Progression-Free but No Overall Survival Benefit for Adult Patients with Bevacizumab Therapy for the Treatment of Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Systematic Literature Review
2.2. Data Extraction for Meta-Analysis
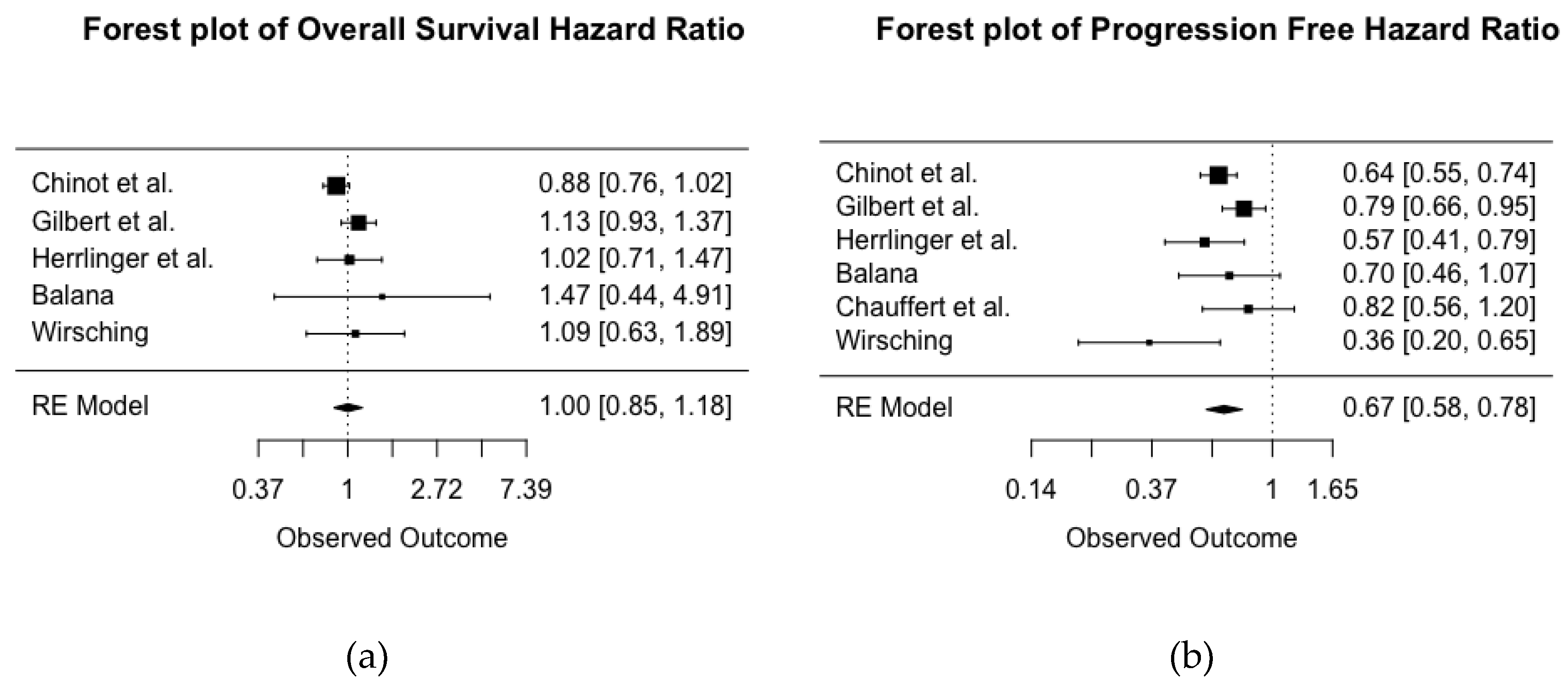
2.3. Meta-Analysis of Hazard Ratios
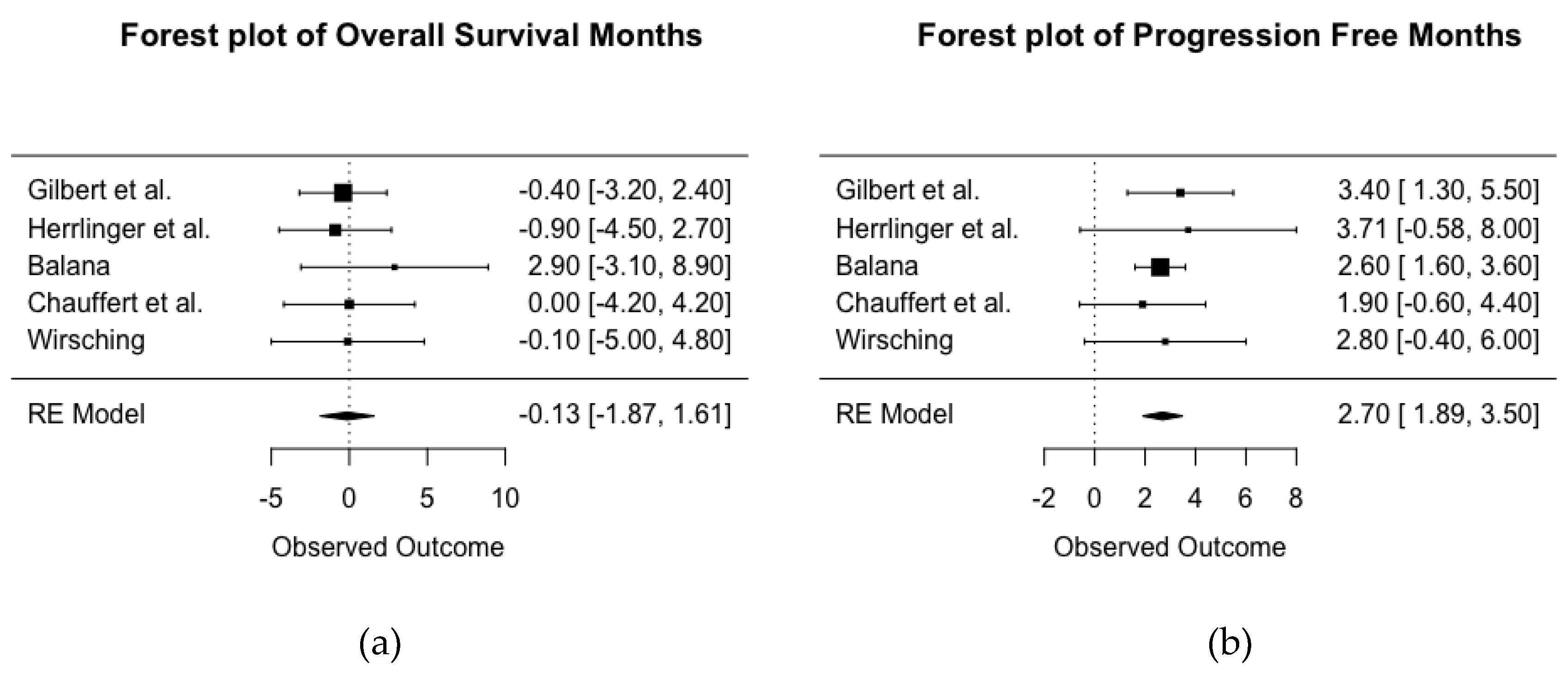
2.4. Meta-Analysis of Months
3. Discussion
4. Materials and Methods
4.1. Systematic Literature Review
4.2. Data Extraction
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. New Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Sadeghi, N.; Camby, I.; Metens, T.; Dewitte, O.; Kiss, R. Present and potential future issues in glioblastoma treatment. Expert Rev. Anticancer Ther. 2006, 6, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., 2nd; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef]
- Norden, A.D.; Young, G.S.; Setayesh, K.; Muzikansky, A.; Klufas, R.; Ross, G.L.; Ciampa, A.S.; Ebbeling, L.G.; Levy, B.; Drappatz, J.; et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology 2008, 70, 779–787. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. New Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. New Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Khasraw, M.; Ameratunga, M.S.; Grant, R.; Wheeler, H.; Pavlakis, N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst. Rev. 2014, CD008218. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA; IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Balana, C.; De Las Penas, R.; Sepulveda, J.M.; Gil-Gil, M.J.; Luque, R.; Gallego, O.; Carrato, C.; Sanz, C.; Reynes, G.; Herrero, A.; et al. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma: The GENOM 009 randomized phase II trial. J. Neurooncol. 2016, 127, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A.; Reddy, K.; Gaspar, L.E.; Ney, D.; Kavanagh, B.D.; Damek, D.; Lillehei, K.; Chen, C. Hypofractionated-intensity modulated radiotherapy (hypo-IMRT) and temozolomide (TMZ) with or without bevacizumab (BEV) for newly diagnosed glioblastoma multiforme (GBM): A comparison of two prospective phase II trials. J. Neuro Oncol. 2015, 123, 251–257. [Google Scholar] [CrossRef]
