Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer
Abstract
:1. Introduction
2. Results
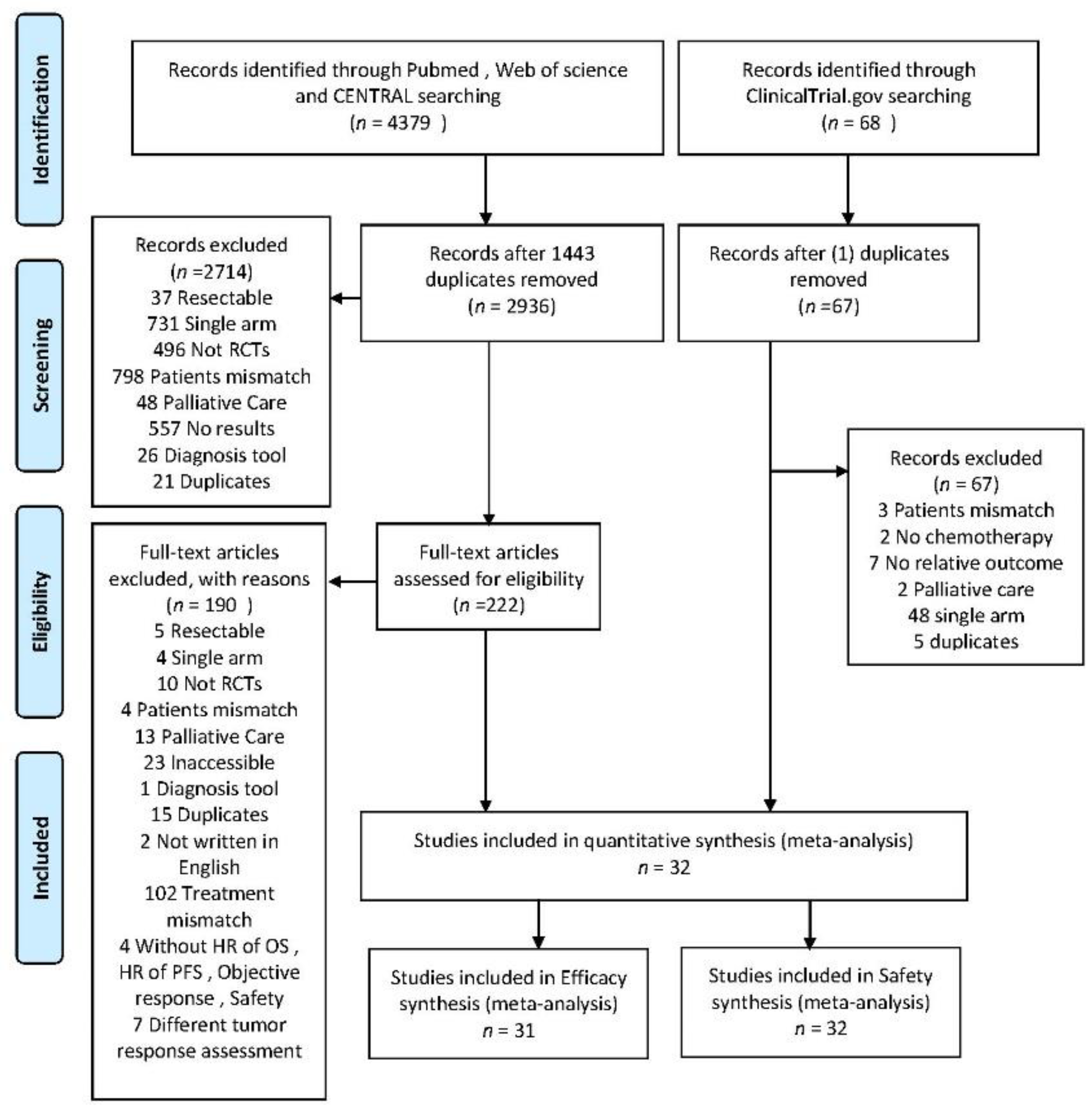
2.1. Search Results
2.2. Risk of Bias
2.3. Overall Survival (OS)
2.4. Progression-Free Survival (PFS)
2.5. Objective Response Rate (ORR)
2.6. Toxicity
2.7. Publication Bias
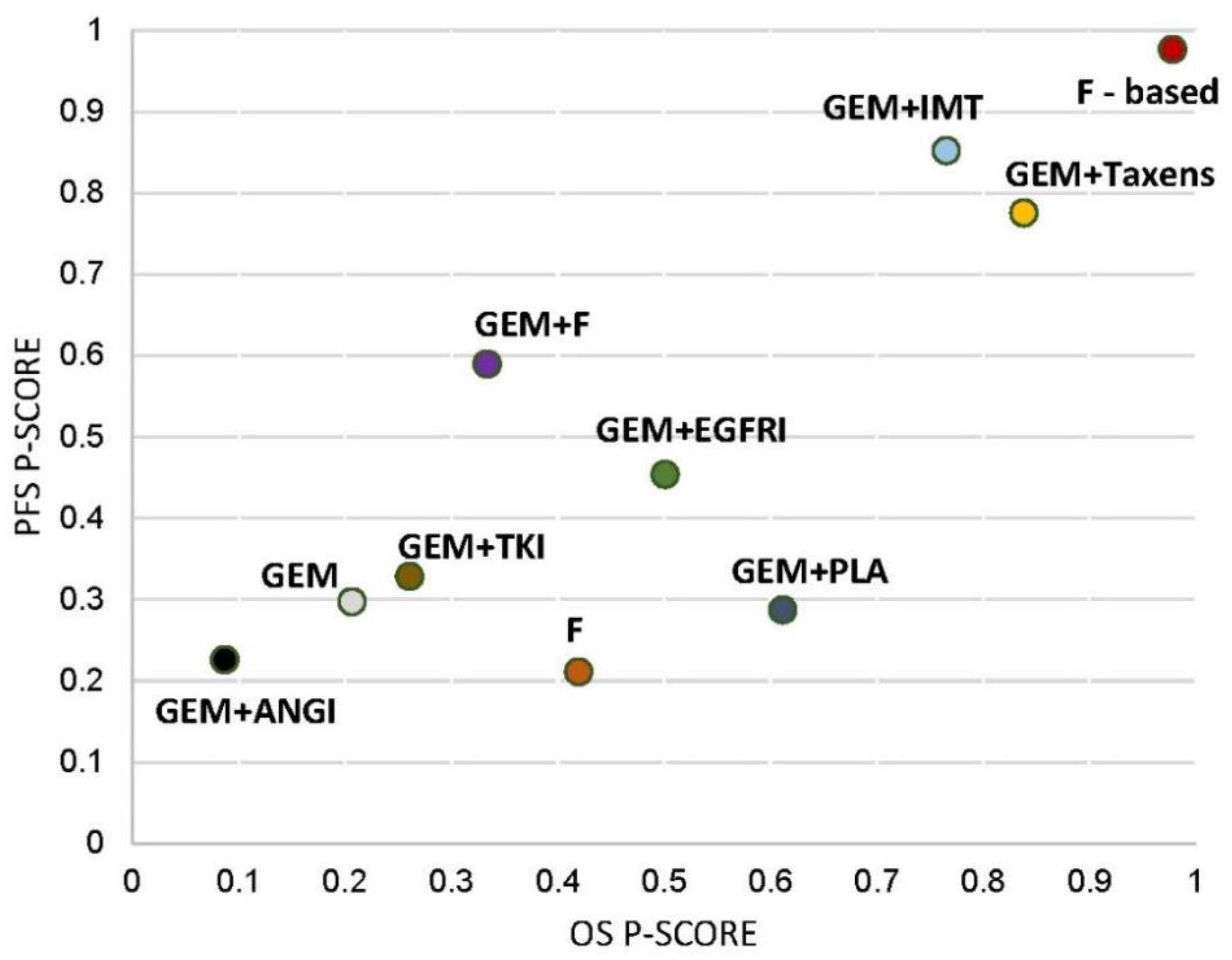
2.8. Finding of Ranking
3. Discussion
4. Materials and Methods
4.1. Literature Search and Study Selection
4.2. Data Extraction and Risk-of-Bias Assessment
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goere, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef]
- Lakkakula, B.; Farran, B.; Lakkakula, S.; Peela, S.; Yarla, N.S.; Bramhachari, P.V.; Kamal, M.A.; Saddala, M.S.; Nagaraju, G.P. Small molecule tyrosine kinase inhibitors and pancreatic cancer-Trials and troubles. Semin. Cancer Biol. 2019, 56, 149–167. [Google Scholar] [CrossRef]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Middleton, G.; Palmer, D.H.; Greenhalf, W.; Ghaneh, P.; Jackson, R.; Cox, T.; Evans, A.; Shaw, V.E.; Wadsley, J.; Valle, J.W.; et al. Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): A prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 2017, 18, 486–499. [Google Scholar] [CrossRef]
- Goncalves, A.; Gilabert, M.; Francois, E.; Dahan, L.; Perrier, H.; Lamy, R.; Re, D.; Largillier, R.; Gasmi, M.; Tchiknavorian, X.; et al. BAYPAN study: A double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann. Oncol. 2012, 23, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Angiogenesis in Pancreatic Cancer: Pre-Clinical and Clinical Studies. Cancers (Basel) 2019, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Barau, A.; Ruiz-Sauri, A.; Valencia, G.; Gomez-Mateo Mdel, C.; Sabater, L.; Ferrandez, A.; Llombart-Bosch, A. High microvessel density in pancreatic ductal adenocarcinoma is associated with high grade. Virchows Arch. 2013, 462, 541–546. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, S.; Zhang, B.; Liu, J.; Qin, Y.; Xu, J.; Yu, X. Role of angiogenesis in pancreatic cancer biology and therapy. Biomed. Pharmacother. 2018, 108, 1135–1140. [Google Scholar] [CrossRef]
