Association of Polyps with Early-Onset Colorectal Cancer and Throughout Surveillance: Novel Clinical and Molecular Implications
Abstract
:1. Introduction
2. Results
2.1. Overall Features
2.2. Comparative Analysis of EOCRC with and without Associated Polyps
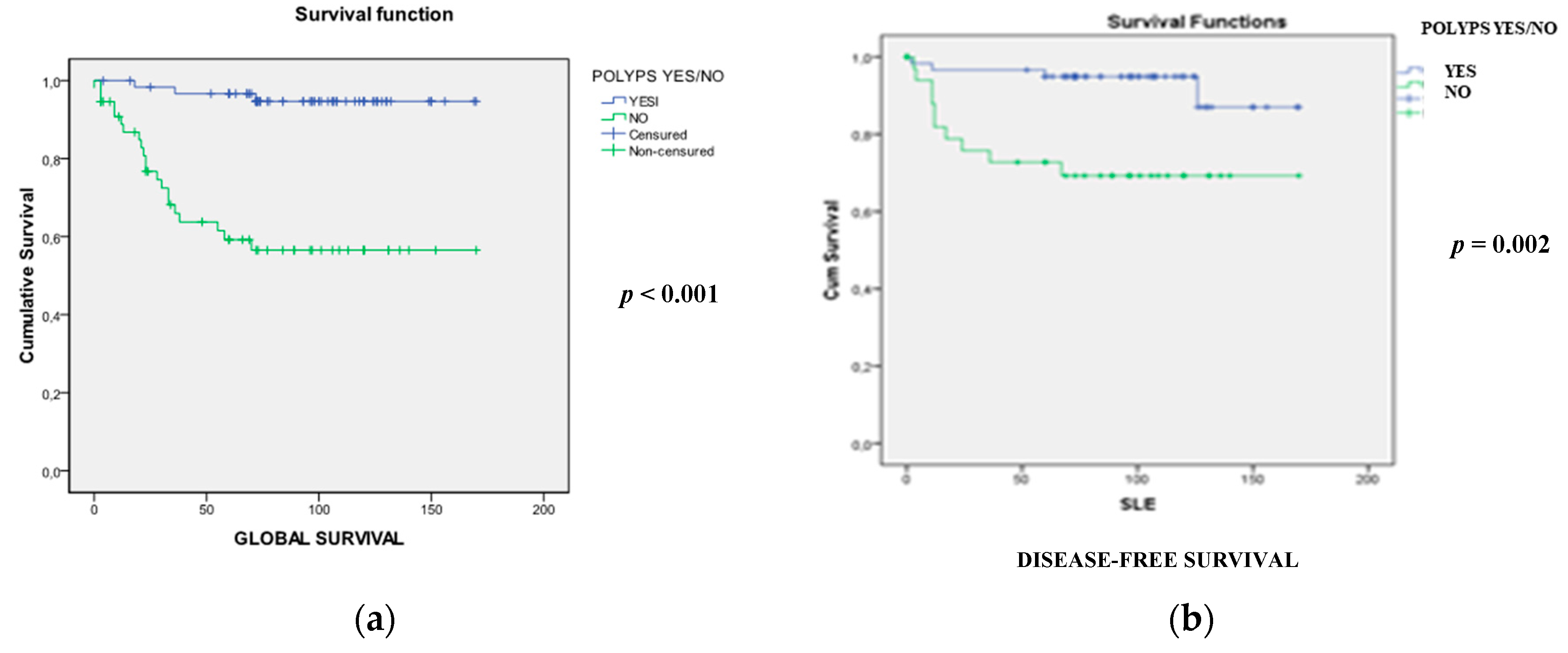
2.2.1. Clinicopathological and Familial Differences
2.2.2. Molecular Differences
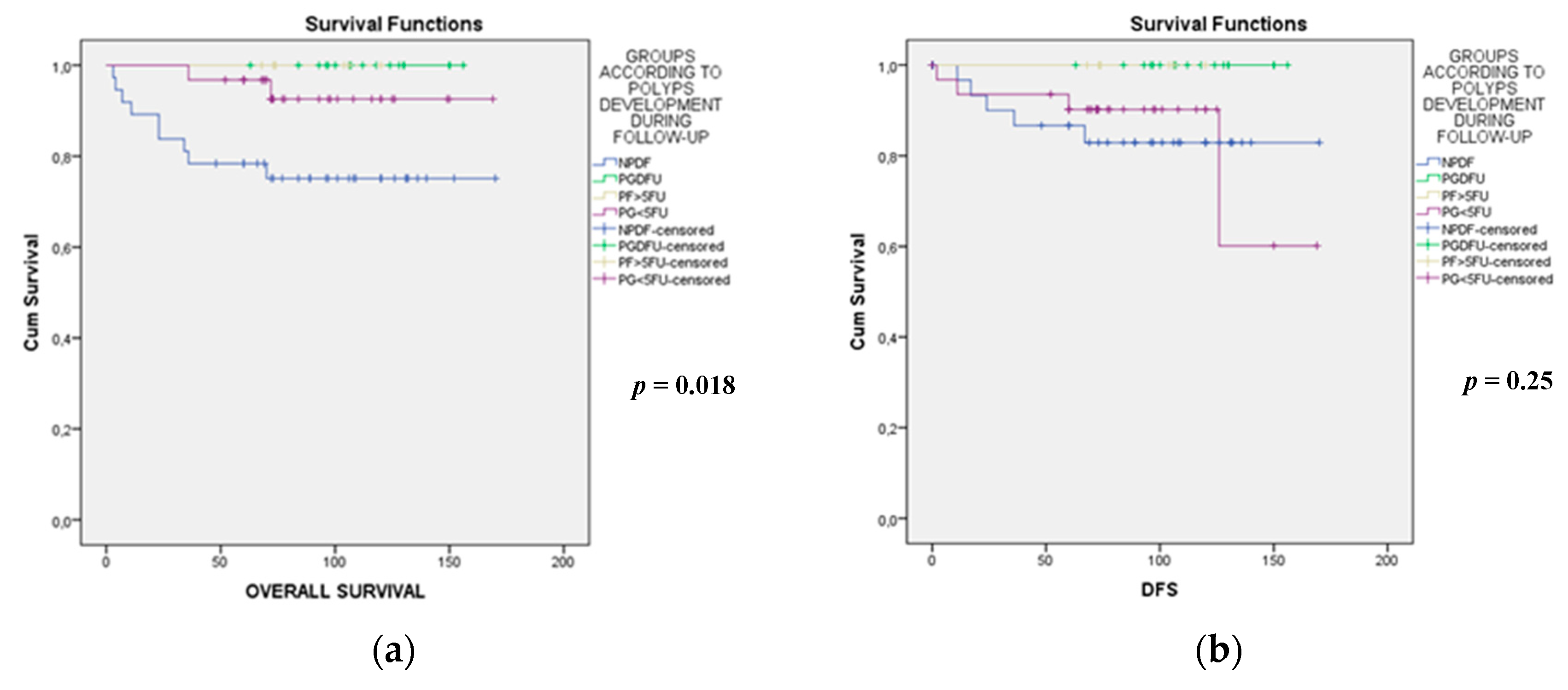
2.3. Comparative Analysis of Patients with EOCRC with the Development of Polyps during the Subsequent Follow-Up
3. Discussion
4. Materials and Methods
4.1. Families, Samples and Data Collection
4.2. Analysis of Microsatellite Instability (MSI) and Mutations in Mismatch Repair (MMR) Genes
4.3. Other Molecular Analyses
4.3.1. Mutational Status Analysis by Next Generation Sequencing
4.3.2. Chromosomal Instability
4.3.3. CpG Island Methylator Phenotype
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Kupfer, S.S.; Brim, H.; Carethers, J.M. Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology 2017, 153, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Lund, J.L.; Sandler, R.S. Young-Onset Colorectal Cancer: Earlier Diagnoses or Increasing Disease Burden? Gastroenterology 2017, 152, 1809–1812.e3. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68. [Google Scholar] [CrossRef]
- Vuik, F.E.R.; Nieuwenburg, S.A.V.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Austin, H.; Henley, S.J.; King, J.; Richardson, L.C.; Eheman, C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control 2014, 25, 191–201. [Google Scholar] [CrossRef]
- Rex, D.K.; Johnson, D.A.; Anderson, J.C.; Schoenfeld, P.S.; Burke, C.A.; Inadomi, J.M.; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am. J. Gastroenterol. 2009, 104, 739–750. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Liu, J.H.; Etzioni, D.A.; Livingston, E.H.; Ko, C.Y. Rates of colon and rectal cancers are increasing in young adults. Am. Surg. 2003, 69, 866–872. [Google Scholar] [PubMed]
- The American Cancer Society Medical and Editorial Content Team. American Cancer Society Guidelines for the Early Detection of Cancer. Available online: https://www.cancer.org. (accessed on 30 May 2018).
