The Prognostic Role of CD8+ T Lymphocytes in Childhood Adrenocortical Carcinomas Compared to Ki-67, PD-1, PD-L1, and the Weiss Score
Abstract
:1. Introduction
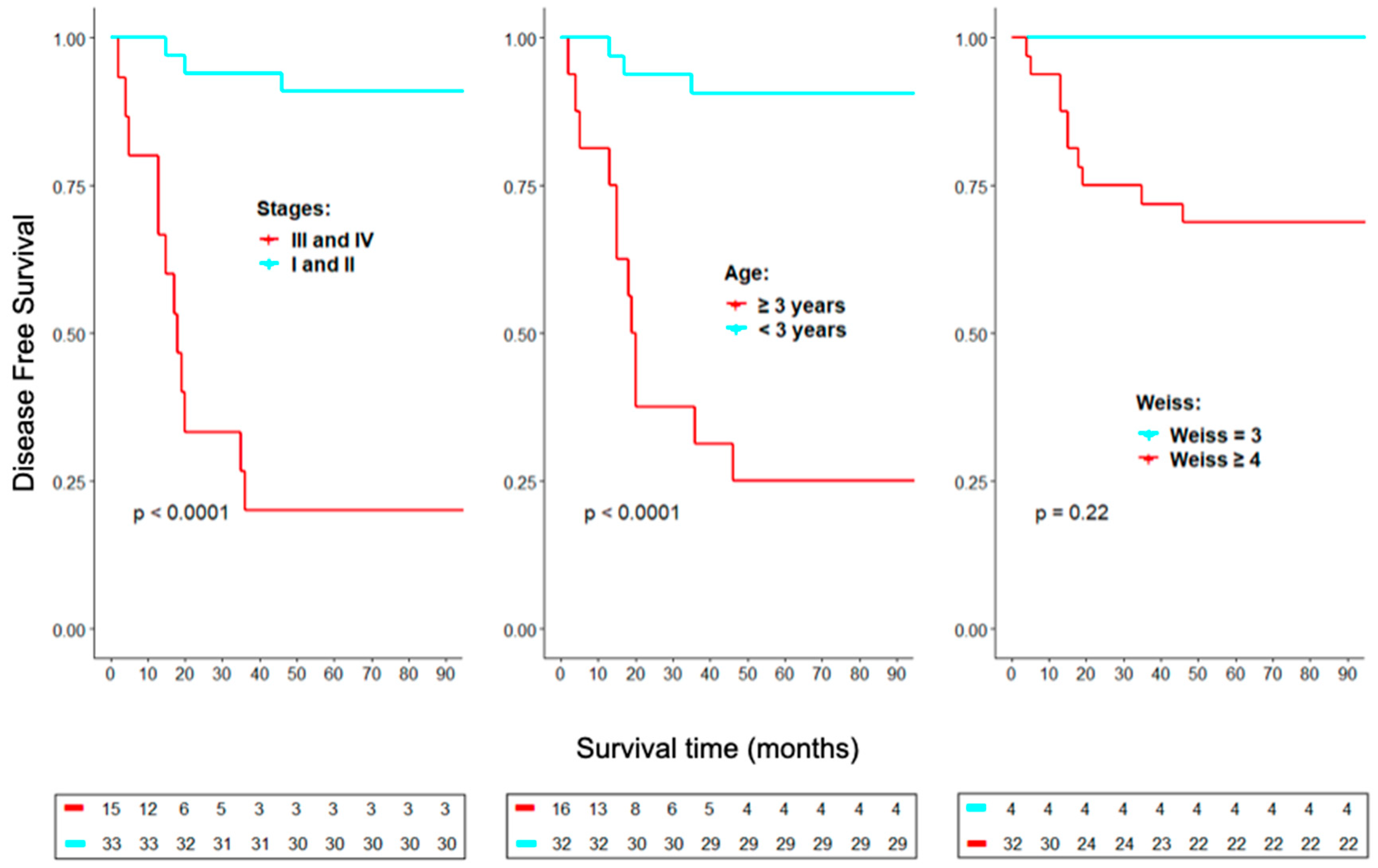
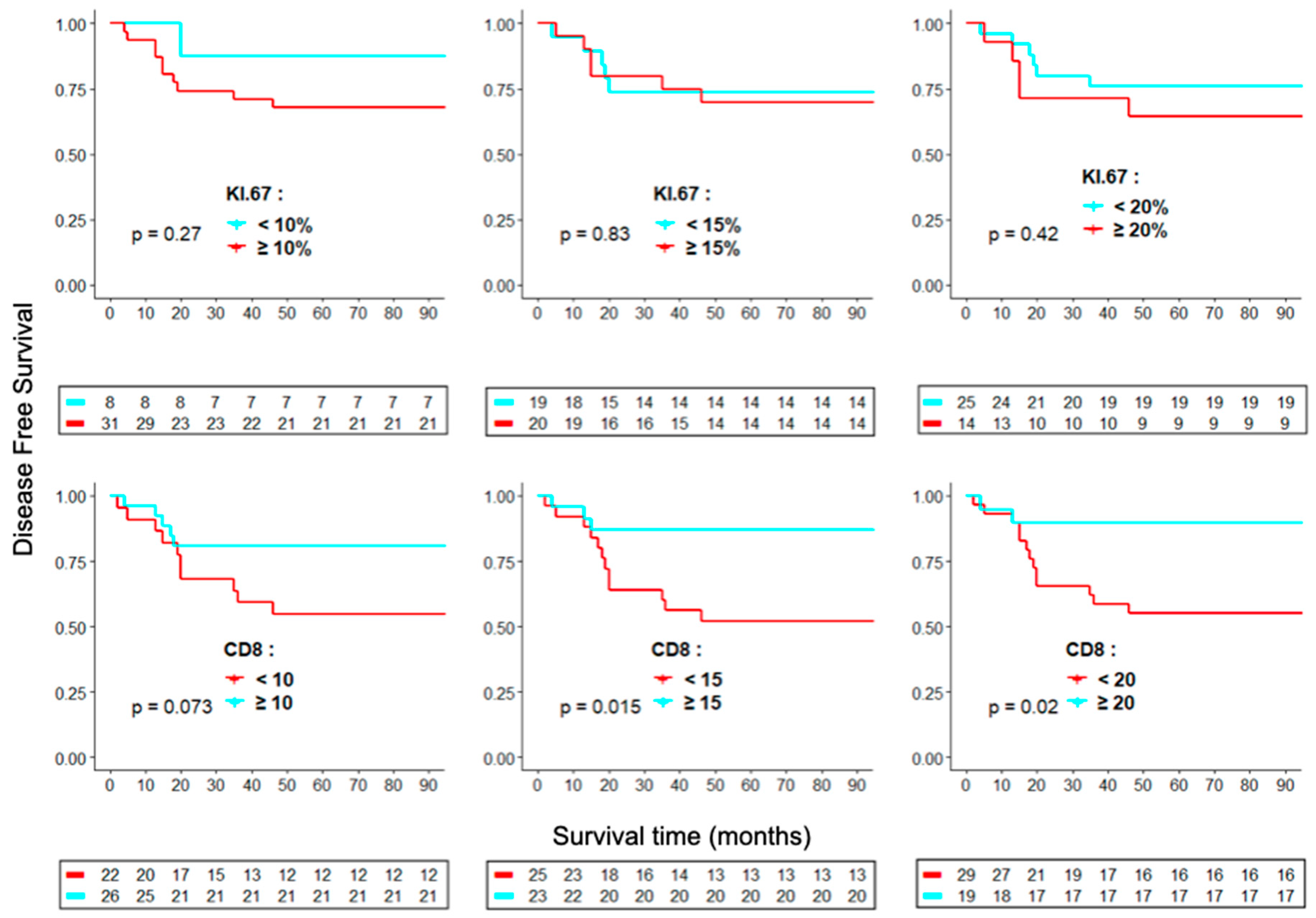
2. Results
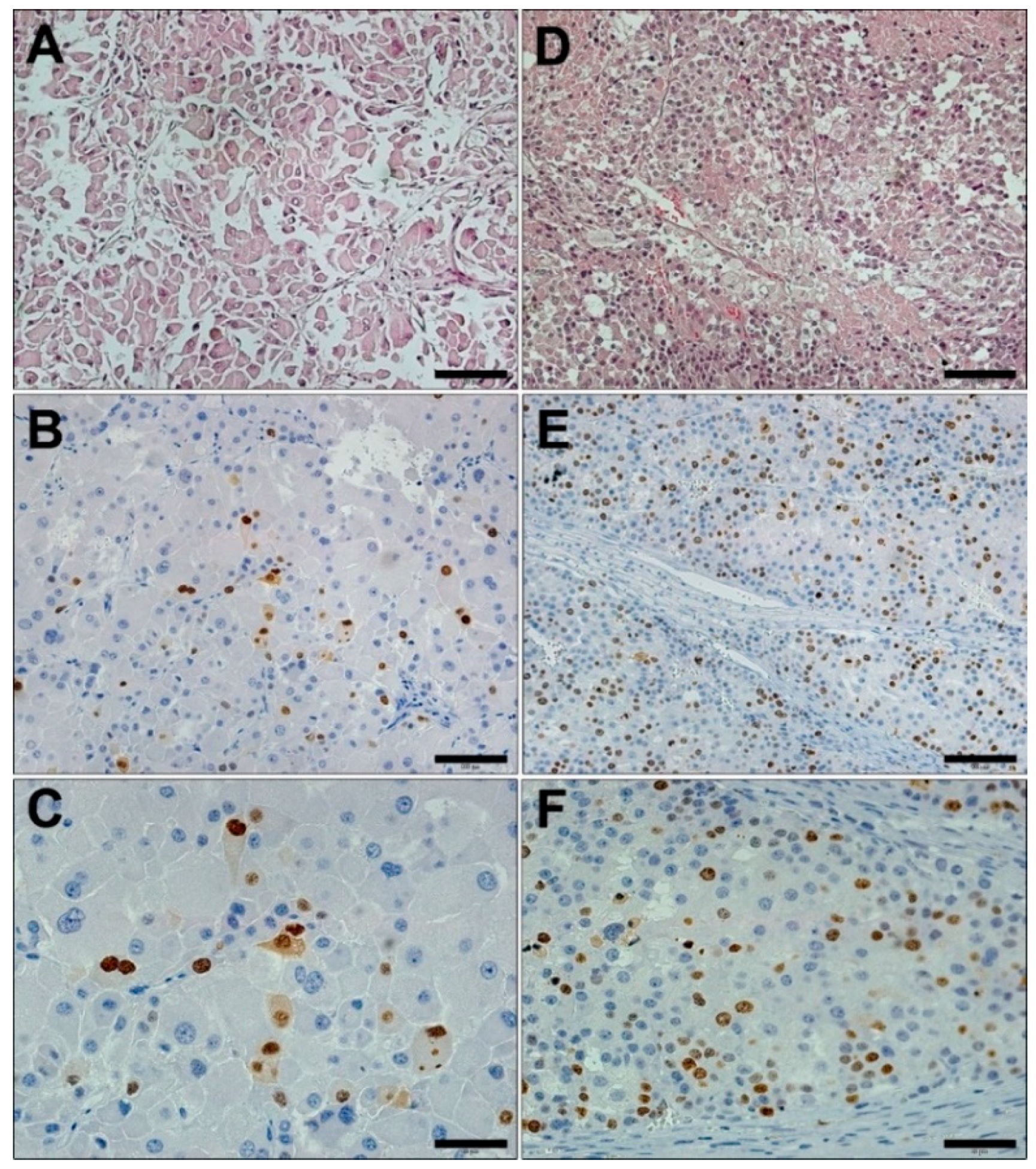
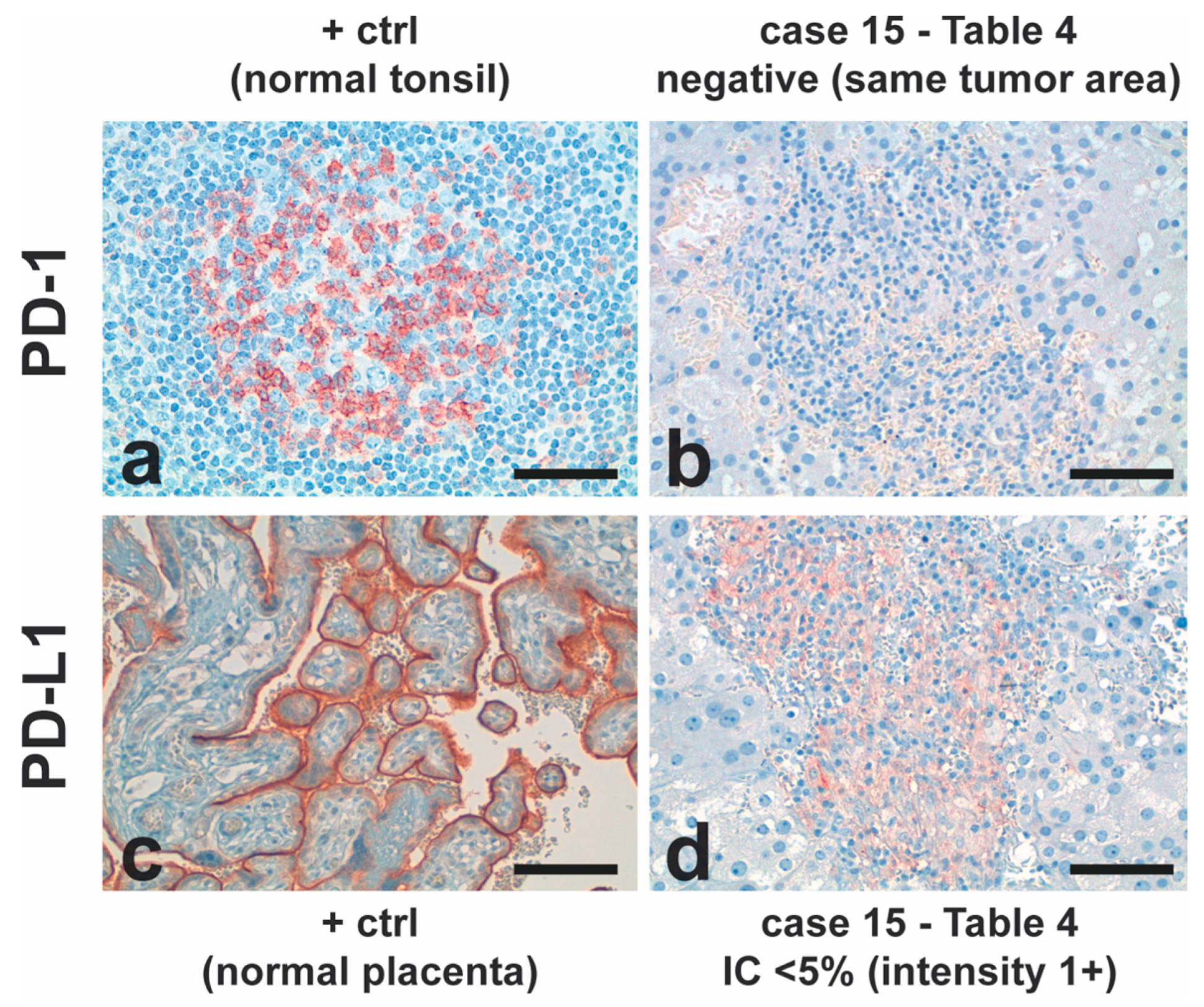
2.1. Histology and Immunostaining of ACC Tumors
Ki-67, CD8 and PD-1/PD-L1 as markers for ACC prognosis
3. Discussion
4. Methods
4.1. Subjects and Samples
4.2. Immunohistochemistry
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribeiro, R.C.; Sandrini, F.; Figueiredo, B.; Zambetti, G.P.; Michalkiewicz, E.; Lafferty, A.R.; DeLacerda, L.; Rabin, M.; Cadwell, C.; Sampaio, G.; et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc. Natl. Acad. Sci. USA 2001, 98, 9330–9335. [Google Scholar] [CrossRef] [PubMed]
- Latronico, A.C.; Pinto, E.M.; Domenice, S.; Fragoso, M.C.; Martin, R.M.; Zerbini, M.C.; Lucon, A.M.; Mendonca, B.B. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 2001, 86, 4970–4973. [Google Scholar] [CrossRef] [PubMed]
- Pianovski, M.A.; Maluf, E.M.; de Carvalho, D.S.; Ribeiro, R.C.; Rodriguez-Galindo, C.; Boffetta, P.; Zancanella, P.; Figueiredo, B.C. Mortality rate of adrenocortical tumors in children under 15 years of age in Curitiba, Brazil. Pediatr. Blood Cancer 2006, 47, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Custodio, G.; Parise, G.A.; Kiesel Filho, N.; Komechen, H.; Sabbaga, C.C.; Rosati, R.; Grisa, L.; Parise, I.Z.; Pianovski, M.A.; Fiori, C.M.; et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J. Clin. Oncol. 2013, 31, 2619–2626. [Google Scholar] [CrossRef]
- Michalkiewicz, E.; Sandrini, R.; Figueiredo, B.; Miranda, E.C.; Caran, E.; Oliveira-Filho, A.G.; Marques, R.; Pianovski, M.A.; Lacerda, L.; Cristofani, L.M.; et al. Clinical and outcome characteristics of children with adrenocortical tumors: A report from the International Pediatric Adrenocortical Tumor Registry. J. Clin. Oncol. 2004, 22, 838–845. [Google Scholar] [CrossRef]
- Dackiw, A.P.; Lee, J.E.; Gagel, R.F.; Evans, D.B. Adrenal cortical carcinoma. World J. Surg. 2001, 25, 914–926. [Google Scholar] [CrossRef]
- Figueiredo, B.C.; Ribeiro, R.C.; Zambetti, G.; Haddad, B.; Pianovsky, M.D.; Pereira, R.M.; DeLacerda, L.; Sandrini, R. Amplification of 9q34 in childhood adrenocortical tumors: A specific feature unrelated to ethnic origin or living conditions. Braz. J. Med. Biol. Res. 2000, 33, 1217–1224. [Google Scholar] [CrossRef]
- Custodio, G.; Komechen, H.; Figueiredo, F.R.; Fachin, N.D.; Pianovski, M.A.; Figueiredo, B.C. Molecular epidemiology of adrenocortical tumors in southern Brazil. Mol. Cell. Endocrinol. 2012, 351, 44–51. [Google Scholar] [CrossRef]
