Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics
Abstract
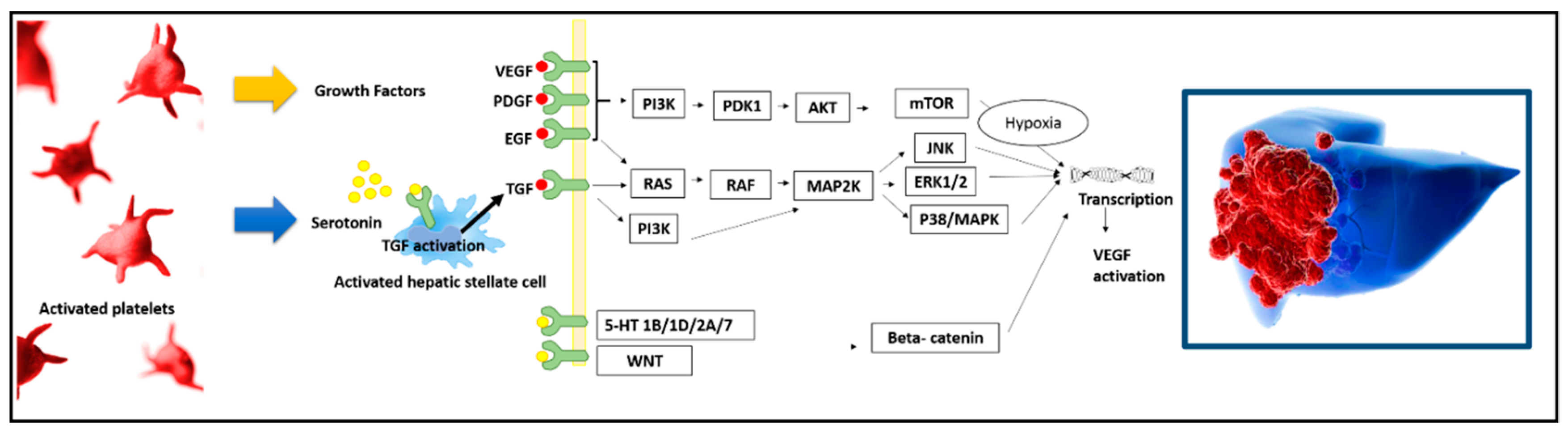
1. The Role of Platelets in the In Vitro/In Vivo HCC Oncogenic Process
2. The Specific Role of Platelet-Derived Growth Factor
3. The Specific Role of Intra-Platelet Serotonin
4. The Connection between Platelets and Epidermal Growth Factor
5. The Connection between Platelets and Vascular Endothelial Growth Factor
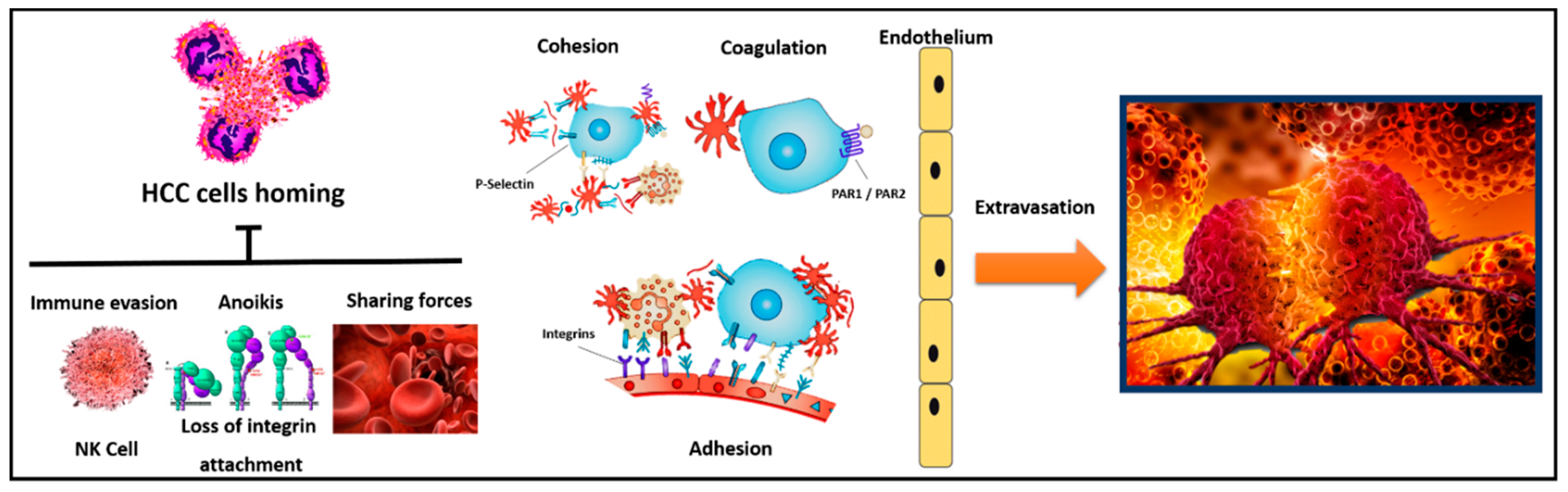
6. The Role of Platelets in HCC Cells Homing
7. The Specific Role of Platelets in the Diagnosis and Prognosis of HCC
8. Platelet-to-Lymphocytes Ratio as Prognostic Index for HCC
9. Prognostic Role of Platelets in Patients with HCC Candidate to Liver Resection
10. Prognostic Role of Platelets in Patients with HCC Candidate to Liver Transplant and in Liver-Transplant Recipients
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| 95%CI | 95% confidence intervals |
| AFP | alpha-fetoprotein |
| APRI | AST-to-platelet ratio index |
| EGF | epidermal growth factor |
| HCC | hepatocellular carcinoma |
| HR | hazard ratio |
| NASH | non-alcoholic steatohepatitis |
| OR | odds ratio |
| PDGF | platelet-derived growth factor |
| PLR | platelet-to-lymphocyte ratio |
| SSRIs | selective serotonin reuptake inhibitors |
| VEGF | vascular endothelial growth factor |
| TACE | trans-arterial chemo-embolization |
References
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; LaValle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Feys, H.B.; De Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Lievens, D.; von Hundelshausen, P. Platelets in atherosclerosis. Thromb. Haemost. 2011, 106, 827–838. [Google Scholar] [PubMed]
- Watson, S.P.; Lowe, K.; Finney, B.A. Platelets in lymph vessel development and integrity. Adv. Anat. Embryol. Cell Biol. 2014, 214, 93–105. [Google Scholar]
- Walsh, T.G.; Metharom, P.; Berndt, M.C. The functional role of platelets in the regulation of angiogenesis. Platelets 2015, 26, 199–211. [Google Scholar] [CrossRef]
- Ikushima, S.; Ono, R.; Fukuda, K.; Sakayori, M.; Awano, N.; Kondo, K. Trousseau’s syndrome: Cancer-associated thrombosis. Jpn. J. Clin. Oncol. 2016, 46, 204–208. [Google Scholar] [CrossRef]
- Lin, R.J.; Afshar-Kharghan, V.; Schafer, A.I. Paraneoplastic thrombocytosis: The secrets of tumor self-promotion. Blood 2014, 124, 184–187. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, C.; Huang, B.; Zhou, F.L.; Huang, H.D.; Li, Q. Correlation between bone metastasis and thrombocytosis in pulmonary adenocarcinoma patients. Oncol. Lett. 2015, 9, 762–768. [Google Scholar] [CrossRef][Green Version]
- Long, Y.; Wang, T.; Gao, Q.; Zhou, C. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: A meta-analysis. Oncotarget 2016, 7, 81849–81861. [Google Scholar] [CrossRef] [PubMed]
- Menczer, J. Preoperative elevated platelet count and thrombocytosis in gynecologic malignancies. Arch. Gynecol. Obstet. 2017, 295, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Pang, Z.; Shen, H.; Ni, Y.; Du, J.; Liu, Q. The Prognostic Value of PLR in Lung Cancer, a Meta-analysis Based on Results from a Large Consecutive Cohort. Sci. Rep. 2016, 6, 34823. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Lam, M.; Menter, D.G.; Kopetz, S. Platelets, circulating tumor cells, and the circulome. Cancer Metastasis Rev. 2017, 36, 235–248. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Sierko, E.; Hempel, D.; Tucker, S.C.; Honn, K.V. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017, 36, 249–262. [Google Scholar] [CrossRef]
- Moeini, A.; Cornellà, H.; Villanueva, A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer 2012, 1, 83–93. [Google Scholar] [CrossRef]
- Höpfner, M.; Schuppan, D.; Scherübl, H. Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J. Gastroenterol. 2008, 14, 1–14. [Google Scholar] [CrossRef]
- Stock, P.; Monga, D.; Tan, X.; Micsenyi, A.; Loizos, N.; Monga, S.P. Platelet-derived growth factor receptor-alpha: A novel therapeutic target in human hepatocellular cancer. Mol. Cancer Ther. 2007, 6, 1932–1941. [Google Scholar] [CrossRef]
- Lau, C.K.; Yang, Z.F.; Ho, D.W.; Ng, M.N.; Yeoh, G.C.; Poon, R.T.; Fan, S.T. An Akt/hypoxia-inducible factor-1alpha/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin. Cancer Res. 2009, 15, 3462–3471. [Google Scholar] [CrossRef]
- Okada, H.; Honda, M.; Campbell, J.S.; Sakai, Y.; Yamashita, T.; Takebuchi, Y.; Hada, K.; Shirasaki, T.; Takabatake, R.; Nakamura, M.; et al. Acyclic Retinoid Targets Platelet-Derived Growth Factor Signaling in the Prevention of Hepatic Fibrosis and Hepatocellular Carcinoma Development. Cancer Res. 2012, 72, 4459–4471. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, R.; Yang, Q.; Hou, X.; Chen, S.; Hou, Y.; Chen, C.; Yang, Y.; Miele, L.; Sarkar, F.H.; et al. Chemoresistance to gemcitabine in hepatoma cells induces epithelial-mesenchymal transition and involves activation of PDGF-D pathway. Oncotarget 2013, 4, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Yamashita, T.; Oishi, N.; Nio, K.; Hayashi, T.; Nomura, Y.; Yoshida, M.; Hayashi, T.; Hashiba, T.; Asahina, Y.; et al. TSU-68 ameliorates hepatocellular carcinoma growth by inhibiting microenvironmental platelet-derived growth factor signaling. Anticancer Res. 2015, 35, 1423–1431. [Google Scholar] [PubMed]