- Chauffert, B.; Feuvret, L.; Bonnetain, F.; Taillandier, L.; Frappaz, D.; Taillia, H.; Schott, R.; Honnorat, J.; Fabbro, M.; Tennevet, I.; et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: Final results of the TEMAVIR study from ANOCEFdagger. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1442–1447. [Google Scholar] [CrossRef]
- Herrlinger, U.; Schafer, N.; Steinbach, J.P.; Weyerbrock, A.; Hau, P.; Goldbrunner, R.; Friedrich, F.; Rohde, V.; Ringel, F.; Schlegel, U.; et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed o6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: The randomized GLARIUS trial. J. Clin. Oncol. 2016, 34, 1611–1619. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Tabatabai, G.; Roelcke, U.; Hottinger, A.F.; Jörger, F.; Schmid, A.; Plasswilm, L.; Schrimpf, D.; Mancao, C.; Capper, D.; et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: The randomized, open-label, phase II ARTE trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1423–1430. [Google Scholar] [CrossRef]
- Narayana, A.; Kelly, P.; Golfinos, J.; Parker, E.; Johnson, G.; Knopp, E.; Zagzag, D.; Fischer, I.; Raza, S.; Medabalmi, P.; et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: Impact on local control and patient survival. J. Neurosurg. 2009, 110, 173–180. [Google Scholar] [CrossRef]
- Vahedi, K.; Hofmeijer, J.; Juettler, E.; Vicaut, E.; George, B.; Algra, A.; Amelink, G.J.; Schmiedeck, P.; Schwab, S.; Rothwell, P.M.; et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007, 6, 215–222. [Google Scholar] [CrossRef]
- Fleming, T.R.; Rothmann, M.D.; Lu, H.L. Issues in using progression-free survival when evaluating oncology products. J. Clin. Oncol. 2009, 27, 2874–2880. [Google Scholar] [CrossRef]
- Pope, W.B.; Lai, A.; Nghiemphu, P.; Mischel, P.; Cloughesy, T.F. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 2006, 66, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Frenkel, E.P.; Neuwelt, E.A. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology 2011, 76, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.Q.; Beal, K.; Goenka, A.; Karimi, S.; Iwamoto, F.M.; Yamada, Y.; Zhang, Z.; Lassman, A.B.; Abrey, L.E.; Gutin, P.H. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.; Buter, J.; Honkoop, A.H.; Boerman, D.; de Vos, F.Y.; et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef]
