encoding for E6/E7 antigen. Hum. Vaccin. Immunother. 2012, 8, 470–478. [Google Scholar] [CrossRef]
| Denomination | Description/Antigen | Adjuvant | Additional Treatment | Administration | Trial Design |
|---|---|---|---|---|---|
| TA-HPV [39] | Vaccinia Virus expressing E6 and E7 of HPV16 and 18 | surgical procedure radiation therapy | i.m. injection | Phase II in patients with early CC | |
| TVGV-1 GPI-0100 Placebo [40] | HPV16E7-PE (Pseudomonas exotoxin A); KDEL (ER retention signal) fusion protein | GPI-0100 (triterpene glycoside derived from saponins) | i.m. injection | Phase II randomized in double-blind patients with confirmed HPV-induced cervical HSIL | |
| HspE7/ Poly-ICLC [41] | HSP65 of Mycobacterium bovis and E7 HPV16 fusion protein | Poly-ICLC/synthetic complex of carboxy-methylcellulose, polyinosinic-polycytidylic acid, and poly-L-lysine double-stranded RNA | i.m. injection | Phase I/II in patients with CIN III | |
| Vvax001 [42] | Semliki Forest Virus vector encoding HPV-derived tumor antigens | Irradiated viral particles | i.m. injection | Phase I in patients with CIN 2, CIN 3, and CC | |
| INO-3112 (VGX3100 INO-9012) [43,44,45] | DNA plasmids expressing E6 and E7 of HPV16 and 18 | IL-12 | Cisplatin; Radiotherapy; with or without Durvalumab (anti- PD-L1 mAb) | i.m. electroporation | Phase I/II in patients with CC, Head and Neck cancer; uterine cervical neoplasms |
| ISA101/ ISA101b [46] | 13 overlapping 25-35-mer peptides from HPV16 E6 and E7 proteins | Pegylated IFN-γ | Carboplatin and paclitaxel; bevacizumab (anti VEGF-A mAb) | i.m. injection | Phase I/II in patients with Advanced or Recurrent HPV16 CC |
| DPX-E7 vaccine [47] | HPV16 E7-specific CTL peptide delivered by proprietary liposome formulation | i.m. injection | Phase I/II in HLA-A02 patients with head and Neck cancer, CC, Cancer of anus | ||
| PepCan [48,49] | 4 HPV16-E6 peptides escalation doses | Candida albicans extract (Candin®) | i.d. injection | Phase I/II in women with HSIL | |
| ADXS11-001 [50] | HPV 16 E7 fused to non-hemolytic listeriolysin O protein | i.m. injection | Phase II in patients with persistent, recurrent SCC or non-SCC | ||
| VB10.16 vaccine [51] | DNA expressing HPV16 E6-E7, a dimerization domain and an APC targeting domain | needle-free injection | Phase I/II in patients with CIN 2 | ||
| GX-188E [52] Placebo | DNA expressing the E6/E7 fusion protein of HPV16 and 18, plus Flt3L and tPA sequences signals | Pembrolizumab (anti-PD1 mAb) | i.m. electroporation | Phase II randomized, double-blind, multi-center in patients with CIN II and CIN III | |
| NGVL4a-Sig/E7(detox)/HSP70 [53] | Vaccinia virus expressing E6/E7; DNA plasmid encoding signal peptide, a detox form of HPV-16 E7 and the HSP70 | Imiquimod | i.m. injection | Phase I in patients with HPV- precancerous lesions and CC | |
| GX-188E GX-I7 [54] | DNA E6/E7 fusion proteins of HPV16 and 18 plus GX-I7 | GX-I7 (IL-7 and hybrid Fc) | Imiquimod | i.m. electroporation | Phase 1 in patients HPV-positive |
| pNGVL4a-CRT/E7-Detox DNA Vaccine [55] | DNA HPV16 E7detox linked to calreticulin (CRT) | Cyclophosphamide intravenously up to 24 h | i.m. electroporation | Phase I in patients with Head and Neck Cancer | |
| Ad-E6E7 MG1-E6E7 [56,57] | Adenovirus expressing E6 and E7 plus Oncolytic Maraba virus expressing E6 and E7 | Atezolizumab (anti-PD-L1 mAb) | i.m. | Phase I |
| Nef Function | Nefmut [68,69] | Wild-Type(wt)-Nef [70,71,72,73,74,75] |
|---|---|---|
| CD4 down-regulation | − | +++ |
| Increase of HIV-1 (Human Immunodeficiency Virus 1) infectivity | − | +++ |
| Class I MHC (Major Histocompatibility Complex) down regulation | − | ++ |
| PAK (p21-activated kinase) activation | − | +++ |
| NAK (NF-kappaB-activating kinase) activation | − | +++ |
| Exosome association | +++ | +/− |
| Strengths of the Nefmut-Based CTL Vaccine Platform |
|---|
| In vivo, endogenously engineering of exosomes with high therapeutic efficacy. |
| Overcoming the pitfalls of ex vivo or in vitro exosome production and isolation approaches. |
| Specificity of the immune response for the antigen of interest, with low risk of an immunogenic response to endogenously engineered exosomes. |
| Advantages in terms of development, production, costs, and safety compared to other exosome-based approaches. |
| Impact of the data obtained from the breast cancer HER2/Neu model of primary carcinogenesis. |
| Description DNA Vaccine Approach | Antigen | Administration | % CD8+ Activation |
|---|---|---|---|
| Nefmut EV anchoring protein to generate immunogenic EVs Nefmut-EVs | HPV16 E7 | i.m. injection | 1.38% [77] |
| Intracellular targeting by LAMP-1, HSP70, CRT, Herpes Simplex Virus (HSV) VP22 sorting signals to enhance Ag presentation by APC | HPV16 E6 | i.d. injection gold particles by gene gun | 3% [33] |
| Simultaneous vaccination with E6+E7 fused to CRT to enhance Ag presentation by APC CRT sorting | HPV16 E6+E7 | i.d. injection gold particles by gene gun | 0.7% E6 0.4% E7 [34] |
| codon optimized E6 | HPV16 E6 | i.d. injection gold particles by gene gun | 0.77% [35] |
| E6/E7 consensus sequences | HPV18 E6/E7 | electroporation | 0.21% [36] |
| E6/E7 consensus sequences | HPV16 E6/E7 | i.m. injection | 0.50% [81] |
| E6/E7 consensus sequences | HPV6 and HPV11 E6/E7 | electroporation | 0.5% HPV6 0.9% HPV11 [37] |
| GX-188: Shuffled E6 and E7 fragments+Flt3L and tPA signals to promote trafficking and Ag presentation | HPV16 and HPV18 E6/E7 | electroporation | 0.08% [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bonito, P.; Accardi, L.; Galati, L.; Ferrantelli, F.; Federico, M. Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes. Cancers 2019, 11, 138. https://doi.org/10.3390/cancers11020138
Di Bonito P, Accardi L, Galati L, Ferrantelli F, Federico M. Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes. Cancers. 2019; 11(2):138. https://doi.org/10.3390/cancers11020138
Chicago/Turabian StyleDi Bonito, Paola, Luisa Accardi, Luisa Galati, Flavia Ferrantelli, and Maurizio Federico. 2019. "Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes" Cancers 11, no. 2: 138. https://doi.org/10.3390/cancers11020138
APA StyleDi Bonito, P., Accardi, L., Galati, L., Ferrantelli, F., & Federico, M. (2019). Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes. Cancers, 11(2), 138. https://doi.org/10.3390/cancers11020138