- Jeffery, M.; Hickey, B.E.; Hider, P.N.; See, A.M. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst. Rev. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- van der Stok, E.P.; Spaander, M.C.W.; Grünhagen, D.J.; Verhoef, C.; Kuipers, E.J. Surveillance after curative treatment for colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Pita-Fernández, S.; Alhayek-Aí, M.; González-Martín, C.; López-Calviño, B.; Seoane-Pillado, T.; Pértega-Díaz, S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 644–656. [Google Scholar] [CrossRef]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Mannucci, A.; Zuppardo, R.A.; Di Leo, M.; Stoffel, E.; Tonon, G. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig. Liver Dis. 2018, 50, 521–532. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Livingston, E.H.; Yo, C.K. Colorectal cancer in the young. Am. J. Surg. 2004, 187, 343–348. [Google Scholar] [CrossRef]
- Khan, S.A.; Morris, M.; Idrees, K.; Gimbel, M.I.; Rosenberg, S.; Zeng, Z.; Li, F.; Gan, G.; Shia, J.; LaQuaglia, M.P.; et al. Colorectal cancer in the very young: A comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J. Pediatr. Surg. 2016, 51, 1812–1817. [Google Scholar] [CrossRef]
- Siegel, R.L.; Jemal, A.; Ward, E.M. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1695–1698. [Google Scholar] [CrossRef]
- Russo, A.; Bazan, V.; Iacopetta, B.; Kerr, D.; Soussi, T.; Gebbia, N.; TP53-CRC Collaborative Study Group. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: Influence of tumor site, type of mutation, and adjuvant treatment. J. Clin. Oncol. 2005, 23, 7518–7528. [Google Scholar] [CrossRef]
- Westra, J.L.; Schaapveld, M.; Hollema, H.; de Boer, J.P.; Kraak, M.M.J.; de Jong, D.; ter Elst, A.; Mulder, N.H.; Buys, C.H.C.M.; Hofstra, R.M.W.; et al. Determination of TP53 mutation is more relevant than microsatellite instability status for the prediction of disease-free survival in adjuvant-treated stage III colon cancer patients. J. Clin. Oncol. 2005, 23, 5635–5643. [Google Scholar] [CrossRef] [PubMed]