- Wang, W.Q.; Liu, L.; Xu, H.X.; Luo, G.P.; Chen, T.; Wu, C.T.; Xu, Y.F.; Xu, J.; Liu, C.; Zhang, B.; et al. Intratumoral alpha-SMA enhances the prognostic potency of CD34 associated with maintenance of microvessel integrity in hepatocellular carcinoma and pancreatic cancer. PLoS ONE 2013, 8, e71189. [Google Scholar] [CrossRef]
- Tong, M.; Wang, J.; Zhang, H.; Xing, H.; Wang, Y.; Fang, Y.; Pan, H.; Li, D. Efficacy and safety of gemcitabine plus anti-angiogenesis therapy for advanced pancreatic cancer: A systematic review and meta-analysis of clinical randomized phase III trials. J. Cancer 2019, 10, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Weniger, M.; Jiang, K.; Boeck, S.; Zhang, K.; Bazhin, A.; Miao, Y.; Werner, J.; D’Haese, J.G. Therapies Targeting the Tumor Stroma and the VEGF/VEGFR Axis in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Target. Oncol. 2018, 13, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Fuchs, C.S.; Tabernero, J.; Ohtsu, A.; Zhu, A.X.; Garon, E.B.; Mackey, J.R.; Paz-Ares, L.; Baron, A.D.; Okusaka, T.; et al. Meta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumab. Ann. Oncol. 2017, 28, 2932–2942. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Sahin, I.H.; Askan, G.; Hu, Z.I.; O’Reilly, E.M. Immunotherapy in pancreatic ductal adenocarcinoma: An emerging entity? Ann. Oncol. 2017, 28, 2950–2961. [Google Scholar] [CrossRef]
- Brunet, L.R.; Hagemann, T.; Andrew, G.; Mudan, S.; Marabelle, A. Have lessons from past failures brought us closer to the success of immunotherapy in metastatic pancreatic cancer? Oncoimmunology 2016, 5, e1112942. [Google Scholar] [CrossRef]
- Deplanque, G.; Demarchi, M.; Hebbar, M.; Flynn, P.; Melichar, B.; Atkins, J.; Nowara, E.; Moye, L.; Piquemal, D.; Ritter, D.; et al. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann. Oncol. 2015, 26, 1194–1200. [Google Scholar] [CrossRef]
- Evans, T.R.J.; Van Cutsem, E.; Moore, M.J.; Bazin, I.S.; Rosemurgy, A.; Bodoky, G.; Deplanque, G.; Harrison, M.; Melichar, B.; Pezet, D.; et al. Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann. Oncol. 2017, 28, 354–361. [Google Scholar] [CrossRef]