- Lalli, E.; Figueiredo, B.C. Pediatric adrenocortical tumors: What they can tell us on adrenal development and comparison with adult adrenal tumors. Front. Endocrinol. (Lausanne) 2015, 6, e23. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Figueiredo, B.C.; Zambetti, G.P.; Ribeiro, R.C. Biology, clinical characteristics, and management of adrenocortical tumors in children. Pediatr. Blood Cancer 2005, 45, 265–273. [Google Scholar] [CrossRef]
- Berruti, A.; Terzolo, M.; Pia, A.; Angeli, A.; Dogliotti, L. Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. Cancer 1998, 83, 2194–2200. [Google Scholar] [CrossRef]
- Zancanella, P.; Pianovski, M.A.; Oliveira, B.H.; Ferman, S.; Piovezan, G.C.; Lichtvan, L.L.; Voss, S.Z.; Stinghen, S.T.; Callefe, L.G.; Parise, G.A.; et al. Mitotane associated with cisplatin, etoposide, and doxorubicin in advanced childhood adrenocortical carcinoma: Mitotane monitoring and tumor regression. J. Pediatr. Hematol. Oncol. 2006, 28, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galindo, C.; Pappo, A.S.; Krailo, M.D.; Pashankar, F.; Caran, E.M.M.; Hicks, J.; McCarville, M.B.; Weldon, C.B.; Malkin, D.; Zambetti, G.; et al. Treatment of childhood adrenocortical carcinoma (ACC) with surgery plus retroperitoneal lymph node dissection (RPLND) and multiagent chemotherapy: Results of the Children’s Oncology Group ARAR0332 protocol. J. Clin. Oncol. 2016, 34, 10515. [Google Scholar] [CrossRef]
- Pinto, E.M.; Chen, X.; Easton, J.; Finkelstein, D.; Liu, Z.; Pounds, S.; Rodriguez-Galindo, C.; Lund, T.C.; Mardis, E.R.; Wilson, R.K.; et al. Genomic landscape of paediatric adrenocortical tumours. Nat. Commun. 2015, 6, e6302. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, C.L.; Sbiera, S.; Leich, E.; Henzel, K.; Rosenwald, A.; Allolio, B.; Fassnacht, M. Single nucleotide polymorphism array profiling of adrenocortical tumors--evidence for an adenoma carcinoma sequence? PLoS ONE 2013, 8, e73959. [Google Scholar] [CrossRef]
- Dehner, L.P. Pediatric adrenocortical neoplasms: On the road to some clarity. Am. J. Surg. Pathol. 2003, 27, 1005–1007. [Google Scholar] [CrossRef]
- Wieneke, J.A.; Thompson, L.D.; Heffess, C.S. Adrenal cortical neoplasms in the pediatric population: A clinicopathologic and immunophenotypic analysis of 83 patients. Am. J. Surg. Pathol. 2003, 27, 867–881. [Google Scholar] [CrossRef]
- McAteer, J.P.; Huaco, J.A.; Gow, K.W. Predictors of survival in pediatric adrenocortical carcinoma: A Surveillance, Epidemiology, and End Results (SEER) program study. J. Pediatr. Surg. 2013, 48, 1025–1031. [Google Scholar] [CrossRef]
- Morimoto, R.; Satoh, F.; Murakami, O.; Suzuki, T.; Abe, T.; Tanemoto, M.; Abe, M.; Uruno, A.; Ishidoya, S.; Arai, Y.; et al. Immunohistochemistry of a proliferation marker Ki67/MIB1 in adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr. J. 2008, 55, 49–55. [Google Scholar] [CrossRef]
