Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation
Abstract
1. Introduction
2. Results
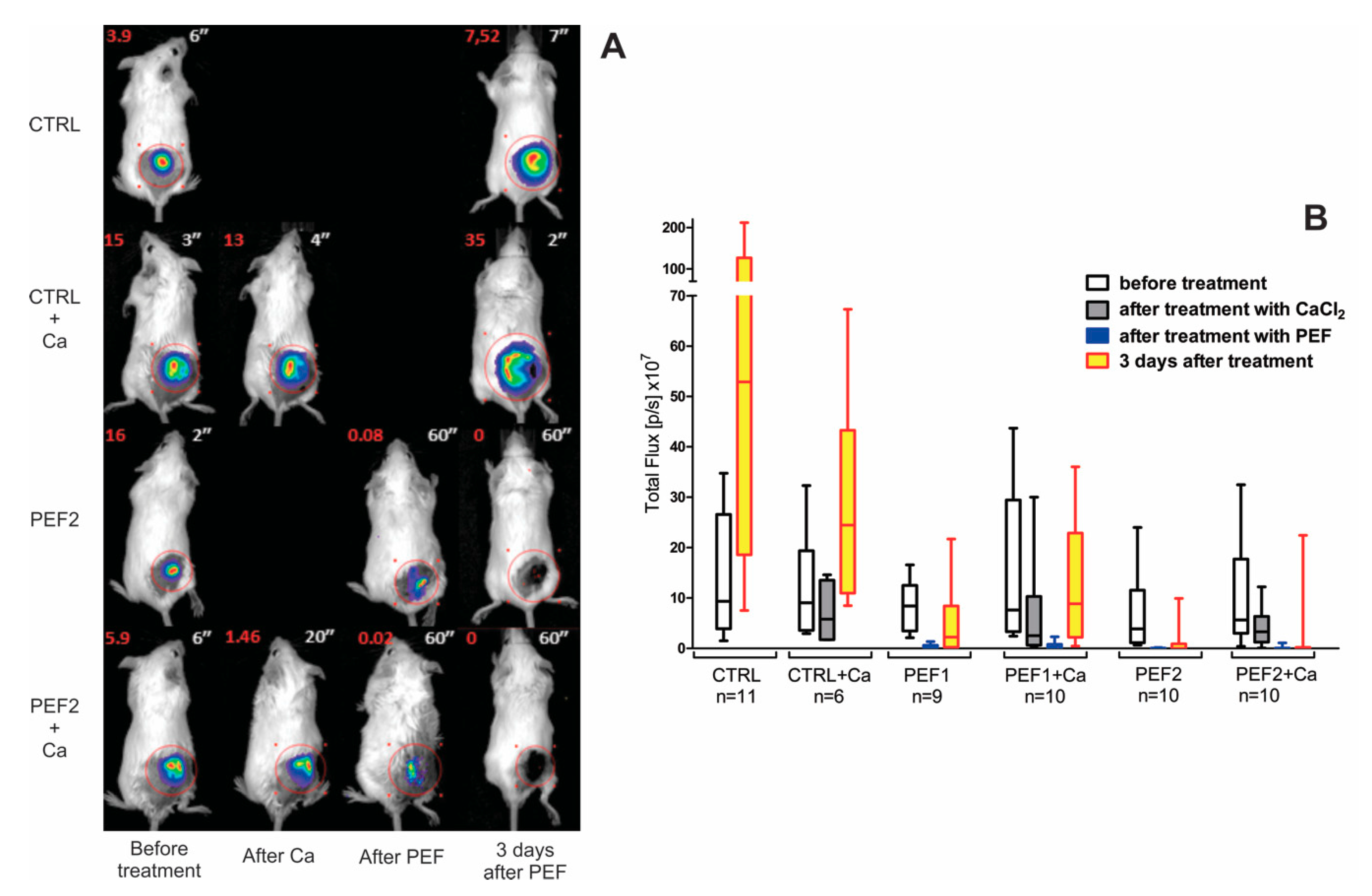
2.1. Bioluminescence Assay
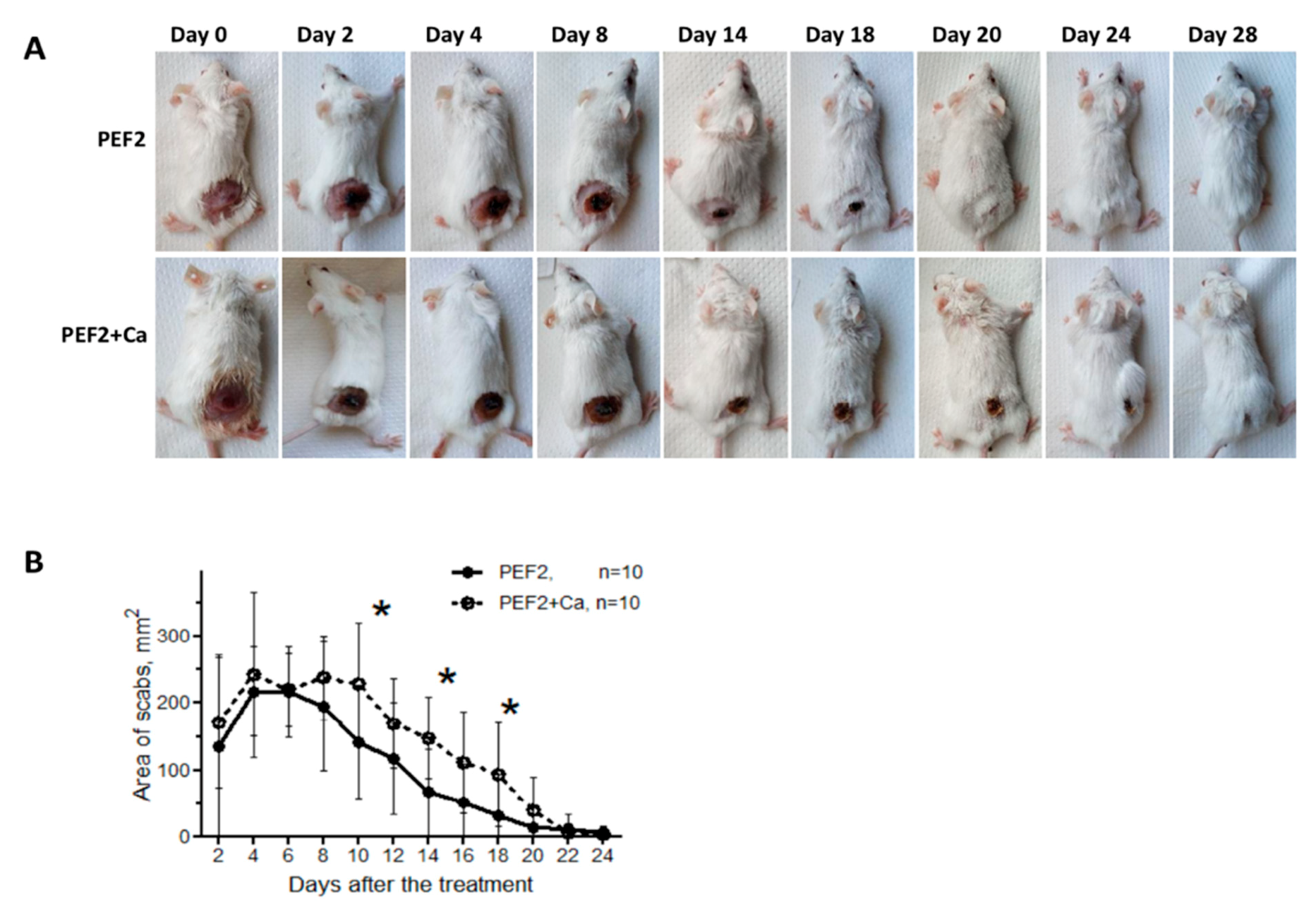
2.2. Scab Formation
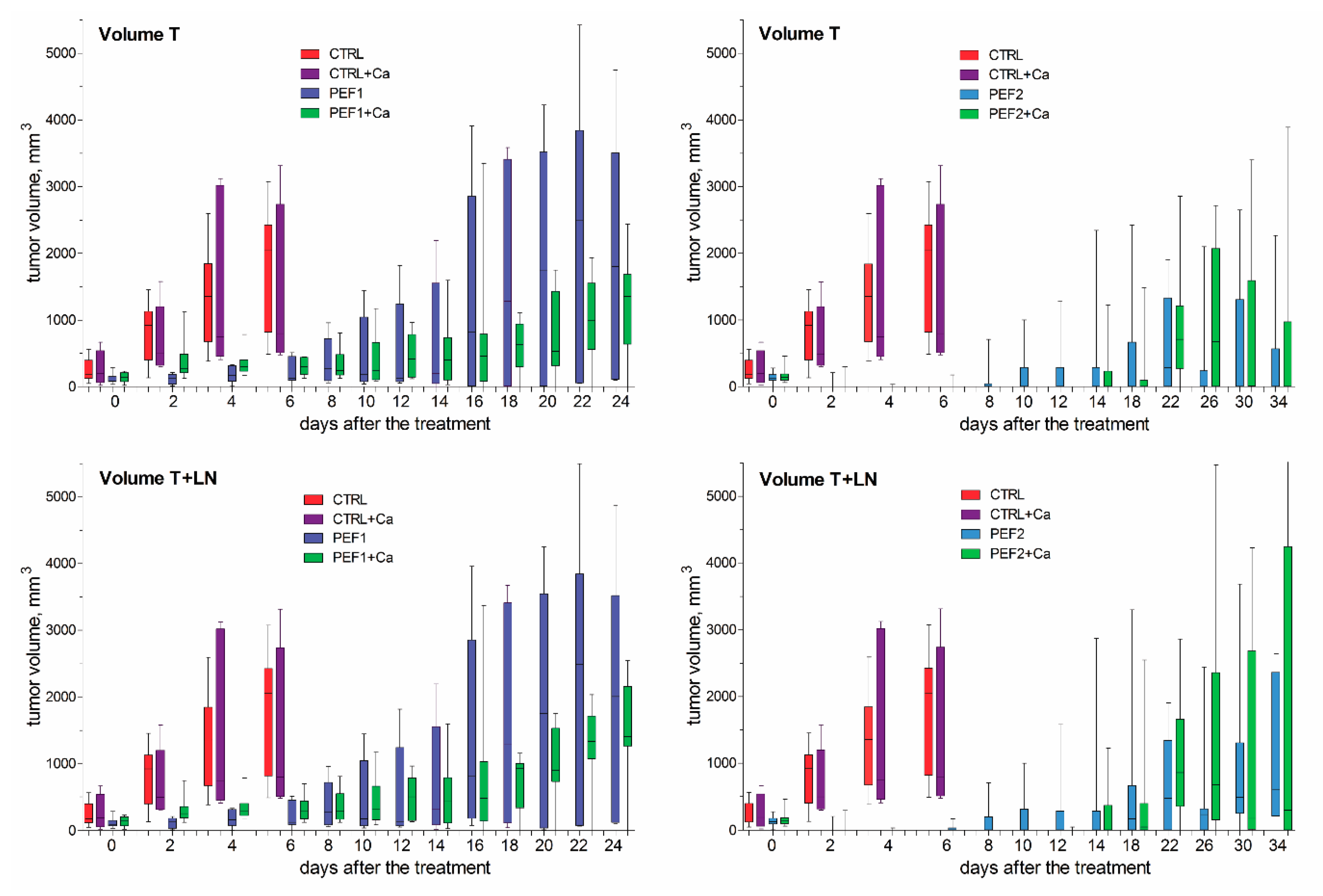
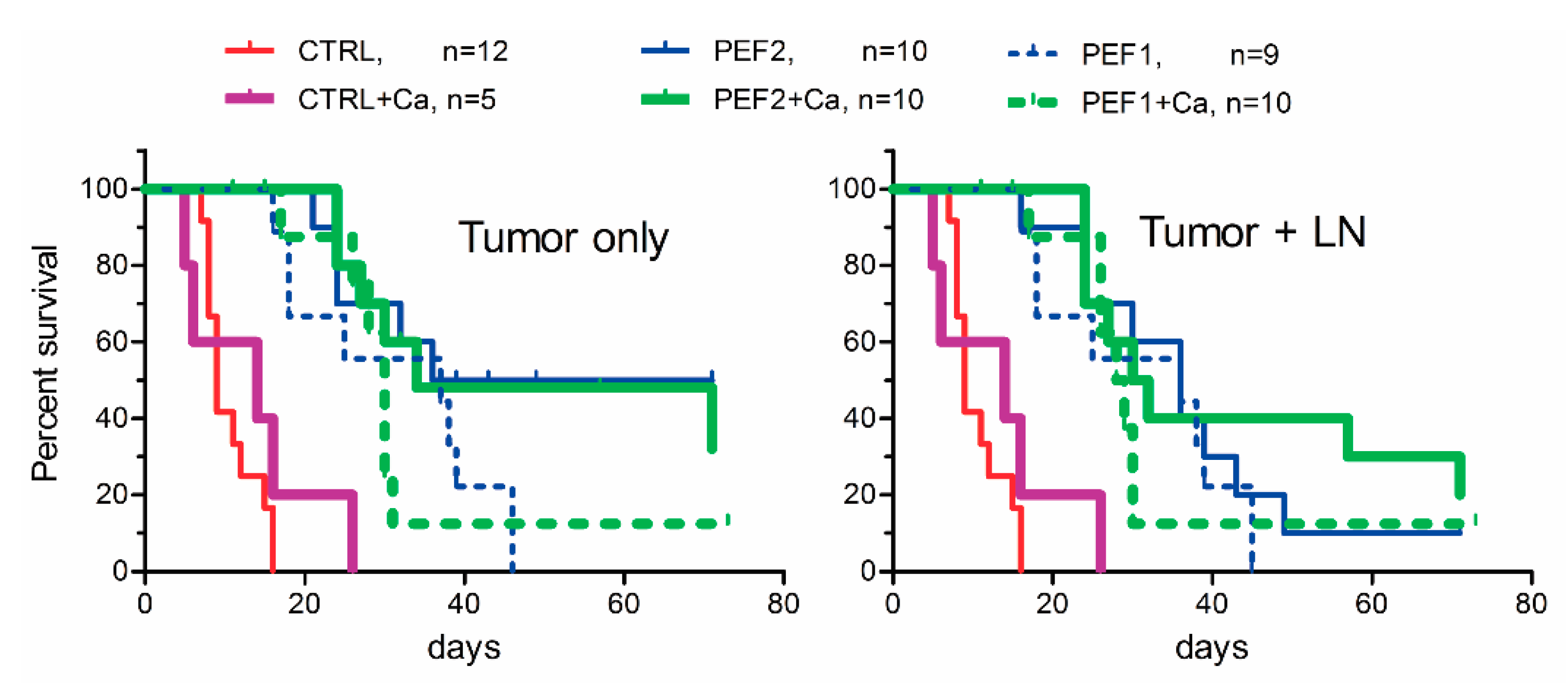
2.3. Volumetric Tumor Changes and Survival
2.4. Lymphocyte Subsets in Spleen, Lymph Nodes and Tumors
2.5. Immune Response
3. Discussion
4. Materials and Methods
4.1. Generation of Luciferase Expressing Sp2/0 Myeloma Cells
4.2. Mice and Tumor Induction
4.3. Electroporation
4.4. Experimental Scheme
4.5. Evaluation of Tumor Sizes
4.6. Flow Cytometric Analysis
4.7. Cytotoxic T Lymphocyte (CTL) Killing Assay
4.8. Determination of Tumor Cell-Specific Antibodies
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagstaff, P.G.K.; Buijs, M.; de Bruin, D.M.; Zondervan, P.J.; Jmch, J.; Rosette, D.; Pes, M.P.L. Irreversible electroporation: State of the art. Onco Targets Ther. 2016, 9, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Orlowski, S.; Belehradek, J.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer Clin. Oncol. 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gehl, J. Effect of calcium electroporation in combination with metformin in vivo and correlation between viability and intracellular ATP level after calcium electroporation in vitro. PLoS ONE 2017, 12, e0181839. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Eriksen, J.; Gehl, J. Calcium electroporation in three cell lines: A comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Davalos, R.V.; Mir, L.M.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223. [Google Scholar] [CrossRef]