- Lohr, J.M.; Haas, S.L.; Bechstein, W.O.; Bodoky, G.; Cwiertka, K.; Fischbach, W.; Folsch, U.R.; Jager, D.; Osinsky, D.; Prausova, J.; et al. Cationic liposomal paclitaxel plus gemcitabine or gemcitabine alone in patients with advanced pancreatic cancer: A randomized controlled phase II trial. Ann. Oncol. 2012, 23, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Kumari, P.; Beniwal, S.; Samdariya, S. Gemcitabine in combination with capecitabine compared with gemcitabine combined with erlotinib in locally advanced or metastatic pancreatic cancer. Ann. Oncol. 2016, 27, 92. [Google Scholar] [CrossRef]
- Schultheis, B.; Reuter, D.; Ebert, M.P.; Siveke, J.; Kerkhoff, A.; Berdel, W.E.; Hofheinz, R.; Behringer, D.M.; Schmidt, W.E.; Goker, E.; et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: A multicenter, randomized phase IIb study. Ann. Oncol. 2017, 28, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, A.G.; Stebbing, J.; Adamson, D.J.; Arif, S.S.; Bidoli, P.; Chang, D.; Cheeseman, S.; Diaz-Beveridge, R.; Fernandez-Martos, C.; Glynne-Jones, R.; et al. Randomised, open-label, phase II study of gemcitabine with and without IMM-101 for advanced pancreatic cancer. Br. J. Cancer 2016, 115, 789–796. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Sasaki, T.; Sasahira, N.; Tsujino, T.; Toda, N.; Kogure, H.; Matsubara, S.; Ito, Y.; Togawa, O.; et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br. J. Cancer 2012, 106, 1934–1939. [Google Scholar] [CrossRef] [Green Version]
- Ozaka, M.; Matsumura, Y.; Ishii, H.; Omuro, Y.; Itoi, T.; Mouri, H.; Hanada, K.; Kimura, Y.; Maetani, I.; Okabe, Y.; et al. Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother. Pharmacol. 2012, 69, 1197–1204. [Google Scholar] [CrossRef]
- Sudo, K.; Ishihara, T.; Hirata, N.; Ozawa, F.; Ohshima, T.; Azemoto, R.; Shimura, K.; Nihei, T.; Nishino, T.; Nakagawa, A.; et al. Randomized controlled study of gemcitabine plus S-1 combination chemotherapy versus gemcitabine for unresectable pancreatic cancer. Cancer Chemother. Pharmacol. 2014, 73, 389–396. [Google Scholar] [CrossRef]
- Nishida, S.; Ishikawa, T.; Egawa, S.; Koido, S.; Yanagimoto, H.; Ishii, J.; Kanno, Y.; Kokura, S.; Yasuda, H.; Oba, M.S.; et al. Combination Gemcitabine and WT1 Peptide Vaccination Improves Progression-Free Survival in Advanced Pancreatic Ductal Adenocarcinoma: A Phase II Randomized Study. Cancer Immunol. Res. 2018, 6, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Yamaue, H.; Tsunoda, T.; Tani, M.; Miyazawa, M.; Yamao, K.; Mizuno, N.; Okusaka, T.; Ueno, H.; Boku, N.; Fukutomi, A.; et al. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study. Cancer Sci. 2015, 106, 883–890. [Google Scholar] [CrossRef]