- Wang, R.; Li, Y.; Hou, Y.; Yang, Q.; Chen, S.; Wang, X.; Wang, Z.; Yang, Y.; Chen, C.; Wang, Z.; et al. The PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget 2015, 6, 7000–7010. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lin, N.; Chen, Z.; Xu, R. Hypoxia-induced secretion of platelet-derived growth factor-BB by hepatocellular carcinoma cells increases activated hepatic stellate cell proliferation, migration and expression of vascular endothelial growth factor-A. Mol. Med. Rep. 2015, 11, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Cho, E.J.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; Kim, C.Y.; Yoon, J.-H. Hypoxia Enhances Tumor-Stroma Crosstalk that Drives the Progression of Hepatocellular Carcinoma. Dig. Dis. Sci. 2016, 61, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Han, C.-C.; Li, Y.; Wang, Y.; Wei, W. Insulin-like growth factor-binding protein-3 inhibits IGF-1-induced proliferation of human hepatocellular carcinoma cells by controlling bFGF and PDGF autocrine/paracrine loops. Biochem. Biophys. Res. Commun. 2016, 478, 964–969. [Google Scholar] [CrossRef]
- Lv, X.; Fang, C.; Yin, R.; Qiao, B.; Shang, R.; Wang, J.; Song, W.; He, Y.; Chen, Y. Agrin para-secreted by PDGF-activated human hepatic stellate cells promotes hepatocarcinogenesis in vitro and in vivo. Oncotarget 2017, 8, 105340–105355. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Ding, H. XPD suppresses cell proliferation and migration via miR-29a-3p-Mdm2/PDGF-B axis in HCC. Cell Biosci. 2019, 9, 6. [Google Scholar] [CrossRef]
- Campbell, J.S.; Johnson, M.M.; Bauer, R.L.; Hudkins, K.L.; Gilbertson, D.G.; Riehle, K.J.; Yeh, M.M.; Alpers, C.E.; Fausto, N. Targeting stromal cells for the treatment of platelet-derived growth factor C-induced hepatocellular carcinogenesis. Differentiation 2007, 75, 843–852. [Google Scholar] [CrossRef]
- Maass, T.; Thieringer, F.R.; Mann, A.; Longerich, T.; Schirmacher, P.; Strand, D.; Hansen, T.; Galle, P.R.; Teufel, A.; Kanzler, S. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. Int. J. Cancer 2011, 128, 1259–1268. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Sun, H.-C.; Jia, W.-D.; Zhuang, P.-Y.; Qian, Y.-B.; Zhu, X.-D.; Kong, L.-Q.; Wang, L.; Wu, W.-Z.; Tang, Z.-Y. Up-regulation of platelet-derived growth factor-A is responsible for the failure of re-initiated interferon alpha treatment in hepatocellular carcinoma. BMC Cancer 2012, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.H.; Johnson, M.M.; Shimizu-Albergine, M.; Bauer, R.L.; Hayes, B.J.; Surapisitchat, J.; Hudkins, K.L.; Riehle, K.J.; Johnson, S.C.; Yeh, M.M.; et al. Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: Evidence for stromal induction of hepatocellular carcinoma. Int. J. Cancer 2014, 15, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Mas, V.R.; Maluf, D.G.; Archer, K.J.; Yanek, K.C.; Fisher, R.A. Angiogenesis Soluble Factors as Hepatocellular Carcinoma Noninvasive Markers for Monitoring Hepatitis C Virus Cirrhotic Patients Awaiting Liver Transplantation. Transplantation 2007, 84, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Pan, H.-B.; Tseng, H.-H.; Hung, Y.-T.; Huang, J.-S.; Chou, C.-P. Assessment of Blood Flow in Hepatocellular Carcinoma: Correlations of Computed Tomography Perfusion Imaging and Circulating Angiogenic Factors. Int. J. Mol. Sci. 2013, 14, 17536–17552. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, L.-N.; Lv, Y.; Ma, X.-Y.; Zhi, L.; Liu, C.; Ma, F.; Zhang, X.-F. Overexpression of platelet-derived growth factor receptor alpha promotes tumor progression and indicates poor prognosis in hepatocellular carcinoma. Oncotarget 2014, 5, 10307–10317. [Google Scholar] [CrossRef]
- Talaat, R.M.; Salem, T.A.; El-Masry, S.; Imbarek, A.; Mokhles, M.; Abdel-Aziz, A.; El-Masry, S.; Abdel-Aziz, A. Circulating pro- and anti-angiogenic mediators in patients infected with hepatitis C at different stages of hepatocellular carcinoma. J. Med. Virol. 2014, 86, 1120–1129. [Google Scholar] [CrossRef]
- Alkozai, E.M.; Porte, R.J.; Adelmeijer, J.; Zanetto, A.; Simioni, P.; Senzolo, M.; Lisman, T. Levels of angiogenic proteins in plasma and platelets are not different between patients with hepatitis B/C-related cirrhosis and patients with cirrhosis and hepatocellular carcinoma. Platelets 2015, 26, 577–582. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Andreoli, J.M.; Hickey, R.; Kallini, J.R.; Gabr, A.; Baker, T.; Kircher, S.; Salem, R.; Kulik, L. Angiogenic Response following Radioembolization: Results from a Randomized Pilot Study of Yttrium-90 with or without Sorafenib. J. Vasc. Interv. Radiol. 2016, 27, 1329–1336. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamashita, T.; Terashima, T.; Suda, T.; Okada, H.; Asahina, Y.; Hayashi, T.; Hara, Y.; Nio, K.; Sunagozaka, H.; et al. Serum cytokine profiles predict survival benefits in patients with advanced hepatocellular carcinoma treated with sorafenib: A retrospective cohort study. BMC Cancer 2017, 17, 870. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Wang, X.; Shen, Q.; Li, C.; Dai, C. Co-expression of PDGF-B and VEGFR-3 strongly correlates with poor prognosis in hepatocellular carcinoma patients after hepatectomy. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 126–133. [Google Scholar] [CrossRef]
