The Role of 18F-FDG PET/CT in Staging and Prognostication of Mantle Cell Lymphoma: An Italian Multicentric Study
Abstract
1. Introduction
2. Results
2.1. Tumor Characteristics
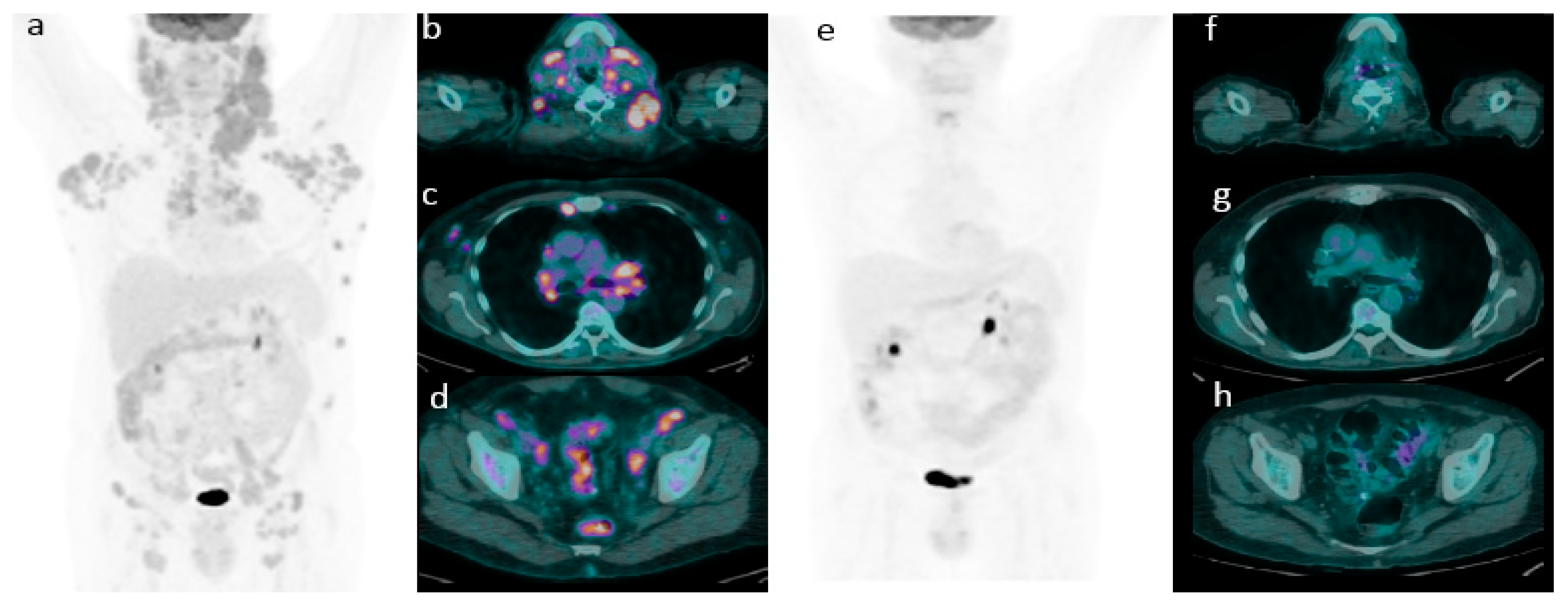
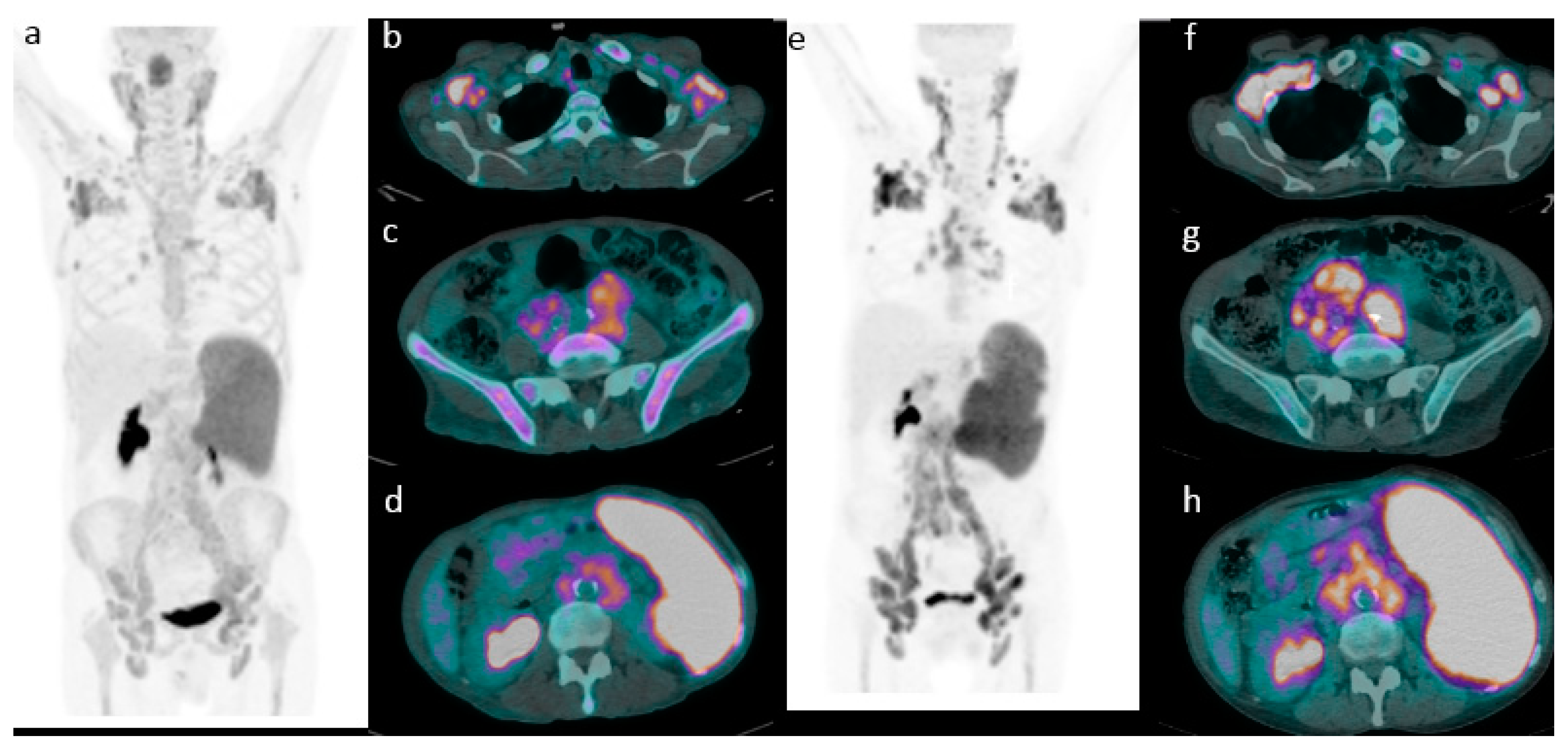
2.2. 18F-FDG PET/CT Evaluation for Initial Staging
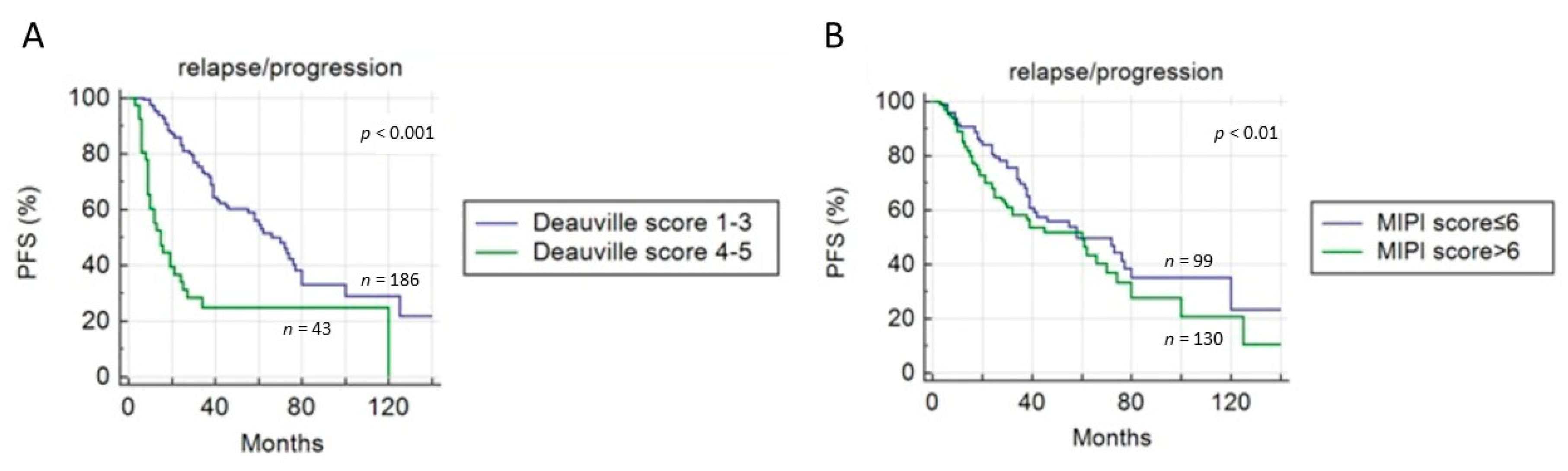
2.3. Role of 18F-FDG PET/CT in Predicting Survival
Application of Deauville Criteria
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. 18F-FDG PET/CT Imaging and Interpretation
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vose, J.M. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2017, 92, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Hoster, E.; Dreyling, M.; Klapper, W.; Gisselbrecht, C.; van Hoof, A.; Kluin-Nelemans, H.C.; Pfreundschuh, M.; Reiser, M.; Metzner, B.; Einsele, H.; et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008, 111, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Carlier, T.; Touzeau, C.; Arlicot, N.; Kraeber-Bodéré, F.; Le Gouill, S.; Bodet-Milin, C. Interest of FDG-PET in the Management of Mantle Cell Lymphoma. Front Med (Lausanne) 2019, 6, 70. [Google Scholar] [CrossRef]
- Maddocks, K. Update on Mantle cell lymphoma. Blood 2018, 132, 1647–1656. [Google Scholar] [CrossRef]
- Gill, S.; Wolf, M.; Prince, H.M.; Januszewicz, H.; Ritchie, D.; Hicks, R.J.; Seymour, J.F. 18F Fluorodeoxyglucose positron emission tomography scanning for staging, response assessment, and disease surveillance in patients with Mantle cell lymphoma. Clin. Lymphoma Myeloma 2008, 8, 158–165. [Google Scholar] [CrossRef]
- Alavi, A.; Shrikanthan, S.; Aydin, A.; Talanow, R.; Schuster, S. Fluorodeoxyglucose-positron-emission tomography findings in Mantle cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2011, 11, 261–266. [Google Scholar] [CrossRef]
- Albano, D.; Ferro, P.; Bosio, G.; Fallanca, F.; Re, A.; Tucci, A.; Ferreri, A.J.M.; Angelillo, P.; Gianolli, L.; Giubbini, R.; et al. Diagnostic and Clinical Impact of Staging 18F-FDG PET/CT in Mantle-Cell Lymphoma: A Two-Center Experience. Clin. Lymphoma Myeloma Leuk. 2019, 19, e457–e464. [Google Scholar] [CrossRef]