- Duregon, E.; Molinaro, L.; Volante, M.; Ventura, L.; Righi, L.; Bolla, S.; Terzolo, M.; Sapino, A.; Papotti, M.G. Comparative diagnostic and prognostic performances of the hematoxylin-eosin and phospho-histone H3 mitotic count and Ki-67 index in adrenocortical carcinoma. Mod. Pathol. 2014, 27, 1246–1254. [Google Scholar] [CrossRef] [Green Version]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libe, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Rodriguez-Galindo, C.; Pounds, S.B.; Wang, L.; Clay, M.R.; Neale, G.; Garfinkle, E.A.R.; Lam, C.G.; Levy, C.F.; Pappo, A.S.; et al. Identification of Clinical and Biologic Correlates Associated With Outcome in Children With Adrenocortical Tumors Without Germline TP53 Mutations: A St Jude Adrenocortical Tumor Registry and Children’s Oncology Group Study. J. Clin. Oncol. 2017, 35, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Libe, R.; Borget, I.; Ronchi, C.L.; Zaggia, B.; Kroiss, M.; Kerkhofs, T.; Bertherat, J.; Volante, M.; Quinkler, M.; Chabre, O.; et al. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): An European Network for the Study of Adrenal Tumor (ENSAT) study. Ann. Oncol. 2015, 26, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Weiss, L.M. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum. Pathol. 2009, 40, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Pennanen, M.; Heiskanen, I.; Sane, T.; Remes, S.; Mustonen, H.; Haglund, C.; Arola, J. Helsinki score-a novel model for prediction of metastases in adrenocortical carcinomas. Hum. Pathol. 2015, 46, 404–410. [Google Scholar] [CrossRef]
- Duregon, E.; Cappellesso, R.; Maffeis, V.; Zaggia, B.; Ventura, L.; Berruti, A.; Terzolo, M.; Fassina, A.; Volante, M.; Papotti, M. Validation of the prognostic role of the “Helsinki Score” in 225 cases of adrenocortical carcinoma. Hum. Pathol. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Bergada, I.; Venara, M.; Maglio, S.; Ciaccio, M.; Diez, B.; Bergada, C.; Chemes, H. Functional adrenal cortical tumors in pediatric patients: A clinicopathologic and immunohistochemical study of a long term follow-up series. Cancer 1996, 77, 771–777. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Chamuleau, M.E.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. MHC class II molecules in tumour immunology: Prognostic marker and target for immune modulation. Immunobiology 2006, 211, 619–625. [Google Scholar] [CrossRef]
- Campoli, M.; Ferrone, S. HLA antigen and NK cell activating ligand expression in malignant cells: A story of loss or acquisition. Semin. Immunopathol. 2011, 33, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Chamoto, K.; Al-Habsi, M.; Honjo, T. Role of PD-1 in Immunity and Diseases. Curr. Top. Microbiol. Immunol. 2017, 410, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Leahy, D.J.; Axel, R.; Hendrickson, W.A. Crystal structure of a soluble form of the human T cell coreceptor CD8 at 2.6 A resolution. Cell 1992, 68, 1145–1162. [Google Scholar] [CrossRef]