- Tejpar, S.; Bertagnolli, M.; Bosman, F.; Lenz, H.-J.; Garraway, L.; Waldman, F.; Warren, R.; Bild, A.; Collins-Brennan, D.; Hahn, H.; et al. Prognostic and predictive biomarkers in resected colon cancer: Current status and future perspectives for integrating genomics into biomarker discovery. Oncologist 2010, 15, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tang, H.; Yan, D.; Fan, J.; Sun, H.; Wen, Y.; Yu, F.; Cui, F.; Zhang, D.; Xue, Y.; et al. DDA1 promotes stage IIB-IIC colon cancer progression by activating NFκB/CSN2/GSK-3β signaling. Oncotarget 2016, 7, 19794–19812. [Google Scholar] [PubMed]
- Glinsky, G.V.; Ivanova, Y.A.; Glinskii, A.B. Common malignancy-associated regions of transcriptional activation (MARTA) in human prostate, breast, ovarian, and colon cancers are targets for DNA amplification. Cancer Lett. 2003, 201, 67–77. [Google Scholar] [CrossRef]
- Thean, L.F.; Low, Y.S.; Lo, M.; Teo, Y.-Y.; Koh, W.-P.; Yuan, J.-M.; Chew, M.H.; Tang, C.L.; Cheah, P.Y. Genome-wide association study identified copy number variants associated with sporadic colorectal cancer risk. J. Med. Genet. 2018, 55, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Canavese, M.; Ngo, D.T.M.; Maddern, G.J.; Hardingham, J.E.; Price, T.J.; Hauben, E. Biology and therapeutic implications of VEGF-A splice isoforms and single-nucleotide polymorphisms in colorectal cancer. Int. J. Cancer 2017, 140, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.; Wang, N.; Zhang, M.; Zeng, X.; Zhao, W. MicroRNA-1299 is a negative regulator of STAT3 in colon cancer. Oncol. Rep. 2017, 37, 3227–3234. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Lu, J.; Wang, P.; Feng, H.; Zong, Y.; Ou, B.; Zheng, M.; Lu, A. Cadherin-12 enhances proliferation in colorectal cancer cells and increases progression by promoting EMT. Tumour Biol. 2016, 37, 9077–9088. [Google Scholar] [CrossRef]
- Farrington, S.M.; Lin-Goerke, J.; Ling, J.; Wang, Y.; Burczak, J.D.; Robbins, D.J.; Dunlop, M.G. Systematic analysis of hMSH2 and hMLH1 in young colon cancer patients and controls. Am. J. Hum. Genet. 1998, 63, 749–759. [Google Scholar] [CrossRef]