- Brepoels, L.; Stroobants, S.; De Wever, W.; Dierickx, D.; Vandenberghe, P.; Thomas, J.; Mortelmans, L.; Verhoef, G.; De Wolf-Peeters, C. Positron emission tomography in mantle cell lymphoma. Leuk. Lymphoma 2008, 49, 1693–1701. [Google Scholar] [CrossRef]
- Cohen, J.B.; Hall, N.C.; Ruppert, A.S.; Jones, J.A.; Porcu, P.; Baiocchi, R.; Christian, B.A.; Penza, S.; Benson, D.M.; Flynn, J.; et al. Association of pre-transplantation positron emission tomography/computed tomography and outcome in mantle cell lymphoma. Bone Marrow Transplant. 2013, 48, 1212–1217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosein, P.J.; Pastorini, V.H.; Paes, F.M.; Eber, D.; Chapman, J.R.; Serafini, A.N.; Alizadeh, A.A.; Lossos, I.S. Utility of positron emission tomography scans in mantle cell lymphoma. Am. J. Hematol. 2011, 86, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Perry, M.; Kwak, J.; Jensen, A.; Kamdar, M. Positron emission tomography-based analysis can accurately predict bone marrow involvement with Mantle Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2018, 18, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.J.A.; Kwee, T.C.; de Keizer, B.; Fijnheer, R.; de Klerk, J.M.; Littooij, A.S.; Nievelstein, R.A. Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: Is bone marrow biopsy still necessary? Ann. Oncol. 2014, 25, 921–927. [Google Scholar] [CrossRef]
- Adams, H.J.A.; Kwee, T.C.; de Keizer, B.; Fijnheer, R.; de Klerk, J.M.; Nievelstein, R.A. FDG PET/CT for the detection of bone marrow involvement in diffuse large B cell lymphoma: Systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 565–574. [Google Scholar] [CrossRef]
- Bodet-Milin, C.; Touzeau, C.; Leux, C.; Sahin, M.; Moreau, A.; Maisonneuve, H.; Morineau, N.; Jardel, H.; Moreau, P.; Gallazini-Crépin, C.; et al. Prognostic impact of 18F-fluorodeoxyglucose positron emission tomography in untreated mantle cell lymphoma: A retrospective study from GOELAMS group. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1633–1642. [Google Scholar] [CrossRef]
- Gontier, E.; Fourme, E.; Wartski, M.; Blondet, C.; Bonardel, G.; Le Stanc, E.; Mantzarides, M.; Foehrenbach, H.; Pecking, A.P.; Alberini, J.L. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 95–99. [Google Scholar] [CrossRef]