- Okazaki, T.; Honjo, T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007, 19, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [Green Version]
- Swart, M.; Verbrugge, I.; Beltman, J.B. Combination Approaches with Immune-Checkpoint Blockade in Cancer Therapy. Front. Oncol. 2016, 6, e233. [Google Scholar] [CrossRef]
- Ohigashi, Y.; Sho, M.; Yamada, Y.; Tsurui, Y.; Hamada, K.; Ikeda, N.; Mizuno, T.; Yoriki, R.; Kashizuka, H.; Yane, K.; et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 2005, 11, 2947–2953. [Google Scholar] [CrossRef]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012, 2012, e656340. [Google Scholar] [CrossRef]
- Fay, A.P.; Signoretti, S.; Callea, M.; Telomicron, G.H.; McKay, R.R.; Song, J.; Carvo, I.; Lampron, M.E.; Kaymakcalan, M.D.; Poli-de-Figueiredo, C.E.; et al. Programmed death ligand-1 expression in adrenocortical carcinoma: An exploratory biomarker study. J. Immunother. Cancer 2015, 3, e3. [Google Scholar] [CrossRef]
- Tierney, J.F.; Vogle, A.; Poirier, J.; Min, I.M.; Finnerty, B.; Zarnegar, R.; Pappas, S.G.; Scognamiglio, T.; Ghai, R.; Gattuso, P.; et al. Expression of programmed death ligand 1 and 2 in adrenocortical cancer tissues: An exploratory study. Surgery 2019, 165, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Hoimes, C.; Zarwan, C.; Wong, D.J.; Bauer, S.; Claus, R.; Wermke, M.; Hariharan, S.; von Heydebreck, A.; Kasturi, V.; et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: Phase 1b results from the JAVELIN solid tumor trial. J. Immunother. Cancer 2018, 6, e111. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, B.J.; Boon, T. Tumor antigens recognized by T lymphocytes. Int. J. Clin. Lab. Res. 1997, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, A.F.; Dzhandzhugazyan, K.; Zeuthen, J. Melanoma-associated antigens recognized by cytotoxic T lymphocytes. APMIS 1998, 106, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G. MHC class II-restricted tumor antigens recognized by CD4+ T cells: New strategies for cancer vaccine design. J. Immunother. 2001, 24, 195–204. [Google Scholar] [CrossRef]
- Naito, Y.; Saito, K.; Shiiba, K.; Ohuchi, A.; Saigenji, K.; Nagura, H.; Ohtani, H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998, 58, 3491–3494. [Google Scholar]
- Vesalainen, S.; Lipponen, P.; Talja, M.; Syrjanen, K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur. J. Cancer 1994, 30, 1797–1803. [Google Scholar] [CrossRef]
- Setala, L.P.; Kosma, V.M.; Marin, S.; Lipponen, P.K.; Eskelinen, M.J.; Syrjanen, K.J.; Alhava, E.M. Prognostic factors in gastric cancer: The value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br. J. Cancer 1996, 74, 766–772. [Google Scholar] [CrossRef]
- Ishigami, S.; Natsugoe, S.; Tokuda, K.; Nakajo, A.; Higashi, H.; Iwashige, H.; Aridome, K.; Hokita, S.; Aikou, T. CD3-zetachain expression of intratumoral lymphocytes is closely related to survival in gastric carcinoma patients. Cancer 2002, 94, 1437–1442. [Google Scholar] [CrossRef]