- Aryal, B.; Yamakuchi, M.; Shimizu, T.; Kadono, J.; Furoi, A.; Gejima, K.; Komokata, T.; Koriyama, C.; Hashiguchi, T.; Imoto, Y. Predictive Value of Diminished Serum PDGF-BB after Curative Resection of Hepatocellular Cancer. J. Oncol. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Yamakuchi, M.; Shimizu, T.; Kadono, J.; Furoi, A.; Gejima, K.; Komokata, T.; Hashiguchi, T.; Imoto, Y. Deciphering Platelet Kinetics in Diagnostic and Prognostic Evaluation of Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Shi, X.; Lin, Z.; Chen, G.Q.; Pan, X.H.; Wu, J.C.; Ho, J.W.; Lee, N.P.; Gao, H.; Zhang, G.; et al. 5-Hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing β-catenin. Mol. Oncol. 2016, 10, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razik, A.; Elhelaly, R.; Elzehery, R.; El-Diasty, A.; Abed, S.; Elhammady, D.; Tawfik, A. Could serotonin be a potential marker for hepatocellular carcinoma? A prospective single-center observational study. Eur. J. Gastroenterol. Hepatol. 2016, 28, 599–605. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.; Shehata, D.E.; Abdel-Ghany, A.A.; Ragaa, A.; Wahid, A. Serum serotonin as unexpected potential marker for staging of experimental hepatocellular carcinoma. Biomed. Pharmacother. 2016, 83, 407–411. [Google Scholar] [CrossRef]
- Aryal, B.; Shimizu, T.; Kadono, J.; Furoi, A.; Komokata, T.; Kitazono, I.; Koriyama, C.; Yamakuchi, M.; Hashiguchi, T.; Imoto, Y. Post-Resection Exhaustion of Intra-Platelet Serotonin: Also an Indicator of Early Hepatocellular Carcinoma Recurrence? J. Cancer 2017, 8, 3984–3991. [Google Scholar] [CrossRef]
- Chan, H.L.; Chiu, W.C.; Chen, V.C.; Huang, K.Y.; Wang, T.N.; Lee, Y.; McIntyre, R.S.; Hsu, T.C.; Lee, C.T.; Tzang, B.S. SSRIs associated with decreased risk of hepatocellular carcinoma: A population-based case-control study. Psychooncology 2018, 27, 187–192. [Google Scholar] [CrossRef]
- Chang, C.-M.; Hsieh, M.-S.; Yang, T.-C.; Hsieh, V.C.-R.; Chiang, J.-H.; Huang, H.-H.; How, C.-K.; Hu, S.-Y.; Yen, D.H.-T. Selective serotonin reuptake inhibitors and the risk of hepatocellular carcinoma in hepatitis B virus-infected patients. Cancer Manag. Res. 2017, 9, 709–720. [Google Scholar] [CrossRef]
- Liu, S.; Miao, R.; Zhai, M.; Pang, Q.; Deng, Y.; Liu, S.; Qu, K.; Liu, C.; Zhang, J. Effects and related mechanisms of serotonin on malignant biological behavior of hepatocellular carcinoma via regulation of Yap. Oncotarget 2017, 8, 47412–47424. [Google Scholar] [CrossRef]
- Yang, Q.; Yan, C.; Yin, C.; Gong, Z. Serotonin Activated Hepatic Stellate Cells Contribute to Sex Disparity in Hepatocellular Carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 484–499. [Google Scholar] [CrossRef]
- Zuo, X.; Chen, Z.; Cai, J.; Gao, W.; Zhang, Y.; Han, G.; Pu, L.; Wu, Z.; You, W.; Qin, J.; et al. 5-Hydroxytryptamine Receptor 1D Aggravates Hepatocellular Carcinoma Progression Through FoxO6 in AKT-Dependent and Independent Manners. Hepatology 2019, 69, 2031–2047. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, X.; Wang, L.; Zhu, B.; Wang, X.; Xia, J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J. Cell Mol. Med. 2014, 18, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Hoshida, Y.; Fujii, T.; Wei, L.; Yamada, S.; Lauwers, G.Y.; McGinn, C.M.; Deperalta, D.K.; Chen, X.; Kuroda, T.; et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 2014, 59, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, R.; Refolo, M.G.; Lippolis, C.; Giannuzzi, G.; Carella, N.; Messa, C.; Cavallini, A.; I Carr, B. Antagonism of Sorafenib and Regorafenib actions by platelet factors in hepatocellular carcinoma cell lines. BMC Cancer 2014, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-G.; Hammam, O.; Moussa, M.; Gabal, S.; Said, N.; El-Hindawi, A.; El-Hindawi, A. Impact of epidermal growth factor receptor and transforming growth factor-α on hepatitis C virus-induced hepatocarcinogenesis. APMIS 2015, 123, 823–831. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, I.K.; Park, K.H.; Yoon, S.Y.; Oh, S.C.; Seo, J.H.; Choi, C.W.; Kim, B.S.; Shin, S.W.; Kim, Y.H.; et al. Serum vascular endothelial growth factor per platelet count in hepatocellular carcinoma: Correlations with clinical parameters and survival. Jpn. J. Clin. Oncol. 2004, 34, 184–190. [Google Scholar] [CrossRef]
- Hu, J.; Xu, Y.; Shen, Z.-Z.; Wang, Z.; Lu, Q.; Yang, G.-H.; Ding, Z.-B.; Fan, J.; Zhou, J. High expressions of vascular endothelial growth factor and platelet-derived endothelial cell growth factor predict poor prognosis in alpha-fetoprotein-negative hepatocellular carcinoma patients after curative resection. J. Cancer Res. Clin. Oncol. 2009, 135, 1359–1367. [Google Scholar] [CrossRef]
- Corradini, S.G.; Morini, S.; Liguori, F.; Carotti, S.; Muda, A.O.; Burza, M.A.; Siciliano, M.; Molinaro, A.; Cantafora, A.; Blotta, I.; et al. Differential vascular endothelial growth factor A protein expression between small hepatocellular carcinoma and cirrhosis correlates with serum vascular endothelial growth factor A and alpha-fetoprotein. Liver Int. 2009, 29, 103–112. [Google Scholar] [CrossRef]