- Bybel, B.; Greenberg, I.D.; Paterson, J.; Ducharme, J.; Leslie, W.D. Increased F-18 FDG intestinal uptake in diabetic patients on metformin: A matched case-control analysis. Clin. Nucl. Med. 2011, 36, 452–456. [Google Scholar] [CrossRef]
- Shah, J.J.; Fayad, L.; Romaguera, J. Mantle Cell International Prognostic Index (MIPI) not prognostic after R-hyper-CVAD. Blood 2008, 112, 2583. [Google Scholar] [CrossRef][Green Version]
- Mato, A.R.; Svodoba, J.; Feldman, T.; Zielonka, T.; Agress, H.; Panush, D.; Miller, M.; Toth, P.; Lizotte, P.M.; Nasta, S.; et al. Post-treatment (not interim) positron emission tomography-computed tomography scan status is highly predictive of outcome in mantle cell lymphoma patients treated with R-HyperCVAD. Cancer 2012, 118, 3565–3570. [Google Scholar] [CrossRef]
- Tiemann, M.; Schrader, C.; Klapper, W.; Dreyling, M.H.; Campo, E.; Norton, A.; Berger, F.; Kluin, P.; Ott, G.; Pileri, S.; et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): A clinicopathological study from the European MCL Network. Br. J. Haematol. 2005, 131, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lamonica, D.; Graf, D.A.; Munteanu, M.C.; Czuczman, M.S. 18F-FDG PET for Measurement of Response and Prediction of Outcome to Relapsed or Refractory Mantle Cell Lymphoma Therapy with Bendamustine-Rituximab. J. Nucl. Med. 2017, 58, 62–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailly, C.; Carlier, T.; Barriolo-Rieding, A.; Casasnovas, O.; Gyan, E.; Meignan, M.; Moreau, A.; Burroni, B.; Djaileb, L.; Gressin, R.; et al. Prognostic value of FDG-PET in patients with mantle cell lymphoma: Results from the LyMa-PET Project. Haematologica 2019. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Bosio, G.; Bianchetti, N.; Pagani, C.; Re, A.; Tucci, A.; Giubbini, R.; Bertagna, F. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in mantle cell lymphoma. Ann. Nucl. Med. 2019, 33, 449–458. [Google Scholar] [CrossRef]
- Kedmi, M.; Avivi, I.; Ribakovsky, E.; Benyamini, N.; Davidson, T.; Goshen, E.; Tadmor, T.; Nagler, A.; Avigdor, A. Is there a role for therapy response assessment with 2-[fluorine-18] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in mantle cell lymphoma? Leuk. Lymphoma 2014, 55, 2484–2489. [Google Scholar] [CrossRef]