- Calvet, C.Y.; Famin, D.; André, F.M.; Mir, L.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef]
- Josserand, V.; Kéramidas, M.; Lavaud, J.; Righini, C.; Vollaire, J.; Bellard, E.; Rols, M.P.; Teissié, J.; Coll, J.L.; Golzio, M. Electrochemotherapy guided by intraoperative fluorescence imaging for the treatment of inoperable peritoneal micro-metastases. J. Control. Release 2016, 233, 81–87. [Google Scholar] [CrossRef]
- Romeo, S.; Sannino, A.; Scarfì, M.R.; Vernier, P.T.; Cadossi, R.; Gehl, J.; Zeni, O. ESOPE-Equivalent Pulsing Protocols for Calcium Electroporation: An In Vitro Optimization Study on 2 Cancer Cell Models. Technol. Cancer Res. Treat. 2018. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012, 72, 1336–1341. [Google Scholar] [CrossRef]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319. [Google Scholar] [CrossRef]
- Falk, H.; Lambaa, S.; Johannesen, H.H.; Wooler, G.; Venzo, A.; Gehl, J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma—A case report. Acta Oncol. 2017, 56, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019. [Google Scholar] [CrossRef] [PubMed]
- Al-Sakere, B.; Bernat, C.; André, F.; Connault, E.; Opolon, P.; Davalos, R.V.; Mir, L.M. A study of the immunological response to tumor ablation with irreversible electroporation. Technol. Cancer Res. Treat. 2007, 6, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Edelblute, C.; Arpag, S.; Lundberg, C.; Heller, R. IL-12 gene electrotransfer triggers a change in immune response within mouse tumors. Cancers 2018, 10, 498. [Google Scholar] [CrossRef]