- Perea, J.; Rueda, D.; Canal, A.; Rodríguez, Y.; Álvaro, E.; Osorio, I.; Alegre, C.; Rivera, B.; Martínez, J.; Benítez, J.; et al. Age at onset should be a major criterion for subclassification of colorectal cancer. J. Mol. Diagn. 2014, 16, 116–126. [Google Scholar] [CrossRef]
- Perea, J.; García, J.L.; Corchete, L.; Lumbreras, E.; Arriba, M.; Rueda, D.; Tapial, S.; Pérez, J.; Vieiro, V.; Rodríguez, Y.; et al. Redefining synchronous colorectal cancers based on tumor clonality. Int. J. Cancer 2019, 144, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Arriba, M.; García, J.L.; Inglada-Pérez, L.; Rueda, D.; Osorio, I.; Rodríguez, Y.; Álvaro, E.; Sánchez, R.; Fernández, T.; Pérez, J.; et al. DNA copy number profiling reveals different patterns of chromosomal instability within colorectal cancer according to the age of onset. Mol. Carcinog. 2016, 55, 705–716. [Google Scholar] [CrossRef] [PubMed]
| Global n (%) | No polyps n (%) | Polyps n (%) | p (χ2) | |
|---|---|---|---|---|
| Patients | 119 (100) | 56 (47) | 63 (53) | − |
| Mean age of onset (±SD) 1 (years) | 40.7 (±4.4) | 41.0 (±4.4) | 40.6 (±4.1) | NS |
| Sex: | ||||
| Male | 67 (56) | 37 (55) | 30 (45) | NS |
| Female | 52 (44) | 26 (50) | 26 (50) | |
| Colon location: | 0.064 | |||
| Right | 29 (25) | 9 (16) | 20 (32) | |
| Left | 42 (35) | 19 (34) | 23 (36) | |
| Rectum | 48 (40) | 28 (50) | 20 (32) | |
| Mucin production 2 | 21/79 (26) | 16/48 (33) | 5/31 (16) | NS |
| “Signet ring” cells 2 | 3/98 (3) | 2/51 (4) | 1/47 (2) | NS |
| Stage at diagnosis: | <0.001 | |||
| I | 47 (39) | 9 (16) | 38 (60) | |
| II | 25 (21) | 9 (16) | 16 (25) | |
| III | 18 (15) | 14 (25) | 4 (6) | |
| IV | 29 (24) | 24 (43) | 5 (8) | |
| Overall survival (±SD) 1 (months) | 76.7 (±45.6) | 58.8 (±47.1) | 94.5 (±36.6) | 0.012 |
| Disease-free survival (±SD) 1 (months) | 68.1(±51.9) | 45.3 (±52.2) | 90.6 (±40.7) | <0.001 |
| Synchronous CRCs | 5/119 (4) | 1/56 (2) | 4/63 (6) | NS |
| Family history of cancer | ||||
| Aggregation for Lynch-related neoplasm | 56 (48) | 20 (37) | 36 (58) | 0.027 |
| Aggregation for CRC | 43 (37) | 13 (24) | 30 (48) | 0.007 |
| Aggregation for Lynch-unrelated neoplasm | 10 (13) | 13 (24) | 23 (37) | NS |
| Sporadic cases | 33 (41) | 30 (54) | 16 (26) | 0.002 |