- Ferroni, P.; Spila, A.; D’Alessandro, R.; Martini, F.; Iacovone, F.; Ettorre, G.M.; Vennarecci, G.; Santoro, R.; Puoti, C.; Guadagni, F. Platelet activation and vascular endothelial growth factor 165 release in hepatocellular cancer. Clin. Chim. Acta 2011, 412, 450–454. [Google Scholar] [CrossRef]
- Guo, J.-H.; Zhu, X.; Li, X.-T.; Yang, R.-J. Impact of serum vascular endothelial growth factor on prognosis in patients with unresectable hepatocellular carcinoma after transarterial chemoembolization. Chin. J. Cancer Res. 2012, 24, 36–43. [Google Scholar] [CrossRef]
- Zhan, P.; Qian, Q.; Yu, L.-K. Serum VEGF level is associated with the outcome of patients with hepatocellular carcinoma: A meta-analysis. HepatoBiliary Surg. Nutr. 2013, 2, 209–215. [Google Scholar] [PubMed]
- Suh, Y.-G.; Lee, E.-J.; Cha, H.; Yang, S.-H.; Seong, J. Prognostic Values of Vascular Endothelial Growth Factor and Matrix Metalloproteinase-2 in Hepatocellular Carcinoma after Radiotherapy. Dig. Dis. 2014, 32, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Li, X.; Qin, C.; Li, J. Prognostic Value of VEGF in Hepatocellular Carcinoma Patients Treated with Sorafenib: A Meta-Analysis. Med. Sci. Monit. 2015, 21, 3144–3151. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Shimizu, T.; Kadono, J.; Furoi, A.; Komokata, T.; Inoue, M.; Ikeda, S.; Fukukura, Y.; Nakamura, M.; Yamakuchi, M.; et al. A Switch in the Dynamics of Intra-Platelet VEGF-A from Cancer to the Later Phase of Liver Regeneration after Partial Hepatectomy in Humans. PLoS ONE 2016, 11, e0150446. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Li, N. Platelets in cancer metastasis: To help the “villain” to do evil. Int. J. Cancer 2016, 138, 2078–2087. [Google Scholar] [CrossRef]
- Naderi-Meshkin, H.; Ahmadiankia, N. Cancer metastasis versus stem cell homing: Role of platelets. J. Cell. Physiol. 2018, 233, 9167–9178. [Google Scholar] [CrossRef]
- Läubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Degen, J.L. Mechanisms linking tumor cell-associated procoagulant function to tumor metastasis. Thromb. Res. 2007, 120, S22–S28. [Google Scholar] [CrossRef]
- Dong, Y.; Xie, X.; Wang, Z.; Hu, C.; Zheng, Q.; Wang, Y.; Chen, R.; Xue, T.; Chen, J.; Gao, D.; et al. Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin β1. Biochem. Biophys. Res. Commun. 2014, 444, 427–432. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Nouso, K.; Wada, N.; Takeuchi, Y.; Kinugasa, H.; Miyahara, K.; Yasunaka, T.; Kuwaki, K.; Onishi, H.; Ikeda, F.; et al. Involvement of platelets in extrahepatic metastasis of hepatocellular carcinoma. Hepatol. Res. 2014, 44, E353–E359. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lin, Y.-J.; Lin, C.-C.; Yen, C.-L.; Shen, C.-H.; Chang, C.-J.; Hsieh, S.-Y. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015, 35, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Savarino, V. Thrombocytopenia in liver disease. Curr. Opin. Hematol. 2008, 15, 473–480. [Google Scholar] [CrossRef]
- Giannini, E.G.; Zaman, A.; Ceppa, P.; Mastracci, L.; Risso, D.; Testa, R. A simple approach to noninvasively identifying significant fibrosis in chronic hepatitis C patients in clinical practice. J. Clin. Gastroenterol. 2006, 40, 521–527. [Google Scholar] [CrossRef]
- Burton, J.R., Jr.; Liangpunsakul, S.; Lapidus, J.; Giannini, E.; Chalasani, N.; Zaman, A. Validation of a multivariate model predicting presence and size of varices. J. Clin. Gastroenterol. 2007, 41, 609–615. [Google Scholar] [CrossRef]
- Giannini, E.G.; Moscatelli, A.; Brunacci, M.; Zentilin, P.; Savarino, V.; Information, P.E.K.F.C. Prognostic role of mean platelet volume in patients with cirrhosis. Dig. Liver Dis. 2016, 48, 409–413. [Google Scholar] [CrossRef]
- Lu, S.-N.; Wang, J.-H.; Liu, S.-L.; Hung, C.-H.; Chen, C.-H.; Tung, H.-D.; Chen, T.-M.; Huang, W.-S.; Lee, C.-M.; Chen, C.-C.; et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer 2006, 107, 2212–2222. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, C.F.; Chang, K.C.; Hung, S.F.; Wang, J.H.; Hung, C.H.; Chen, C.H.; Tseng, P.L.; Kee, K.M.; Yen, Y.H.; et al. Community-based screening for hepatocellular carcinoma in elderly residents in a hepatitis B- and C-endemic area. J. Gastroenterol. Hepatol. 2011, 26, 129–134. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F.; Davila, J.A.; Kramer, J.; Richardson, P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 2014, 146, 1249–1255. [Google Scholar] [CrossRef]
- Nathan, H.; Herlong, H.F.; Gurakar, A.; Li, Z.; Koteish, A.A.; Bridges, J.F.; Pawlik, T.M. Clinical Decision-Making by Gastroenterologists and Hepatologists for Patients with Early Hepatocellular Carcinoma. Ann. Surg. Oncol. 2014, 21, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Peck-Radosavljevic, M.; Giannini, E.G.; Vibert, E.; Sieghart, W.; Van Poucke, S.; Pawlik, T.M. Personalized treatment of patients with very early hepatocellular carcinoma. J. Hepatol. 2017, 66, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Qu, K.; Zhang, J.Y.; Song, S.D.; Liu, S.S.; Tai, M.H.; Liu, H.C.; Liu, C. The prognostic value of platelet count in patients with hepatocellular carcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 2015, 94, e1431. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Qu, K.; Bi, J.-B.; Liu, S.-S.; Zhang, J.-Y.; Song, S.-D.; Lin, T.; Xu, X.-S.; Wan, Y.; Tai, M.-H.; et al. Thrombocytopenia for prediction of hepatocellular carcinoma recurrence: Systematic review and meta-analysis. World J. Gastroenterol. 2015, 21, 7895–7906. [Google Scholar] [CrossRef]