- Pisarra, P.; Mortarini, R.; Salvi, S.; Anichini, A.; Parmiani, G.; Sensi, M. High frequency of T cell clonal expansions in primary human melanoma. Involvement of a dominant clonotype in autologous tumor recognition. Cancer Immunol. Immunother. 1999, 48, 39–46. [Google Scholar] [CrossRef]
- Ladanyi, A.; Somlai, B.; Gilde, K.; Fejos, Z.; Gaudi, I.; Timar, J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin. Cancer Res. 2004, 10, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Echchakir, H.; Vergnon, I.; Dorothee, G.; Grunenwald, D.; Chouaib, S.; Mami-Chouaib, F. Evidence for in situ expansion of diverse antitumor-specific cytotoxic T lymphocyte clones in a human large cell carcinoma of the lung. Int. Immunol. 2000, 12, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kondratiev, S.; Sabo, E.; Yakirevich, E.; Lavie, O.; Resnick, M.B. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin. Cancer Res. 2004, 10, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, T.; Siegal, G.P.; Wei, S. The CD4/CD8 ratio of tumor-infiltrating lymphocytes at the tumor-host interface has prognostic value in triple-negative breast cancer. Hum. Pathol. 2017, 69, 110–117. [Google Scholar] [CrossRef]
- Jouinot, A.; Bertherat, J. Management of Endocrine Disease: Adrenocortical carcinoma: Differentiating the good from the poor prognosis tumors. Eur. J. Endocrinol. 2018, 178, 215–230. [Google Scholar] [CrossRef]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 2016, 29, 723–736. [Google Scholar] [CrossRef]
- Weng, N.P.; Araki, Y.; Subedi, K. The molecular basis of the memory T cell response: Differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 2012, 12, 306–315. [Google Scholar] [CrossRef]
- Zlobec, I.; Koelzer, V.H.; Dawson, H.; Perren, A.; Lugli, A. Next-generation tissue microarray (ngTMA) increases the quality of biomarker studies: An example using CD3, CD8, and CD45RO in the tumor microenvironment of six different solid tumor types. J. Transl. Med. 2013, 11, e104. [Google Scholar] [CrossRef]
- Marchetti, A.; Barberis, M.; Franco, R.; De Luca, G.; Pace, M.V.; Staibano, S.; Volante, M.; Buttitta, F.; Guerini-Rocco, E.; Righi, L.; et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2017, 12, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br. J. Cancer 2015, 112, 95–102. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014–2019; Available online: http://www.R-project.org/ (accessed on 9 July 2019).
- Weiss, L.M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 1984, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, C.; Grisanti, S.; Cosentini, D.; Abate, A.; Rossini, E.; Berruti, A.; Sigala, S. Molecular Drivers of Potential Immunotherapy Failure in Adrenocortical Carcinoma. J. Oncol. 2019, 2019, e6072863. [Google Scholar] [CrossRef] [PubMed]