| Chr | Start | End | Region | Region | Size (Mb) | P Group % Gains | % Losses | NP Group % Gains | % Losses |
|---|---|---|---|---|---|---|---|---|---|
| chr 1 | 49547237 | 50769506 | p33 | p32.3 | 1,222269 | 5,263157895 | 47,36842105 | 4,761904762 | 14,28571429 |
| chr 1 | 50819987 | 52195285 | p32.3 | p32.3 | 1,375298 | 26,31578947 | 26,31578947 | 23,80952381 | 0 |
| chr 1 | 204672629 | 208121292 | q32.1 | q32.2 | 3,448663 | 0 | 21,05263158 | 9,523809524 | 0 |
| chr 3 | 38942563 | 46420768 | p22.2 | p21.31 | 7,478205 | 5,263157895 | 31,57894737 | 19,04761905 | 5,263157895 |
| chr 5 | 14473935 | 16465030 | p15.2 | p15.1 | 1,991095 | 15,78947368 | 21,05263158 | 14,28571429 | 0 |
| chr 5 | 20785106 | 21941459 | p14.3 | p14.3 | 1,156353 | 5,263157895 | 47,36842105 | 14,28571429 | 14,28571429 |
| No Polyps During Follow-Up (NPDF) n (%) | Polyp Development During Follow-Up (PDFU) n (%) | Polyp Development after 5 Years of Follow-Up (P > 5FU) n (%) | Polyp Development before 5 Years of Follow-Up (P < 5FU) n (%) | p (χ2) | |
|---|---|---|---|---|---|
| Patients | 37 | 17 | 7 | 32 | − |
| Mean age of onset (±SD) 1 (years) | 40.8 (±4.4) | 39.2 (±4.4) | 40.4 (±5) | 41.5 (±3.6) | NS |
| Sex: | |||||
| Male | 20 (54) | 8 (47) | 5 (71) | 21 (66) | |
| Female | 17 (46) | 9 (53) | 2 (29) | 11 (34) | NS |
| Colon location: | NS | ||||
| Right | 7 (19) | 9 (53) | 2 (29) | 8 (25) | |
| Left | 13 (35) | 3 (18) | 4 (57) | 13 (41) | |
| Rectum | 17 (46) | 5 (29) | 1 (14) | 11 (34) | |
| Type of surgery: | |||||
| Curative | 30 (81) | 17 (100) | 7 (100) | 31 (97) | 0.037 |
| Palliative | 7 (19) | 0 (0) | 0 (0) | 1 (3) | |
| Mucin production 2 | 9/29 (31) | 3/8 (38) | 3/6 (50) | 0/14 (0) | 0.055 |
| “Signet ring” cells | 2/31 (7) | 0/11 (0) | 0/7 (0) | 1/26 (4) | NS |
| Stage at diagnosis: | |||||
| I | 12 (33) | 10 (59) | 2 (28) | 21 (65) | |
| II | 6 (16) | 4 (24) | 5 (71) | 8 (25) | |
| III | 10 (27) | 2 (12) | 0 (0) | 2 (6) | 0.003 |
| IV | 9 (24) | 1 (6) | 0 (0) | 1 (3) | |
| Overall survival (±SD) 1 (months) | 84 (±45) | 114 (±25) | 88 (±21) | 88 (±34) | 0.04 |
| Disease-free survival (±SD) 1 (months) | 74 (±51) | 114 (±25) | 88 (±21) | 81 (±38) | 0.012 |