- Fattovich, G.; Pantalena, M.; Zagni, I.; Realdi, G.; Schalm, S.W.; Christensen, E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: A cohort study of 297 patients. Am. J. Gastroenterol. 2002, 97, 2886–2895. [Google Scholar] [CrossRef]
- Qamar, A.A.; Grace, N.D.; Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Burroughs, A.K.; Ripoll, C.; Maurer, R.; Planas, R.; Escorsell, A.; et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin. Gastroenterol. Hepatol. 2009, 7, 689–695. [Google Scholar] [CrossRef]
- Carr, B.I.; Guerra, V. Thrombocytosis and hepatocellular carcinoma. Dig. Dis. Sci. 2013, 58, 1790–1796. [Google Scholar] [CrossRef]
- Carr, B.I.; Guerra, V.; Giannini, E.G.; Farinati, F.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Zoli, M.; Borzio, F.; et al. Significance of platelet and AFP levels and liver function parameters for HCC size and survival. Int. J. Biol. Mark. 2014, 29, e215–e223. [Google Scholar] [CrossRef]
- Carr, B.I.; Pancoska, P.; Giannini, E.G.; Farinati, F.; Ciccarese, F.; Rapaccini, G.L.; Marco, M.D.; Benvegnù, L.; Zoli, M.; Borzio, F.; et al. Identification of two clinical hepatocellular carcinoma patient phenotypes from results of standard screening parameters. Semin. Oncol. 2014, 41, 406–414. [Google Scholar] [CrossRef]
- Akkiz, H.; Carr, B.I.; Yalçın, K.K.; Guerra, V.; Kuran, S.; Altıntaş, E.; Üsküdar, O.; Karaoğullarından, Ü.; Özakyol, A.; Tokmak, S.; et al. Characteristics of Hepatocellular Carcinoma Aggressiveness Factors in Turkish Patients. Oncology 2018, 94, 116–124. [Google Scholar] [CrossRef]
- I Carr, B.; Cavallini, A.; D’Alessandro, R.; Refolo, M.G.; Lippolis, C.; Mazzocca, A.; Messa, C. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and cancer: What is the link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, N.; Chang, Q.; Meng, X.; Wang, W. The role of IDO, IL-10, and TGF-β in the HCV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J. Med. Virol. 2019, 91, 265–271. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, X.; Sun, R.; Wei, H.; Tian, Z. Natural Killer Cell–Derived Interferon-Gamma Promotes Hepatocellular Carcinoma Through the Epithelial Cell Adhesion Molecule–Epithelial-to-Mesenchymal Transition Axis in Hepatitis B Virus Transgenic Mice. Hepatology 2019, 69, 1735–1750. [Google Scholar] [CrossRef]
- Benfeitas, R.; Bidkhori, G.; Mukhopadhyay, B.; Klevstig, M.; Arif, M.; Zhang, C.; Lee, S.; Cinar, R.; Nielsen, J.; Uhlen, M.; et al. Characterization of heterogeneous redox responses in hepatocellular carcinoma patients using network analysis. EBioMedicine 2019, 40, 471–487. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019, 39, 26–42. [Google Scholar] [CrossRef]
- Siu, E.H.-L.; Chan, A.W.-H.; Chong, C.C.-N.; Chan, S.L.; Lo, K.-W.; Cheung, S.T. Treatment of advanced hepatocellular carcinoma: Immunotherapy from checkpoint blockade to potential of cellular treatment. Transl. Gastroenterol. Hepatol. 2018, 3, 89. [Google Scholar] [CrossRef]
- Busato, D.; Mossenta, M.; Baboci, L.; Di Cintio, F.; Toffoli, G.; Bo, M.D. Novel immunotherapeutic approaches for hepatocellular carcinoma treatment. Expert Rev. Clin. Pharmacol. 2019, 12, 453–470. [Google Scholar] [CrossRef]
- Li, G.; Ni, A.; Yu, M. Pretumor microenvironment of hepatocellular carcinoma: Cancerization or anticancerization? Gene 2019, 701, 46–54. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Miele, L.; Oben, J.; Grieco, A.; Vinciguerra, M. Biology, Epidemiology, Clinical Aspects of Hepatocellular Carcinoma and the Role of Sorafenib. Curr. Drug Targets 2016, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, H.C.; Lindholm, P.F. Fibrin and Fibrinolysis in Cancer. Semin. Thromb. Hemost. 2019, 45, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Mezouar, S.; Frere, C.; Darbousset, R.; Mege, D.; Crescence, L.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb. Res. 2016, 139, 65–76. [Google Scholar] [CrossRef]
- Huszno, J.; Kolosza, Z.; Mrochem-Kwarciak, J.; Rutkowski, T.; Skladowski, K. The Role of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Platelets in the Prognosis of Metastatic Renal Cell Carcinoma. Oncology 2019, 97, 7–17. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, Y.; Zhang, R.; Dong, X.; Shen, S.; Zhao, Y.; Bai, J.; Albanes, D.; Caporaso, N.E.; Landi, M.T.; et al. Elevated Platelet Count Appears to Be Causally Associated with Increased Risk of Lung Cancer: A Mendelian Randomization Analysis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 935–942. [Google Scholar] [CrossRef]
- Adhyatma, K.P.; Warli, S.M. Diagnostic Value of Platelet-To-Lymphocyte Ratio in Prostate Cancer. Open Access Maced. J. Med. Sci. 2019, 7, 1093–1096. [Google Scholar] [CrossRef]