- Boon, T.; Cerottini, J.C.; Van den Eynde, B.; van der Bruggen, P.; Van Pel, A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994, 12, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Old, L.J.; Chen, Y.T. New paths in human cancer serology. J. Exp. Med. 1998, 187, 1163–1167. [Google Scholar] [CrossRef] [PubMed]

| Code | Gender | Age at Diagnosis | Clinical Manif. | Staging | Surgical Ressection | CT/M ** | Recurrence | DFS | Outcome | Weight (g) | Weiss | CD8 Counts (Cells/HPF) | Ki-67 LI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 1y10m | V | 1 | Total | No | No | 3y1m | Well | 77 | 8 | 17,8 | 10 |
| 2 | F | 1y6m | V | 2 | Total | No | No | 8y7m | Well | 127 | 5 | 22,7 | 7 |
| 3 | F | 3y4m | V | 3 | Total | CT/M | Yes | 5m | DD | 125 | 9 | 1,2 | 50 |
| 4 | F | 8y | V | 3 | Total | CT/M | No | 10y3m | Well | 300 | 3 | 22 | 14 |
| 5 | M | 2y4m | V+C | 4 | Total | CT/M | No | 12y6m | Well | 80 | 5 | 8,8 | 14 |
| 6 | M | 9m | NF | 2 | Total | M | No | 6y | Well | 300 | 6 | 18,2 | 15 |
| 7 | F | 8m | V+C | 1 | Total | No | No | 3y | Well | 62 | 7 | 3,2 | 53 |
| 8 | F | 2y4m | V | 2 | Total | M | No | 3y3m | Well | 275 | 5 | 4,2 | 18 |
| 9 | M | 1y1m | V | 1 | Total | No | No | 6y9m | Well | 12 | 5 | 37,8 | 37 |
| 10 | F | 1y9m | V+C | 4 | Total | CT/M | Yes | 1y1m | DD | 392 | 8 | 48,2 | 55 |
| 11 | F | 11m | V+C | 1 | Total | No | No | 3y11m | Well | 33 | 5 | 7,7 | 22 |
| 12 | F | 4y5m | V | 3 | Partial | CT/M | Yes | 1y6m | DD | 440 | 4 | 12,3 | 13 |
| 13 | F | 1y2m | V+C | 2 | Total | No | No | 6y7m | Well | 212.21 | 7 | 9,7 | 18 |
| 14 | M | 10m | V+C | 2 | Total | No | No | 3y | Well | 105 | 4 | 1,6 | 19 |
| 15 | M | 2y11m | V | 2 | Total | M | No | 12y4m | Well | 300 | 5 | 52,9 | 17 |
| 16 | M | 1y | ABM | 2 | Total | No | No | 3y4m | Well | 126 | 5 | 25,6 | 70 |
| 17 | M | 7y2m | No | 2 | Total | CT/M | No | 4y10m | Well | 238 | 6 | 24,9 | 35 |
| 18 | F | 4y5m | V | 2 | Total | CT/M | No | 3y8m | Well | 318 | 8 | 27,9 | 56 |
| 19 | F | 8m | V | 1 | Total | No | No | 3y | Well | 16 | 4 | 24,6 | 48 |
| 20 | F | 6y8m | V | 4 | Total | CT/M | Yes | 1y3m | DD | 342 | 9 | 16 | 70 |
| 21 | F | 10m | V+C | 2 | Total | No | No | 11y1m | Well | 135 | 3 | 3,2 | 2 |
| 22 | F | 1y8m | V+C | 1 | Total | No | No | 6y8m | Well | * | * | 25,3 | 11 |
| 23 | M | 9y9m | V+C | 1 | Total | CT/M | Yes | 1y8m | DD | * | * | 6 | 7 |
| 24 | M | 2y9m | V+H | 4 | Total | CT/M | Yes | 2y11m | DD | 625 | 7 | 0,7 | 15 |
| 25 | M | 8y5m | No | 1 | Total | CT/M | Yes | 1y3m | DD | 22 | 7 | 1,9 | 22 |
| 26 | M | 4y5m | V | 1 | Total | No | No | 25y8m | Well | 15 | 4 | 48,1 | 4 |
| 27 | M | 11m | ABM | 2 | Total | No | * | 11y6m | Well | 320 | 5 | 1 | 3 |
| 28 | F | 2y3m | V | 1 | Total | No | No | 14y8m | Well | 60 | 4 | 17,6 | 1 |
| 29 | F | 5y6m | V | 2 | Total | CT/M | Yes | 3y10m | DD | 690 | 8 | 1,9 | 20 |
| 30 | F | 5days | ABM | 1 | Total | No | No | 15y1m | Well | 10 | 3 | 39,7 | 10 |