- Meignan, M.; Gallamini, A.; Haioun, C.; Polliack, A. Report on the Second International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma. 2010, 51, 2171–2180. [Google Scholar] [CrossRef]
- Fallanca, F.; Alongi, P.; Incerti, E.; Ginaolli, L.; Picchio, M.; Kayani, I.; Bomanji, J. Diagnostic accuracy of FDG PET/CT for clinical evaluation at the end of treatment of HL and NHL: A comparison of the Deauville Criteria (DC) and the International Harmonization Project Criteria (IHPC). Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1837–1840. [Google Scholar] [CrossRef]
- Klener, P.; Fronkova, E.; Belada, D.; Forsterova, K.; Pytlik, R.; Kalinova, M.; Simkovic, M.; Salek, D.; Mocikova, H.; Prochazka, V.; et al. Alternating R-CHOP and R-cytarabine is a safe and effective regimen for transplant-ineligible patients with a newly diagnosed mantle cell lymphoma. Hematol. Oncol. 2018, 36, 110–115. [Google Scholar] [CrossRef]
- Czuczman, M.S.; Goy, A.; Lamonica, D.; Graf, D.A.; Munteanu, M.C.; van der Jagt, R.H. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: Efficacy, tolerability, and safety findings. Ann. Hematol. 2015, 94, 2025–2032. [Google Scholar] [CrossRef]
- Karam, M.; Ata, A.; Irish, K.; Feustel, P.J.; Mottaghy, F.M.; Stroobants, S.G.; Verhoef, G.E.; Chundru, S.; Douglas-Nikitin, V.; Wong, C.O.; et al. FDG positron emission tomography/computed tomography scan may identify mantle cell lymphoma patients with unusually favorable outcome. Nucl. Med. Comm. 2009, 30, 770–778. [Google Scholar] [CrossRef]
- Novelli, A.; Briones, J.; Flotats, A.; Sierra, J. PET/CT Assessment of Follicular Lymphoma and High Grade B Cell Lymphoma-Good Correlation with Clinical and Histological Features at Diagnosis. Adv. Clin. Exp. Med. 2015, 24, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Wienke, A.; Surov, A. Correlations Between Imaging Biomarkers and Proliferation Index Ki-67 in Lymphomas: A Systematic Review and Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2019, 19, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Vardinam, J.W. Tumours of Haematopoietic and Lymphoid Tissues: World Health Organization Classification of Tumours, Pathology, and Genetics; IARC Press: Lyon, France, 2001; Volume 3. [Google Scholar]