- Kamensek, U.; Cemazar, M.; Lampreht Tratar, U.; Ursic, K.; Sersa, G. Antitumor in situ vaccination effect of TNFα and IL-12 plasmid DNA electrotransfer in a murine melanoma model. Cancer Immunol. Immunother. 2018, 67, 785–795. [Google Scholar] [CrossRef]
- Chaobin, H.; Jun, W.; Shuxin, S.; Yu, Z.; Shengping, L. Immunomodulatory Effect after Irreversible Electroporation in Patients with Locally Advanced Pancreatic Cancer. J. Oncol. 2019, 2019, 9346017. [Google Scholar] [CrossRef]
- Falk, H.; Forde, P.F.; Bay, M.L.; Mangalanathan, U.M.; Hojman, P.; Soden, D.M.; Gehl, J. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model. Oncoimmunology 2017, 6, e1301332. [Google Scholar] [CrossRef]
- Narayanan, G.; Hosein, P.J.; Arora, G.; Barbery, K.J.; Froud, T.; Livingstone, A.S.; Franceschi, D.; Rocha Lima, C.M.; Yrizarry, J. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J. Vasc. Interv. Radiol. 2012, 23, 613–621. [Google Scholar] [CrossRef]
- Nesin, O.M.; Pakhomova, O.N.; Xiao, S.; Pakhomov, A.G. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim. Biophys. Acta Biomembr. 2011, 1808, 792–801. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Shevin, R.; White, J.A.; Kolb, J.F.; Pakhomova, O.N.; Joshi, R.P.; Schoenbach, K.H. Membrane permeabilization and cell damage by ultrashort electric field shocks. Arch. Biochem. Biophys. 2007, 465, 109–118. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Labib, C.; Saulis, G.; Novickij, V.; Pakhomova, O.N.; Pakhomov, A.G. Selective susceptibility to nanosecond pulsed electric field (nsPEF) across different human cell types. Cell. Mol. Life Sci. 2016, 74, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; Lui, K.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanosecond pulsed electric field stimulation of reactive oxygen species in human pancreatic cancer cells is Ca2+-dependent. Biochem. Biophys. Res. Commun. 2013, 435, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; Chen, X.; Pakhomov, A.G.; Baldwin, W.H.; Sheikh, S.; Pomicter, J.L.; Ren, W.; Osgood, C.; Swanson, R.J.; Kolb, J.F.; et al. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int. J. Cancer 2009, 125, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Skeate, J.G.; Da Silva, D.M.; Chavez-Juan, E.; Anand, S.; Nuccitelli, R.; Martin Kast, W. Nano-Pulse Stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS ONE 2018, 13, e0191311. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Tran, K.; Sheikh, S.; Athos, B.; Kreis, M.; Nuccitelli, P. Optimized nanosecond pulsed electric field therapy can cause murine malignant melanomas to self-destruct with a single treatment. Int. J. Cancer 2010, 127, 1727–17236. [Google Scholar] [CrossRef]
- Guo, S.; Burcus, N.I.; Edelblute, C.M.; Hornef, J.; Jiang, C.; Schoenbach, K.; Heller, R.; Beebe, S.J. Enhanced Electric Pulse Technology for the Ablation of Pancreatic Cancer. In Advances in Pancreatic Cancer; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2018, 142, 629–640. [Google Scholar] [CrossRef]