| 31 | F | 6m | V | 4 | Total | CT/M | Yes | 11/8m | Well | 50 | 6 | 0,1 | 12 |
| 32 | F | 1y6m | V+H | 1 | Total | No | No | 11y | Well | 20 | * | 5,5 | 4 |
| 33 | F | 2y8m | V | 1 | Total | No | No | 4y7m | Well | 18 | 4 | 66,4 | 8 |
| 34 | M | 1y4m | V | 2 | Total | No | No | 14y4m | Well | 165 | 7 | 36,1 | 25 |
| 35 | F | 7y3m | V+H | 4 | Total | CT/M | No | 4m | DD | 400 | 4 | 34,9 | 10 |
| 36 | F | 5y3m | V | 4 | Total | CT/M | Yes | 1y1m | DD | 250 | 7 | 2,3 | 12 |
| 37 | F | 1y11m | V | 1 | Total | No | No | 8y3m | Well | 18 | 3 | 14,7 | 22 |
| 38 | F | 4y2m | V | 4 | Total | CT/M | Yes | 1y7m | DD | 750 | 6 | 4 | 12 |
| 39 | F | 8m | V | 1 | Total | No | No | 5y8m | Well | 100 | 6 | 4,4 | 12 |
| 40 | F | 2y7m | V | 1 | Total | No | No | 21y | Well | 20 | * | 22 | * |
| 41 | F | 7m | V+C | 1 | Total | M | Yes | 15y3m | Well | 100 | * | 68 | * |
| 42 | M | 2y7m | V | 1 | Total | No | * | 21y | Well | 50 | * | 43 | * |
| 43 | M | 1y10m | V | 2 | Total | No | * | 4y9m | Well | 170 | * | 0 | * |
| 44 | F | 1y3m | ABM | 2 | Total | No | No | 11y9m | Well | 265 | * | 76 | * |
| 45 | M | 3y4m | V | 3 | Total | CT/M | Yes | 2m | DD | 370 | * | 9 | * |
| 46 | M | 3y2m | V+C | 4 | Partial | CT | Yes | 1y8m | DD | * | * | 0 | * |
| 47 | F | 3y6m | ABM | 4 | Total | CT/M | Yes | 3y | DD | 123 | * | 0 | * |
| 48 | M | 2y10m | V+H | 4 | Total | CT/M | Yes | 1y5m | DD | 1050 | * | 10 | * |
| Parameters | Coefficient | Hazard Ratio | Standard Error | z | p Value |
|---|---|---|---|---|---|
| Stages 3 and 4 | 2.149 | 8.578 | 0.704 | 3.055 | 0.002 |
| Age ≥ 3 years | 1.746 | 5.729 | 0.698 | 2.5 | 0.012 |
| Stages 3 and 4 | 2.658 | 14.26 | 0.86 | 3.091 | 0.002 |
| Age ≥ 3 years | 1.914 | 6.78 | 0.818 | 2.34 | 0.019 |
| Ki-67 ≥ 20% | 1.286 | 3.618 | 0.75 | 1.714 | 0.086 |
| Age ≥ 3 years | 2.647 | 14.11 | 0.787 | 3.362 | <0.001 |
| Ki-67 ≥ 15% | 0.223 | 1.25 | 0.609 | 0.367 | 0.714 |
| Age ≥ 3 years | 2.635 | 13.94 | 0.798 | 3.303 | <0.001 |
| Ki-67 ≥ 20% | 0.028 | 1.028 | 0.616 | 0.046 | 0.964 |
| Age ≥ 3 years | 2.552 | 12.84 | 0.659 | 3.872 | <0.001 |
| CD8 < 15 cells/HPF | 1.377 | 3.963 | 0.657 | 2.096 | 0.036 |
| Age ≥ 3 years | 2.661 | 14.31 | 0.665 | 4.005 | <0.001 |
| CD8 < 20 cells/HPF | 1.705 | 5.503 | 0.775 | 2.201 | 0.028 |
| Parameters | Subroup A (Stages 1+2 Well) n (%) | Subgroup B (All Stages DD and 3+4 Alive) n (%) | p-Value |
|---|---|---|---|
| Staging | <0.001 | ||
| 1 | 16 (88.9) | 2 (11.1) | |
| 2 | 14 (93.3) | 1 (6.7) | |
| 3 | 0 (0) | 4 (100) | |
| 4 | 0 (0) | 11 (100) | |
| CD8+-CTL | 0.052 | ||
| <10 | 10 (45.5) | 12 (54.5) | |
| ≥10 | 20 (76.9) | 6 (23.1) | |
| - | 0.008 | ||
| <15 | 11 (44) | 14 (56) | |
| ≥15 | 19 (82.6) | 4 (17.4) | |
| - | 0.016 | ||
| <20 | 14 (48.3) | 15 (51.7) | |
| ≥20 | 16 (84.2) | 3 (15.8) | |
| Ki-67 | 0.218 | ||
| <10% | 7 (87.5) | 1 (12.5) | |
| ≥10% | 18 (58.1) | 13 (41.9) | |
| - | 0.65 | ||