- Albano, D.; Bosio, G.; Camoni, L.; Farina, M.; Re, A.; Tucci, A.; Giubbini, R.; Bertagna, F. Prognostic role of baseline 18F-FDG PET/CT parameters in MALT lymphoma. Hematol. Oncol. 2019, 37, 39–46. [Google Scholar]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]


| Variables | Patients n (%) | Average (Range) | |
|---|---|---|---|
| Age (years) | 65.1 (29–88) | ||
| Sex | |||
| male | 172 (75%) | ||
| female | 57 (25%) | ||
| Tumor stage at diagnosis (Ann Arbor) | |||
| I | 4 (2%) | ||
| II | 9 (4%) | ||
| III | 32 (14%) | ||
| IV | 184 (80%) | ||
| Blastoid variant | 26 (11%) | ||
| B symptoms | 65 (28%) | ||
| LDH | |||
| ≤245 U/L | 121 (57%) | ||
| >245 U/L | 93 (43%) | ||
| β2-microglobulin | |||
| ≤2.8 mg/L | 108 (62%) | ||
| >2.8 mg/L | 65 (38%) | ||
| MIPI score | |||
| low-intermediate (≤6) | 99 (43%) | ||
| high-intermediate (>6) | 130 (57%) | ||
| Bulky disease | 40 (17%) | ||
| Splenomegaly | 101 (44%) | ||
| Ki-67 score | |||
| ≤15% | 65 (36%) | ||
| >15% | 118 (64%) | ||
| PET/CT Findings | BM Biopsy | GI Endoscopy | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Positive | 37 (16%) | 0 (0%) | 28 (12%) | 2 (1%) |
| Negative | 99 (43%) | 93 (41%) | 19 (8%) | 180 (79%) |
| Total | 136 (59%) | 93 (41%) | 47 (21%) | 182 (79%) |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p-Value | HR (95% CI) | p-Value | HR (95% CI) | |
| PFS | ||||
| Sex | 0.451 | 0.845 (0.548–1.306) | ||
| Age | 0.153 | 1.530 (0.838–3.097) | ||
| MIPI score | 0.009 | 0.713 (0.482–1.056) | 0.174 | 1.219 (0.915–1.623) |
| LDH level | 0.163 | 0.742 (0.488–1.128) | ||
| Β2 microglobulin | 0.458 | 0.831 (0.511–1.353) | ||
| Ki-67 score | 0.066 | 0.653 (0.415–1.028) | ||
| Bulky disease | 0.153 | 1.722 (0.992–2.987) | ||
| Splenomegaly | 0.087 | 0.703 (0.472–01049) | ||
| Stage acc Ann Arbor | 0.855 | 0.957 (0.589–1.531) | ||
| Blastoid variant | 0.185 | 0.598 (0.282–1.270) | ||
| Deauville score | <0.001 | 0.137 (0.073–0.259) | <0.001 | 4.059 (2.573–6.403) |
| Treatment regimen | 0.655 | 0.857 (0.519–1.243) | ||
| OS | ||||
| Sex | 0.211 | 1.759 (0.577–6.033) | ||