- Silve, A.; Leray, I.; Poignard, C.; Mir, L.M. Impact of external medium conductivity on cell membrane electropermeabilization by microsecond and nanosecond electric pulses. Sci. Rep. 2016, 6, 19957. [Google Scholar] [CrossRef]
- Silve, A.; Leray, I.; Leguèbe, M.; Poignard, C.; Mir, L.M. Cell membrane permeabilization by 12-ns electric pulses: Not a purely dielectric, but a charge-dependent phenomenon. Bioelectrochemistry 2015, 106, 369–378. [Google Scholar] [CrossRef]
- Di Gennaro, P.; Gerlini, G.; Urso, C.; Sestini, S.; Brandani, P.; Pimpinelli, N.; Borgognoni, L. CD4+FOXP3+ T regulatory cells decrease and CD3+CD8+ T cells recruitment in TILs from melanoma metastases after electrochemotherapy. Clin. Exp. Metastasis 2016, 33, 787–798. [Google Scholar] [CrossRef]
- Dai, J.; El Gazzar, M.; Li, G.Y.; Moorman, J.P.; Yao, Z.Q. Myeloid-derived suppressor cells: Paradoxical roles in infection and immunity. J. Innate Immun. 2015, 7, 16–26. [Google Scholar] [CrossRef]
- Bronte, V.; Apolloni, E.; Cabrelle, A.; Ronca, R.; Serafini, P.; Zamboni, P.; Restifo, N.P.; Zanovello, P. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood 2000, 96, 3838–3846. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Guo, R.; Wang, P. CD4+Foxp3−type 1 regulatory T cells in glioblastoma multiforme suppress T cell responses through multiple pathways and are regulated by tumor-associated macrophages. Int. J. Biochem. Cell Biol. 2016, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, W.G.H.; Schuurhuis, D.H.; Fu, N.; Camps, M.; van