- Bergmann, L.; Maute, L.; Heil, G.; Russel, J.; Weidmann, E.; Koberle, D.; Fuxius, S.; Weigang-Kohler, K.; Aulitzky, W.E.; Wormann, B.; et al. A prospective randomised phase-II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: A study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Eur. J. Cancer 2015, 51, 27–36. [Google Scholar] [CrossRef]
- Rougier, P.; Riess, H.; Manges, R.; Karasek, P.; Humblet, Y.; Barone, C.; Santoro, A.; Assadourian, S.; Hatteville, L.; Philip, P.A. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur. J. Cancer 2013, 49, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Miyakawa, H.; Fujii, H.; Nakamori, S.; Satoh, T.; Hamamoto, Y.; Ito, T.; Maguchi, H.; Matsumoto, S.; Ueno, H.; et al. Updated results from GEST study: A randomized, three-arm phase III study for advanced pancreatic cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Chau, I.; Stocken, D.D.; Valle, J.W.; Smith, D.; Steward, W.; Harper, P.G.; Dunn, J.; Tudur-Smith, C.; West, J.; et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 2009, 27, 5513–5518. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, R.; Bodoky, G.; Ruhstaller, T.; Glimelius, B.; Bajetta, E.; Schuller, J.; Saletti, P.; Bauer, J.; Figer, A.; Pestalozzi, B.; et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J. Clin. Oncol. 2007, 25, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Niedzwiecki, D.; Hollis, D.; Sutherland, S.; Schrag, D.; Hurwitz, H.; Innocenti, F.; Mulcahy, M.F.; O’Reilly, E.; Wozniak, T.F.; et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 2010, 28, 3617–3622. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.A.; Benedetti, J.; Corless, C.L.; Wong, R.; O’Reilly, E.M.; Flynn, P.J.; Rowland, K.M.; Atkins, J.N.; Mirtsching, B.C.; Rivkin, S.E.; et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J. Clin. Oncol. 2010, 28, 3605–3610. [Google Scholar] [CrossRef]
- Poplin, E.; Feng, Y.; Berlin, J.; Rothenberg, M.L.; Hochster, H.; Mitchell, E.; Alberts, S.; O’Dwyer, P.; Haller, D.; Catalano, P.; et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: A trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2009, 27, 3778–3785. [Google Scholar] [CrossRef]