| Age | 0.375 | 1.270 (0.722–2.369) | ||
| MIPI score | 0.025 | 0.711 (0.527–0.959) | 0.017 | 1.204 (1.032–1.403) |
| LDH level | 0.709 | 0.942 (0.690–1.287) | ||
| Β2 microglobulin | 0.524 | 1.128 (0.778–1.635) | ||
| Ki67 score | 0.195 | 1.250 (0.891–1.754) | ||
| Bulky disease | 0.390 | 0.828 (0.539–1.272) | ||
| Splenomegaly | 0.287 | 0.846 (0.622–1.150) | ||
| Stage acc Ann Arbor | 0.393 | 0.859 (0.606–1.217) | ||
| Blastoid variant | 0.075 | 0.618 (0.363–1.051) | ||
| Deauville score | 0.814 | 1.055 (0.671–1.660) | ||
| Treatment regimen | 0.598 | 1.001 (0.571–1.460) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, D.; Laudicella, R.; Ferro, P.; Allocca, M.; Abenavoli, E.; Buschiazzo, A.; Castellino, A.; Chiaravalloti, A.; Cuccaro, A.; Cuppari, L.; et al. The Role of 18F-FDG PET/CT in Staging and Prognostication of Mantle Cell Lymphoma: An Italian Multicentric Study. Cancers 2019, 11, 1831. https://doi.org/10.3390/cancers11121831
Albano D, Laudicella R, Ferro P, Allocca M, Abenavoli E, Buschiazzo A, Castellino A, Chiaravalloti A, Cuccaro A, Cuppari L, et al. The Role of 18F-FDG PET/CT in Staging and Prognostication of Mantle Cell Lymphoma: An Italian Multicentric Study. Cancers. 2019; 11(12):1831. https://doi.org/10.3390/cancers11121831
Chicago/Turabian StyleAlbano, Domenico, Riccardo Laudicella, Paola Ferro, Michela Allocca, Elisabetta Abenavoli, Ambra Buschiazzo, Alessia Castellino, Agostino Chiaravalloti, Annarosa Cuccaro, Lea Cuppari, and et al. 2019. "The Role of 18F-FDG PET/CT in Staging and Prognostication of Mantle Cell Lymphoma: An Italian Multicentric Study" Cancers 11, no. 12: 1831. https://doi.org/10.3390/cancers11121831
APA StyleAlbano, D., Laudicella, R., Ferro, P., Allocca, M., Abenavoli, E., Buschiazzo, A., Castellino, A., Chiaravalloti, A., Cuccaro, A., Cuppari, L., Durmo, R., Evangelista, L., Frantellizzi, V., Kovalchuk, S., Linguanti, F., Santo, G., Bauckneht, M., & Annunziata, S., on behalf of Young Italian Association of Nuclear Medicine (AIMN) Working Group. (2019). The Role of 18F-FDG PET/CT in Staging and Prognostication of Mantle Cell Lymphoma: An Italian Multicentric Study. Cancers, 11(12), 1831. https://doi.org/10.3390/cancers11121831