- Suner, A.; Carr, B.I.; Akkiz, H.; Karakülah, G.; Üsküdar, O.; Yalçın, K.; Kuran, S.; Tokat, Y.; Yilmaz, S.; Özakyol, A.; et al. C-Reactive Protein and Platelet-Lymphocyte Ratio as Potential Tumor Markers in Low-Alpha-Fetoprotein Hepatocellular Carcinoma. Oncology 2019, 96, 25–32. [Google Scholar] [CrossRef]
- Suner, A.; Carr, B.I.; Akkiz, H.; Uskudar, O.; Kuran, S.; Tokat, Y.; Tokmak, S.; Ballı, T.; Ulku, A.; AkCam, T.; et al. Inflammatory markers C-reactive protein and PLR in relation to HCC characteristics. J. Transl. Sci. 2019, 5. [Google Scholar] [CrossRef]
- Lin, W.-F.; Zhong, M.-F.; Zhang, Y.-R.; Wang, H.; Zhao, H.-T.; Cheng, B.-B.; Ling, C.-Q. Prognostic Role of Platelet-to-Lymphocyte Ratio in Hepatocellular Carcinoma with Different BCLC Stages: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Lai, Q.; Castro Santa, E.; Rico Juri, J.M.; Pinheiro, R.S.; Lerut, J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl. Int. 2014, 27, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Melandro, F.; Laureiro, Z.L.; Giovanardi, F.; Corradini, S.G.; Ferri, F.; Hassan, R.; Rossi, M.; Mennini, G. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: A systematic review and meta-analysis. World J. Gastroenterol. 2018, 24, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Tejima, K.; Masuzaki, R.; Ikeda, H.; Yoshida, H.; Tateishi, R.; Sugioka, Y.; Kume, Y.; Okano, T.; Iwai, T.; Gotoh, H.; et al. Thrombocytopenia is more severe in patients with advanced chronic hepatitis C than B with the same grade of liver stiffness and splenomegaly. J. Gastroenterol. 2010, 45, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Capussotti, L.; Ferrero, A.; Viganò, L.; Muratore, A.; Polastri, R.; Bouzari, H. Portal Hypertension: Contraindication to Liver Surgery? World J. Surg. 2006, 30, 992–999. [Google Scholar] [CrossRef]
- Cucchetti, A.; Ercolani, G.; Vivarelli, M.; Cescon, M.; Ravaioli, M.; Ramacciato, G.; Grazi, G.L.; Pinna, A.D. Is Portal Hypertension a Contraindication to Hepatic Resection? Ann. Surg. 2009, 250, 922–928. [Google Scholar] [CrossRef]
- Ishizawa, T.; Hasegawa, K.; Aoki, T.; Takahashi, M.; Inoue, Y.; Sano, K.; Imamura, H.; Sugawara, Y.; Kokudo, N.; Makuuchi, M. Neither Multiple Tumors Nor Portal Hypertension Are Surgical Contraindications for Hepatocellular Carcinoma. Gastroenterology 2008, 134, 1908–1916. [Google Scholar] [CrossRef]
- Giannini, E.G.; Savarino, V.; Farinati, F.; Ciccarese, F.; Rapaccini, G.; Marco, M.D.; Benvegnù, L.; Zoli, M.; Borzio, F.; Caturelli, E.; et al. Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int. 2013, 33, 1594–1600. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Xia, Y.; Yang, T.; Gao, Y.; Li, J.; Wu, Y.; Shen, F. Impact of clinically significant portal hypertension on outcomes after partial hepatectomy for hepatocellular carcinoma: A systematic review and meta-analysis. HPB 2019, 21, 1–13. [Google Scholar] [CrossRef]
- Roayaie, S.; Obeidat, K.; Sposito, C.; Mariani, L.; Bhoori, S.; Pellegrinelli, A.; Labow, D.; Llovet, J.M.; Schwartz, M.; Mazzaferro, V.M. Resection of hepatocellular cancer ≤2 cm: Results from two Western centers. Hepatology 2013, 57, 1426–1435. [Google Scholar] [CrossRef]
- Giannini, E.G.; Savarino, V. Platelet count and survival of patients with compensated cirrhosis and small hepatocellular carcinoma treated with surgery. Hepatology 2014, 59, 1649. [Google Scholar] [CrossRef]
- Padickakudy, R.; Pereyra, D.; Offensperger, F.; Jonas, P.; Oehlberger, L.; Schwarz, C.; Haegele, S.; Assinger, A.; Brostjan, C.; Gruenberger, T.; et al. Bivalent role of intra-platelet serotonin in liver regeneration and tumor recurrence in humans. J. Hepatol. 2017, 67, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Wang, W.; Hua, Y.; Liu, L.; Shen, S.; Peng, B. Thrombocytopenia and the outcomes of hepatectomy for hepatocellular carcinoma: A meta-analysis. J. Surg. Res. 2017, 210, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Jun, M.-J.; Han, S.; Lee, Y.-J.; Lee, S.-G.; Kim, K.M.; Lim, Y.-S.; Lee, H.C. Prognostic Nomograms for Prediction of Recurrence and Survival After Curative Liver Resection for Hepatocellular Carcinoma. Ann. Surg. 2015, 261, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Zhang, J.-Y.; Xu, X.-S.; Song, S.-D.; Qu, K.; Chen, W.; Zhou, Y.-Y.; Miao, R.-C.; Liu, S.-S.; Dong, Y.-F.; et al. Significance of platelet count and platelet-based models for hepatocellular carcinoma recurrence. World J. Gastroenterol. 2015, 21, 5607–5621. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cai, J.; Li, H.; Zeng, K.; He, L.; Fu, H.; Zhang, J.; Chen, L.; Yao, J.; Zhang, Y.; et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: A Meta-Analysis and Systematic Review. Cell. Physiol. Biochem. 2017, 44, 967–981. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, G.; Zhu, F.; Hui, J.; Cai, S.; Huang, C.; Cheng, S.; Fathy, A.H.; Xiang, Y.; Li, J. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 22854–22862. [Google Scholar] [CrossRef]
- Qin, W.; Wang, L.; Hu, B.; Leng, S.; Tian, H.; Luo, H.; Yao, J.; Chen, X.; Wu, C.; Chen, G.; et al. A novel score predicts HBV-related hepatocellular carcinoma recurrence after hepatectomy: A retrospective multicenter study. J. Gastrointest. Surg. 2019, 23, 922–932. [Google Scholar] [CrossRef]