- Ueno, H.; Ioka, T.; Ikeda, M.; Ohkawa, S.; Yanagimoto, H.; Boku, N.; Fukutomi, A.; Sugimori, K.; Baba, H.; Yamao, K.; et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J. Clin. Oncol. 2013, 31, 1640–1648. [Google Scholar] [CrossRef]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouche, O.; Shannon, J.; Andre, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Colucci, G.; Labianca, R.; Di Costanzo, F.; Gebbia, V.; Carteni, G.; Massidda, B.; Dapretto, E.; Manzione, L.; Piazza, E.; Sannicolo, M.; et al. Randomized Phase III Trial of Gemcitabine Plus Cisplatin Compared With Single-Agent Gemcitabine As First-Line Treatment of Patients With Advanced Pancreatic Cancer: The GIP-1 Study. J. Clin. Oncol. 2010, 28, 1645–1651. [Google Scholar] [CrossRef]
- Kulke, M.H.; Tempero, M.A.; Niedzwiecki, D.; Hollis, D.R.; Kindler, H.L.; Cusnir, M.; Enzinger, P.C.; Gorsch, S.M.; Goldberg, R.M.; Mayer, R.J. Randomized Phase II Study of Gemcitabine Administered at a Fixed Dose Rate or in Combination With Cisplatin, Docetaxel, or Irinotecan in Patients With Metastatic Pancreatic Cancer: CALGB 89904. J. Clin. Oncol. 2009, 27, 5506–5512. [Google Scholar] [CrossRef]
- Spano, J.P.; Chodkiewicz, C.; Maurel, J.; Wong, R.; Wasan, H.; Barone, C.; Letourneau, R.; Bajetta, E.; Pithavala, Y.; Bycott, P.; et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: An open-label randomised phase II study. Lancet 2008, 371, 2101–2108. [Google Scholar] [CrossRef]
- Kindler, H.L.; Ioka, T.; Richel, D.J.; Bennouna, J.; Letourneau, R.; Okusaka, T.; Funakoshi, A.; Furuse, J.; Park, Y.S.; Ohkawa, S.; et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: A double-blind randomised phase 3 study. Lancet Oncol. 2011, 12, 256–262. [Google Scholar] [CrossRef]
- Lee, H.S.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Kim, H.G.; Noh, M.H.; Lee, S.H.; Kim, Y.T.; Kim, H.J.; et al. A randomized, multicenter, phase III study of gemcitabine combined with capecitabine versus gemcitabine alone as first-line chemotherapy for advanced pancreatic cancer in South Korea. Medicine 2017, 96, e5702. [Google Scholar] [CrossRef] [PubMed]