Duivenvoorde, L.M.; Louis-Plence, P.; Franken, K.L.M.C.; Huizinga, T.W.J.; Melief, C.J.M.; Toes, R.E.M.; et al. DC-induced CD8+ T-cell response is inhibited by MHC class II-dependent DX5+ CD4+ Treg. Eur. J. Immunol. 2009, 39, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, C.; Exley, M.; Balk, S.; Hohenberger, W.; Kuchroo, V.K. Characterization of the phenotype and function of CD8+, α/β+ NKT cells from tumor-bearing mice that show a natural killer cell activity and lyse multiple tumor targets. Eur. J. Immunol. 2001, 31, 2818–2828. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Li, Z.; Chai, Y.; Jiang, Y.; Wang, Q.; Ji, Y.; Zhu, Z.; Wan, Y.; Yuan, Z.; et al. CD8+ NKT-like cells regulate the immune response by killing antigen-bearing DCs. Sci. Rep. 2015, 5, 14124. [Google Scholar] [CrossRef]
- Kambayashi, T.; Assarsson, E.; Chambers, B.J.; Ljunggren, H.G. Expression of the DX5 antigen on CB8+ T cells is associated with activation and subsequent cell death or memory during influenza virus infection. Eur. J. Immunol. 2001, 31, 1523–1530. [Google Scholar] [CrossRef]
- Maly, K.; Schirmer, M. The story of CD4+CD28- T cells revisited: Solved or still ongoing? J. Immunol. Res. 2015, 2015, 348746. [Google Scholar] [CrossRef]
- Escarra-Senmarti, M.; Bueno-Topete, M.R.; Jave-Suarez, L.F.; Gomez-Bañuelos, E.; Gutierrez-Franco, J.; Vega-Magaña, N.; Aguilar-Lemarroy, A.; Pereira-Suarez, A.L.; Haramati, J.; del Toro-Arreola, S. Loss of CD28 within CD4+ T cell subsets from cervical cancer patients is accompanied by the acquisition of intracellular perforin, and is further enhanced by NKG2D expression. Immunol. Lett. 2017, 182, 30–38. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Xiang, A.P. CD8+CD28- T cells: Not only age-related cells but a subset of regulatory T cells. Cell. Mol. Immunol. 2018, 15, 34. [Google Scholar] [CrossRef]
- Song, Q.; Ren, J.; Zhou, X.; Wang, X.; Song, G.; Hobeika, A.; Yuan, Y.; Lyerly, H.K. Circulating CD8+ CD28− suppressor T cells tied to poorer prognosis among metastatic breast cancer patients receiving adoptive T-cell therapy: A cohort study. Cytotherapy 2018, 20, 126–133. [Google Scholar] [CrossRef]
- Liu, W.; Peng, B.; Lu, Y.; Xu, W.; Qian, W.; Zhang, J.Y. Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmun. Rev. 2011, 10, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Kim, W.Y.; O’Connell, F.; Flippin, L.; Günzler, V.; Horner, J.W.; DePinho, R.A.; Kaelin, W.G. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: Assessment of an oral agent that stimulates erythropoietin production. Proc. Natl. Acad. Sci. USA 2006, 103, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Novickij, V.; Grainys, A.; Butkus, P.; Tolvaišienė, S.; Švedienė, J.; Paškevičius, A.; Novickij, J. High-frequency submicrosecond electroporator. Biotechnol. Biotechnol. Equip. 2016, 30, 607–613. [Google Scholar] [CrossRef]
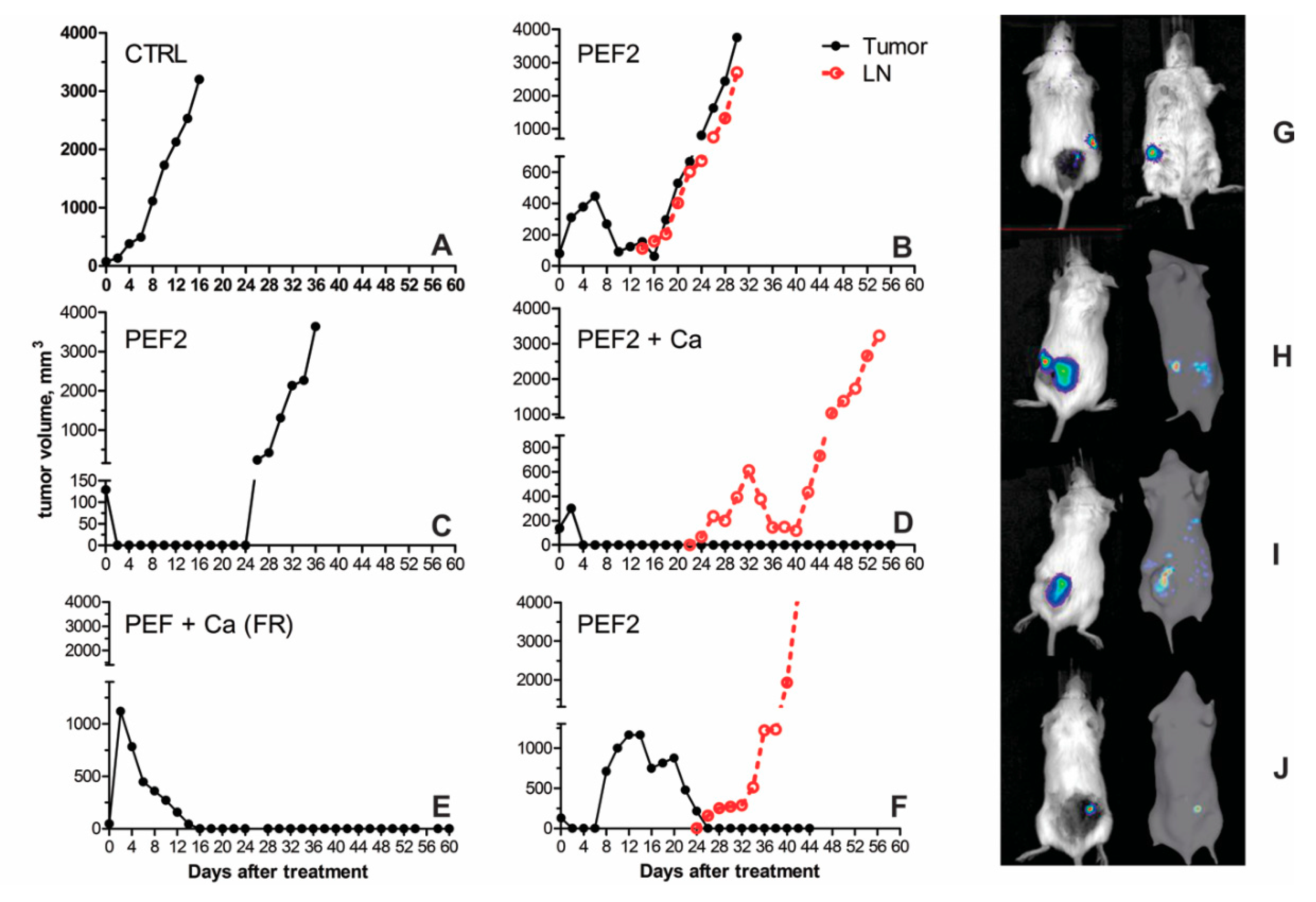

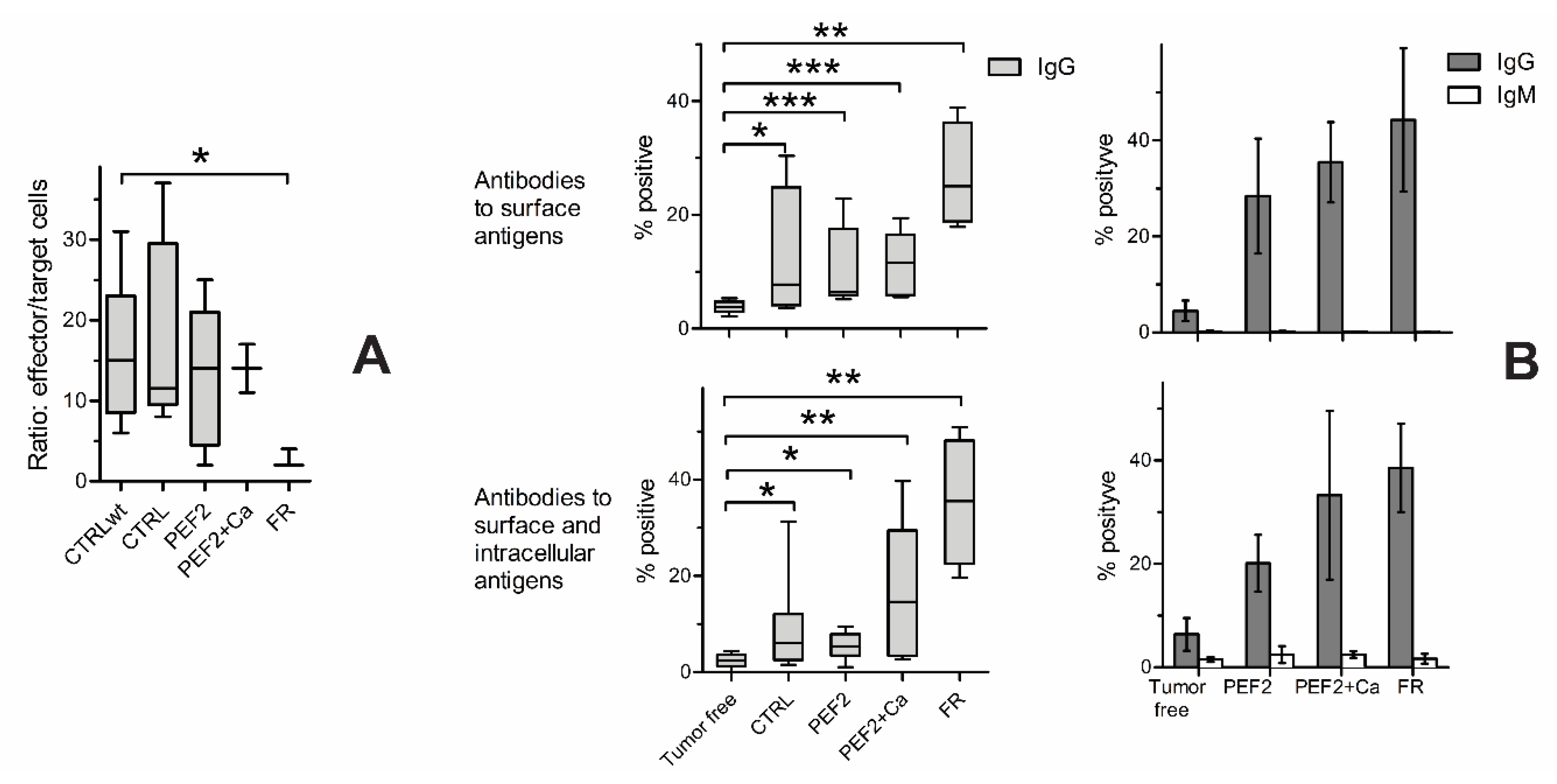
| Mouse Groups | CTRL | CTRL + Ca | PEF1 | PEF1 + Ca | PEF2 | PEF2 + Ca | |
|---|---|---|---|---|---|---|---|