| Synchronous CRCs | 1 (3) | 2 (12) | 0 (0) | 1 (3) | NS |
| Family history of cancer | |||||
| Aggregation for Lynch-related neoplasm | 16/37 (43) | 12/17 (71) | 4/7 (57) | 16/32 (50) | NS |
| Aggregation for CRC neoplasm | 12/37 (32) | 12/17 (71) | 3/7 (43) | 12/32 (38) | 0.032 |
| Aggregation for Lynch-unrelated neoplasm | 11/37 (30) | 8/17 (47) | 3/7 (32) | 7/32 (22) | NS |
| Sporadic cases | 18/37 (49) | 2/17 (12) | 2/7 (29) | 11/32 (34) | 0.069 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perea García, J.; Arribas, J.; Cañete, Á.; García, J.L.; Álvaro, E.; Tapial, S.; Narváez, C.; Vivas, A.; Brandáriz, L.; Hernández-Villafranca, S.; et al. Association of Polyps with Early-Onset Colorectal Cancer and Throughout Surveillance: Novel Clinical and Molecular Implications. Cancers 2019, 11, 1900. https://doi.org/10.3390/cancers11121900
Perea García J, Arribas J, Cañete Á, García JL, Álvaro E, Tapial S, Narváez C, Vivas A, Brandáriz L, Hernández-Villafranca S, et al. Association of Polyps with Early-Onset Colorectal Cancer and Throughout Surveillance: Novel Clinical and Molecular Implications. Cancers. 2019; 11(12):1900. https://doi.org/10.3390/cancers11121900
Chicago/Turabian StylePerea García, José, Julia Arribas, Ángel Cañete, Juan Luis García, Edurne Álvaro, Sandra Tapial, Cristina Narváez, Alfredo Vivas, Lorena Brandáriz, Sergio Hernández-Villafranca, and et al. 2019. "Association of Polyps with Early-Onset Colorectal Cancer and Throughout Surveillance: Novel Clinical and Molecular Implications" Cancers 11, no. 12: 1900. https://doi.org/10.3390/cancers11121900
APA StylePerea García, J., Arribas, J., Cañete, Á., García, J. L., Álvaro, E., Tapial, S., Narváez, C., Vivas, A., Brandáriz, L., Hernández-Villafranca, S., Rueda, D., Rodríguez, Y., Pérez-García, J., Olmedillas-López, S., García-Olmo, D., Cavestro, G. M., Urioste, M., Goel, A., & González-Sarmiento, R. (2019). Association of Polyps with Early-Onset Colorectal Cancer and Throughout Surveillance: Novel Clinical and Molecular Implications. Cancers, 11(12), 1900. https://doi.org/10.3390/cancers11121900