- Boeck, S.; Hoehler, T.; Seipelt, G.; Mahlberg, R.; Wein, A.; Hochhaus, A.; Boeck, H.P.; Schmid, B.; Kettner, E.; Stauch, M.; et al. Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): Final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann. Oncol. 2008, 19, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Li, G.J.; Zhao, H. Efficacy and Toxicity of Different Chemotherapy Regimens in the Treatment of Advanced or Metastatic Pancreatic Cancer: A Network Meta-Analysis. J. Cell. Biochem. 2018, 119, 511–523. [Google Scholar] [CrossRef]
- Zhang, S.H.; Liu, G.F.; Li, X.F.; Liu, L.; Yu, S.N. Efficacy of different chemotherapy regimens in treatment of advanced or metastatic pancreatic cancer: A network meta-analysis. J. Cell. Physiol. 2018, 233, 3352–3374. [Google Scholar] [CrossRef]
- Chan, K.; Shah, K.; Lien, K.; Coyle, D.; Lam, H.; Ko, Y.J. A Bayesian meta-analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer. PLoS ONE 2014, 9, e108749. [Google Scholar] [CrossRef]
- Gresham, G.K.; Wells, G.A.; Gill, S.; Cameron, C.; Jonker, D.J. Chemotherapy regimens for advanced pancreatic cancer: A systematic review and network meta-analysis. BMC Cancer 2014, 14, 471. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Uson Junior, P.L.S.; Rother, E.T.; Maluf, F.C.; Bugano, D.D.G. Meta-analysis of Modified FOLFIRINOX Regimens for Patients With Metastatic Pancreatic Cancer. Clin. Colorectal Cancer 2018, 17, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, S.; Voutsadakis, I.A. FOLFIRINOX Chemotherapy in Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Retrospective and Phase II Studies. J. Clin. Med. 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, H.; Simms, L.; Govindan, R. Biostatistics primer: What a clinician ought to know: Hazard ratios. J. Thorac. Oncol. 2011, 6, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, C.; Sturtz, S.; Bender, R. Indirect Comparisons and Network Meta-Analyses. Dtsch. Arztebl. Int. 2015, 112, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Gurrin, L.; Ademi, Z.; Liew, D. Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. Br. J. Clin. Pharmacol. 2014, 77, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 2012, 3, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Donegan, S.; Williamson, P.; D’Alessandro, U.; Tudur Smith, C. Assessing key assumptions of network meta-analysis: A review of methods. Res. Synth. Methods 2013, 4, 291–323. [Google Scholar] [CrossRef]