- Prados, M.; Cloughesy, T.; Samant, M.; Fang, L.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.A.; Paleologos, N.; et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- Sandmann, T.; Bourgon, R.; Garcia, J.; Li, C.; Cloughesy, T.; Chinot, O.L.; Wick, W.; Nishikawa, R.; Mason, W.; Henriksson, R.; et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: Retrospective analysis of the avaglio trial. J. Clin. Oncol. 2015, 33, 2735–2744. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef]
- Lambrechts, D.; Lenz, H.J.; de Haas, S.; Carmeliet, P.; Scherer, S.J. Markers of response for the antiangiogenic agent bevacizumab. J. Clin. Oncol. 2013, 31, 1219–1230. [Google Scholar] [CrossRef]
- Adilijiang, A.; Hirano, M.; Okuno, Y.; Aoki, K.; Ohka, F.; Maeda, S.; Tanahashi, K.; Motomura, K.; Shimizu, H.; Yamaguchi, J.; et al. Next generation sequencing-based transcriptome predicts bevacizumab efficacy in combination with temozolomide in glioblastoma. Molecules 2019, 24, 3046. [Google Scholar] [CrossRef]
- Gerstner, E.; Emblem, K.E.; Chang, K.; Vakulenko-Lagun, B.; Yen, Y.F.; Beers, A.L.; Dietrich, J.; Plotkin, S.R.; Catana, C.; Hooker, J.M.; et al. Bevacizumab reduces permeability and concurrent temozolomide delivery in a subset of patients with recurrent glioblastoma. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Smith, D.M.; Team, R.D.C. An Introduction to R: A Programming Environment for Data Analysis and Graphics; Network Theory: Bristol, UK, 2009. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 48. [Google Scholar] [CrossRef]

| First Author (Year) | Level of Study | n | Median Age (Years) | Duration of Follow-up (Months) | Treatment | Control | Prior Treatment | |
|---|---|---|---|---|---|---|---|---|
| 1 | Balana [14] (2016) | I | Tx: 49 Control: 53 | Tx: 62.9 Control: 62.0 | >18.0 | BEV + TMZ | TMZ | Biopsy only |