| Mice, total numbers | 13 | 5 | 9 | 8 | 10 | 10 | |
| Primary tumor did not renew | numbers | 0 | 0 | 0 | 1 | 5 | 4 |
| % from total | 0 | 0 | 0 | 12.5 | 50 | 40 | |
| Enlarged lymph nodes due to metastasis | numbers | 3 | 2 | 5 | 7 | 7 | 4 |
| % from total | 23.4 | 40 | 55.6 | 87.5 | 70 | 40 | |
| Fully recovered mice | numbers | 0 | 0 | 0 | 1 | 1 | 2 |
| % from total | 0 | 0 | 0 | 12.5 | 10 | 20 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novickij, V.; Čėsna, R.; Perminaitė, E.; Zinkevičienė, A.; Characiejus, D.; Novickij, J.; Šatkauskas, S.; Ruzgys, P.; Girkontaitė, I. Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation. Cancers 2019, 11, 1763. https://doi.org/10.3390/cancers11111763
Novickij V, Čėsna R, Perminaitė E, Zinkevičienė A, Characiejus D, Novickij J, Šatkauskas S, Ruzgys P, Girkontaitė I. Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation. Cancers. 2019; 11(11):1763. https://doi.org/10.3390/cancers11111763
Chicago/Turabian StyleNovickij, Vitalij, Robertas Čėsna, Emilija Perminaitė, Auksė Zinkevičienė, Dainius Characiejus, Jurij Novickij, Saulius Šatkauskas, Paulius Ruzgys, and Irutė Girkontaitė. 2019. "Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation" Cancers 11, no. 11: 1763. https://doi.org/10.3390/cancers11111763
APA StyleNovickij, V., Čėsna, R., Perminaitė, E., Zinkevičienė, A., Characiejus, D., Novickij, J., Šatkauskas, S., Ruzgys, P., & Girkontaitė, I. (2019). Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation. Cancers, 11(11), 1763. https://doi.org/10.3390/cancers11111763