- White, I.R.; Barrett, J.K.; Jackson, D.; Higgins, J.P. Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res. Synth. Methods 2012, 3, 111–125. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]

| PFS HR (95% CI) # | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| F | 2.32 (1.39 to 3.88) * | 1.09 (0.76 to 1.55) | 1.03 (0.66 to 1.60) | 1.17 (0.79 to 1.76) | 1.27 (0.85 to 1.90) | 1.77 (1.04 to 3.00) * | 1.06 (0.63 to 1.76) | 1.54 (0.98 to 2.45) | 1.10 (0.72 to 1.68) |
| 1.68 (1.20 to 2.35) * | F-Based | 0.47 (0.32 to 0.68) * | 0.44 (0.28 to0.70) * | 0.51 (0.33 to 0.77) * | 0.55 (0.36 to 0.83) * | 0.76 (0.44 to 1.31) | 0.46 (0.27 to 0.77) * | 0.67 (0.41 to 1.07) | 0.47 (0.31 to 0.73) * |
| 0.96 (0.77 to 1.20) | 0.57 (0.44 to 0.73) * | GEM | 0.94 (0.72 to 1.23) | 1.08 (0.89 to 1.30) | 1.17 (0.97 to 1.41) | 1.62 (1.09 to 2.40) * | 0.97 (0.67 to 1.40) | 1.42 (1.06 to 1.90) * | 1.01 (0.80 to 1.27) |
| 0.90 (0.69 to 1.17) | 0.53 (0.40 to 0.71) * | 0.94 (0.81 to 1.08) | GEM + ANGI | 1.14 (0.82 to1.59) | 1.24 (0.89 to 1.72) | 1.72 (1.07 to 2.77) * | 1.03 (0.65 to 1.62) | 1.50 (1.01 to 2.24) * | 1.07 (0.75 to 1.53) |
| 1.03 (0.80 to 1.33) | 0.61 (0.46 to0.81) * | 1.08 (0.95 to 1.22) | 1.15 (0.96 to 1.39) | GEM + EGFRI | 1.08 (0.83 to 1.42) | 1.50 (0.97 to 2.33) | 0.90 (0.59 to 1.36) | 1.32 (0.93 to 1.86) | 0.93 (0.69 to 1.26) |
| 1.13 (0.88 to 1.45) | 0.67 (0.51 to 0.88) * | 1.17 (1.04 to 1.32)* | 1.25 (1.04 to 1.51) * | 1.09 (0.92 to 1.29) | GEM + F | 1.39 (0.89 to 2.15) | 0.83 (0.55 to 1.26) | 1.21 (0.85 to 1.72) | 0.86 (0.64 to 1.16) |
| 1.30 (0.88 to 1.92) | 0.77 (0.51 to 1.16) | 1.36 (0.98 to 1.87) | 1.45 (1.02 to 2.06) * | 1.26 (0.89 to 1.77) | 1.16 (0.82 to 1.63) | GEM + IMT | 0.60 (0.35 to 1.03) | 0.87 (0.54 to 1.43) | 0.62 (0.39 to 0.98) * |
| 1.07 (0.81 to 1.42) | 0.64 (0.47 to 0.86) * | 1.12 (0.95 to 1.33) | 1.20 (0.96 to 1.49) | 1.04 (0.84 to 1.28) | 0.95 (0.78 to 1.17) | 0.83 (0.57 to 1.19) | GEM + PLA | 1.46 (0.91 to 2.34) | 1.04 (0.67 to 1.61) |
| 1.35 (1.01 to 1.80)* | 0.80 (0.59 to 1.10) | 1.41 (1.17 to 1.69) * | 1.50 (1.19 to 1.90) * | 1.31 (1.05 to 1.63) * | 1.20 (0.96 to 1.50) | 1.04 (0.72 to 1.50) | 1.26 (0.98 to 1.61) | GEM + Taxanes | 0.71 (0.49 to 1.03) |