| <15% | 11 (57.9) | 8 (42.1) | |
| ≥15% | 14 (70) | 6 (30) | |
| - | 1 | ||
| <20% | 16 (64) | 9 (36) | |
| ≥20% | 9 (64.3) | 5 (35.7) | |
| Weiss_Score | 1 | ||
| =3 | 3 (75) | 1 (25) | |
| >3 | 20 (62.5) | 12 (37.5) | |
| Age | <0.001 | ||
| ≥3 years | 3 (18.8) | 13 (81.2) | |
| <3 years | 27 (84.4) | 5 (15.6) | |
| Outcome | <0.001 | ||
| DD | 0 (0) | 15 (100) | |
| Well | 30 (90.9) | 3 (9.1) |
| Code | Age of Diagnosis | Clinical Manifestation | Stage | PD-L1 TC | PD-L1 IC | PD-1 |
|---|---|---|---|---|---|---|
| 1 | 1y10m | V | 1 | 0 | <5% (1+) | 0 |
| 2 | 1y6m | V | 1 | 0 | 0 | 0 |
| 3 | 3y4m | V | 3 | 0 | 0 | 0 |
| 4 | 8y | V | 3 | 0 | 0 | 0 |
| 5 | 2y4m | V+C | 4 | 0 | 0 | 0 |
| 6 | 9m | NF | 2 | 0 | 0 | 0 |
| 7 | 8m | V+C | 1 | 0 | 0 | 0 |
| 8 | 2y4m | V | 2 | 0 | 0 | 0 |
| 9 | 1y1m | V | 1 | 0 | 0 | 0 |
| 10 | 1y9m | V+C | 4 | 0 | 0 | 0 |
| 15 | 2y11m | V | 2 | 0 | <5% (1+) | 0 |
| 16 | 1y | ABM | 2 | 0 | <5% (1+) | 0 |
| 17 | 7y2m | No | 2 | 0 | <5% (1+) | 0 |
| 19 | 8m | V | 1 | 0 | 0 | 0 |
| 20 | 6y8m | V | 4 | 0 | 0 | 0 |
| 26 | 4y5m | V | 2 | 0 | 0 | 0 |
| a | 4y5m | V | 3 | 0 | 0 | 0 |
| b | 1m22days | No | 1 | 0 | 0 | 0 |
| c | 11m | V | 2 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parise, I.Z.S.; Parise, G.A.; Noronha, L.; Surakhy, M.; Woiski, T.D.; Silva, D.B.; Costa, T.E.-J.B.; Del-Valle, M.H.C.P.; Komechen, H.; Rosati, R.; et al. The Prognostic Role of CD8+ T Lymphocytes in Childhood Adrenocortical Carcinomas Compared to Ki-67, PD-1, PD-L1, and the Weiss Score. Cancers 2019, 11, 1730. https://doi.org/10.3390/cancers11111730
Parise IZS, Parise GA, Noronha L, Surakhy M, Woiski TD, Silva DB, Costa TE-JB, Del-Valle MHCP, Komechen H, Rosati R, et al. The Prognostic Role of CD8+ T Lymphocytes in Childhood Adrenocortical Carcinomas Compared to Ki-67, PD-1, PD-L1, and the Weiss Score. Cancers. 2019; 11(11):1730. https://doi.org/10.3390/cancers11111730
Chicago/Turabian StyleParise, Ivy Zortéa S., Guilherme A. Parise, Lúcia Noronha, Mirvat Surakhy, Thiago Demetrius Woiski, Denise B. Silva, Tatiana EI-Jaick B. Costa, Maria Helena C. P. Del-Valle, Heloisa Komechen, Roberto Rosati, and et al. 2019. "The Prognostic Role of CD8+ T Lymphocytes in Childhood Adrenocortical Carcinomas Compared to Ki-67, PD-1, PD-L1, and the Weiss Score" Cancers 11, no. 11: 1730. https://doi.org/10.3390/cancers11111730
APA StyleParise, I. Z. S., Parise, G. A., Noronha, L., Surakhy, M., Woiski, T. D., Silva, D. B., Costa, T. E.-J. B., Del-Valle, M. H. C. P., Komechen, H., Rosati, R., Ribeiro, M. G., Nascimento, M. L., Souza, J. A. d., Andrade, D. P., Paraizo, M. M., Galvão, M. M. R., Barbosa, J. R. S., Barbosa, M. L., Custódio, G. C., ... Figueiredo, B. C. (2019). The Prognostic Role of CD8+ T Lymphocytes in Childhood Adrenocortical Carcinomas Compared to Ki-67, PD-1, PD-L1, and the Weiss Score. Cancers, 11(11), 1730. https://doi.org/10.3390/cancers11111730