- Ji, F.; Liang, Y.; Fu, S.J.; Guo, Z.Y.; Shu, M.; Shen, S.L.; Li, S.Q.; Peng, B.G.; Liang, L.J.; Hua, Y.P. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: The neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer 2016, 16, 137. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, B.; Miao, X.Y.; Zhou, J.J.; Xiong, L.; Wen, Y.; Zou, H. Comparison of FIB-4 index and Child-Pugh score in predicting the outcome of hepatic resection for hepatocellular carcinoma. J. Gastrointest. Surg. 2019, in press. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.-X.; Cao, Y.; Zhang, G.; Chen, W.-B.; Jiang, C.-P. Preoperative inflammation-based markers predict early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 266–274. [Google Scholar] [CrossRef]
- Okamura, Y.; Ashida, R.; Yamamoto, Y.; Ito, T.; Sugiura, T.; Bekku, E.; Aramaki, T.; Uesaka, K. The FIB-4 index is a significant prognostic factor in patients with non-B non-C hepatocellular carcinoma after curative surgery. Langenbeck’s Arch. Surg. 2016, 401, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Tada, T.; Kaneoka, Y.; Maeda, A. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery 2015, 157, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, C.; Wen, T.-F.; Yan, L.-N.; Li, B.; Wang, W.-T.; Yang, J.-Y.; Xu, M.-Q. Postoperative aspartate aminotransferase to platelet ratio index change predicts prognosis for hepatocellular carcinoma. Medicine 2016, 95, e4160. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, C.; Zhu, W.-J.; Wen, T.-F.; Yan, L.-N.; Li, B.; Wang, W.-T.; Yang, J.-Y. Prognostic value of the platelet to lymphocyte ratio change in liver cancer. J. Surg. Res. 2015, 194, 464–470. [Google Scholar] [CrossRef]
- Shen, S.-L.; Fu, S.-J.; Chen, B.; Kuang, M.; Li, S.-Q.; Hua, Y.-P.; Liang, L.-J.; Guo, P.; Hao, Y.; Peng, B.-G. Preoperative Aspartate Aminotransferase to Platelet Ratio is an Independent Prognostic Factor for Hepatitis B-Induced Hepatocellular Carcinoma After Hepatic Resection. Ann. Surg. Oncol. 2014, 21, 3802–3809. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Q.; Li, J.; Liao, Y.; Chen, Q.; Liao, W.-J.; Huang, J. The preoperative alkaline phosphatase-to-platelet ratio index is an independent prognostic factor for hepatocellular carcinoma after hepatic resection. Medicine 2016, 95, e5734. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Zheng, X.-L.; Zhang, Z.-Y.; Zhou, Y.; Hao, J.; Tang, G.; Li, O.; Xiang, J.-X.; Wu, Z.; Wang, B. Preoperative γ-glutamyl transpeptidase to platelet ratio (GPR) is an independent prognostic factor for HBV-related hepatocellular carcinoma after curative hepatic resection. Medicine 2016, 95, e4087. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, K.; Shen, J.; Li, B.; Kuang, M.; Cao, Q.; Peng, S. Novel prognostic nomograms based on inflammation-related markers for patients with hepatocellular carcinoma underwent hepatectomy. Cancer Res. Treat. 2019, in press. [Google Scholar] [CrossRef]
- Choi, W.-M.; Lee, J.-H.; Ahn, H.; Cho, H.; Cho, Y.Y.; Lee, M.; Yoo, J.-J.; Cho, Y.; Lee, D.H.; Bin Lee, Y.; et al. Forns index predicts recurrence and death in patients with hepatitis B-related hepatocellular carcinoma after curative resection. Liver Int. 2015, 35, 1992–2000. [Google Scholar] [CrossRef]
- Shindoh, J.; Kawamura, Y.; Kobayashi, Y.; Kiya, Y.; Sugawara, T.; Akuta, N.; Kobayashi, M.; Suzuki, Y.; Ikeda, K.; Hashimoto, M. Platelet-albumin score as a sensitive measure for surgical risk prediction and survival outcomes of patients with hepatocellular carcinoma. J. Gastrointest. Surg. 2019, 23, 76–83. [Google Scholar] [CrossRef]
- Li, W.; Shen, S.-Q.; Wu, S.-M.; Chen, Z.-B.; Hu, C.; Yan, R.-C. Simultaneous hepatectomy and splenectomy versus hepatectomy alone for hepatocellular carcinoma complicated by hypersplenism: A meta-analysis. OncoTargets Ther. 2015, 8, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Takemura, N.; Aoki, T.; Hasegawa, K.; Kaneko, J.; Arita, J.; Akamatsu, N.; Makuuchi, M.; Kokudo, N. Hepatectomy for hepatocellular carcinoma after perioperative management of portal hypertension. Br. J. Surg. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Shen, S.; Wang, W. Synchronous hepatectomy and splenectomy vs hepatectomy for selected patients with hepatocellular carcinoma and clinically significant portal hypertension: A systematic review and meta-analysis. J. Surg. Oncol. 2019, 119, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Takeishi, K.; Kawanaka, H.; Itoh, S.; Harimoto, N.; Ikegami, T.; Yoshizumi, T.; Shirabe, K.; Maehara, Y. Impact of splenic volume and splenectomy on prognosis of hepatocellular carcinoma within Milan criteria after curative hepatectomy. World J. Surg. 2018, 42, 1120–1128. [Google Scholar] [CrossRef]
- Regalia, E.; Doci, R.; Andreola, S.; Montalto, F.; Ammatuna, M.; Mazzaferro, V.M.; Pulvirenti, A.; Bozzetti, F.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar]