| 0.97 (0.74 to 1.27) | 0.58 (0.43 to 0.78) * | 1.01 (0.87 to 1.18) | 1.08 (0.88 to 1.33) | 0.94 (0.77 to 1.15) | 0.86 (0.71 to 1.05) | 0.75 (0.52 to 1.07) | 0.90 (0.72 to 1.14) | 0.72 (0.57 to 0.92) * | GEM + TKI |
| OS HR (95% CI) # | |||||||||
| F | |||||||||
| 0.42 (0.18 to 0.98) * | F-based | ||||||||
| 1.40 (0.83 to 2.36) | 3.37 (1.71 to 6.67) * | GEM | |||||||
| 1.15 (0.55 to 2.41) | 2.76 (1.17 to 6.52) * | 0.82 (0.48 to 1.38) | GEM + ANGI | ||||||
| 1.24 (0.64 to 2.40) | 2.97 (1.34 to 6.62) * | 0.88 (0.58 to 1.34) | 1.08 (0.55 to 2.11) | GEM + EGFRI | |||||
| 0.79 (0.48 to 1.32) | 1.91 (0.91 to 4.00) | 0.57 (0.42 to 0.75) * | 0.69 (0.38 to 1.26) | 0.64 (0.39 to 1.05) | GEM + F | ||||
| 0.87 (0.27 to 2.77) | 2.09 (0.60 to 7.22) | 0.62 (0.22 to 1.74) | 0.76 (0.24 to 2.42) | 0.70 (0.23 to 2.15) | 1.10 (0.37 to 3.21) | GEM + IMT | |||
| 0.93 (0.47 to 1.86) | 2.25 (0.99 to5.09) | 0.67 (0.42 to1.05) | 0.81 (0.41 to 1.63) | 0.76 (0.41 to 1.40) | 1.18 (0.69 to 2.02) | 1.08 (0.35 to 3.33) | GEM + PLA | ||
| 0.65 (0.32 to 1.30) | 1.55 (0.68 to 3.54) | 0.46 (0.29 to 0.73) * | 0.56 (0.28 to 1.14) | 0.52 (0.28 to 0.98) * | 0.81 (0.47 to 1.41) | 0.74 (0.24 to 2.32) | 0.69 (0.38 to 1.27) | GEM + Taxanes | |
| 0.90 (0.43 to 1.90) | 2.17 (0.92 to 5.15) | 0.64 (0.38 to 1.09) | 0.79 (0.37 to 1.66) | 0.73 (0.37 to 1.44) | 1.14 (0.62 to 2.08) | 1.04 (0.32 to 3.33) | 0.97 (0.48 to 1.94) | 1.40 (0.69 to 2.84) | GEM + TKI |
| ORR RR (95% CI) # | |||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-I.; Yang, J.-L.; Lin, Y.-C.; Chou, C.-Y.; Chen, J.-H.; Hung, C.-C. Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer. Cancers 2019, 11, 1746. https://doi.org/10.3390/cancers11111746
Lin K-I, Yang J-L, Lin Y-C, Chou C-Y, Chen J-H, Hung C-C. Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer. Cancers. 2019; 11(11):1746. https://doi.org/10.3390/cancers11111746
Chicago/Turabian StyleLin, Kun-I, Jia-Lian Yang, Yu-Chao Lin, Che-Yi Chou, Jin-Hua Chen, and Chin-Chuan Hung. 2019. "Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer" Cancers 11, no. 11: 1746. https://doi.org/10.3390/cancers11111746
APA StyleLin, K.-I., Yang, J.-L., Lin, Y.-C., Chou, C.-Y., Chen, J.-H., & Hung, C.-C. (2019). Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer. Cancers, 11(11), 1746. https://doi.org/10.3390/cancers11111746