| 2 | Carlson [15] (2015) | I | Tx: 30 Control: 26 | Tx: 56.5 Control: 60.5 | Tx: 14.7 Control: 13.9 | BEV + Hypo-IMRT + TMZ | Hypo-IMRT + TMZ | Biopsy/Sx |
| 3 | Chauffert [16] (2014) | I | Tx: 60 Control: 60 | Tx: 60.2 Control: 60.9 | – | BEV + IRI + RT + TMZ | RT + TMZ | Biopsy only |
| 4 | Chinot [9] (2014) | I | Tx: 458 Control: 463 | Tx: 57.0 Control: 56.0 | Tx: 14.4 Control: 13.7 | BEV + RT + TMZ | PLA + RT + TMZ | Biopsy/Sx |
| 5 | Gilbert [10] (2014) | I | Tx: 312 Control: 309 | Tx: 59.0 Control: 57.0 | 20.5 | BEV + RT + TMZ | PLA + RT + TMZ | Biopsy/Sx |
| 6 | Herrlinger [17] (2016) | I | Tx: 116 Control: 54 | Tx and Control: 56.0 | – | BEV + IRI + RT | RT + TMZ | Biopsy/Sx |
| 7 | Wirsching [18] (2018) | I | Tx: 50 Control: 25 | Tx: 70 Control: 70 | – | BEV + Hypo-RT | Hypo-RT | Sx, steroids |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaka, N.; Hafazalla, K.; Samawi, H.; Simpkin, A.; Perry, J.; Sahgal, A.; Das, S. Progression-Free but No Overall Survival Benefit for Adult Patients with Bevacizumab Therapy for the Treatment of Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1723. https://doi.org/10.3390/cancers11111723
Kaka N, Hafazalla K, Samawi H, Simpkin A, Perry J, Sahgal A, Das S. Progression-Free but No Overall Survival Benefit for Adult Patients with Bevacizumab Therapy for the Treatment of Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis. Cancers. 2019; 11(11):1723. https://doi.org/10.3390/cancers11111723
Chicago/Turabian StyleKaka, Nagham, Karim Hafazalla, Haider Samawi, Andrew Simpkin, James Perry, Arjun Sahgal, and Sunit Das. 2019. "Progression-Free but No Overall Survival Benefit for Adult Patients with Bevacizumab Therapy for the Treatment of Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis" Cancers 11, no. 11: 1723. https://doi.org/10.3390/cancers11111723
APA StyleKaka, N., Hafazalla, K., Samawi, H., Simpkin, A., Perry, J., Sahgal, A., & Das, S. (2019). Progression-Free but No Overall Survival Benefit for Adult Patients with Bevacizumab Therapy for the Treatment of Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis. Cancers, 11(11), 1723. https://doi.org/10.3390/cancers11111723