- Yao, F.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Mazzaferro, V.M.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; E Schwartz, M.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Raj, A.; McCall, J.; Gane, E. Validation of the “Metroticket” predictor in a cohort of patients transplanted for predominantly HBV-related hepatocellular carcinoma. J. Hepatol. 2011, 55, 1063–1068. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Nicolini, D.; Agostini, A.; Montalti, R.; Mocchegiani, F.; Mincarelli, C.; Mandolesi, A.; Robertson, N.L.; Candelari, R.; Giovagnoni, A.; Vivarelli, M. Radiological response and inflammation scores predict tumour recurrence in patients treated with transarterial chemoembolization before liver transplantation. World J. Gastroenterol. 2017, 23, 3690–3701. [Google Scholar] [CrossRef]
- Xia, W.; Ke, Q.; Guo, H.; Wang, W.; Zhang, M.; Shen, Y.; Wu, J.; Xu, X.; Yan, S.; Yu, J.; et al. Expansion of the Milan criteria without any sacrifice: Combination of the Hangzhou criteria with the pre-transplant platelet-to-lymphocyte ratio. BMC Cancer 2017, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Ke, Q.; Wang, Y.; Wang, W.; Zhang, M.; Shen, Y.; Wu, J.; Xu, X.; Zheng, S. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J. Surg. Oncol. 2015, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Parisi, I.; Tsochatzis, E.; Wijewantha, H.; Rodriguez-Peralvarez, M.; De Luca, L.; Manousou, P.; Fatourou, E.; Pieri, G.; Papastergiou, V.; Davies, N.; et al. Inflammation-based scores do not predict post-transplant recurrence of hepatocellular carcinoma in patients within milan criteria. Liver Transplant. 2014, 20, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, S.; Yang, J.D.; Leise, M.D.; Ahn, J.H.; Kim, S.; Jung, K.; Gwak, M.S.; Kim, G.S.; Ko, J.S. Risk of Risk of posttransplant hepatocellular carcinoma recurrence is greater in recipients with higher platelet counts in living donor liver transplantation. Liver Transplant. 2018, 24, 44–55. [Google Scholar] [CrossRef] [PubMed]
| Year | Author | Results |
|---|---|---|
| In Vitro | ||
| 2007 | Stock P [18] | 63% of HCC tissues showed up to 7-fold increase in total PDGFRa levels compared with adjacent tissue controls; anti-PDGFa decreases cell proliferation in HCC cell lines. |
| 2009 | Lau CK [19] | HCC cell lines and hepatic progenitor cell lines treated with cisplatin under normo- or hypoxic conditions; blockade of the Akt/HIF-1α/PDGF-BB autocrine signaling increases the chemosensitivity of HCC cells and hepatic progenitor cells under hypoxic conditions. |
| 2012 | Okada H [20] | Peretinoin represses the expression of PDGF-A/B in primary mouse hepatoma cells, preventing the progression of fibrosis and the subsequent development of HCC. |
| 2013 | Wu Q [21] | Gemcitabine-resistant HCC cells; PDGF-D highly expressed in these cells, with down-regulation of PDGF-D leading to partial reversal of the epithelial-mesenchymal transition. |
| 2015 | Hara Y [22] | Multikinase-inhibitor TSU-68 inhibits stromal PDGF signaling activated by HCC cells and inhibits HCC growth. |
| 2015 | Wang R [23] | PDGF-D is highly expressed in gemcitabine-resistant HCC cells. |
| 2015 | Lu Y [24] | Hepatic stellate cells in hypoxic condition; PDGF-BB expression markedly increased, while PDGF-BB blocking abolished cell proliferation, migration, and vascular endothelial growth-factor-A expression. |
| 2016 | Cho Y [25] | Human HCC cell lines cocultured with activated human hepatic stellate cell line under normo- or hypoxic conditions; hypoxic stellate cell-derived PDGF-BB stimulates the proliferation of HCC cells through activation of the PI3K/Akt pathway, while the inhibition of PDGF-BB or PI3K/Akt pathways enhances apoptotic cell death. |
| 2016 | Ma Y [26] | Human HCC cell lines; insulin-like growth factor-binding protein-3 suppresses PDGF expression. |
| 2017 | Lv X [27] | Cocultured hepatic stellate cells and HCC; PDGF is an effective activator of hepatic stellate cells. |
| 2019 | Xiao Z [28] | HCC cells; XPD suppresses cell proliferation and migration via regulating miR-29a-3p-Mdm2/PDGF-B axis |
| In Vivo (animal models) | ||
| 2007 | Campbell JS [29] | Transgenic PDGF-C mice with HCC; imatinib treatment decreases PDGFRa and stromal cell proliferation. |
| 2011 | Maass T [30] | Transgenic PDGF-B mice with HCC; PDGF-B mice present HCC larger than wild-type. |
| 2012 | Zhang JB [31] | HCC nude mouse model; PDGF-A up-regulated when IFN-α treatment re-initiated. |
| 2014 | Wright JH [32] | Mouse model; PDGF-C induces progressive fibrosis, chronic inflammation, neoangiogenesis, sinusoidal congestion, and global changes in gene expression. |
| In Vivo (Human) | ||
| 2007 | Mas VR [33] | From the gene expression analysis of the HCV–HCC tumors compared to normal livers, an important number of genes related to angiogenesis was differentially expressed, including PDGF. PDGF was also statistically differentially expressed between HCV cirrhosis and HCV–HCC groups. |
| 2013 | Chen YW [34] | HCC cases = 21 vs. controls = 8; circulating PDGF not higher in HCC. |
| 2014 | Wei T [35] | HCC = 57 vs. adjacent nontumor tissue = 57; PDGFRα overexpression strongly correlated with HCC microvessel density (p < 0.05), macroscopic vascular invasion (p < 0.05), shorter overall survival, and higher recurrence rate (p < 0.05, respectively). |
| 2014 | Talaat RM [36] | HCC cases = 135 vs. healthy cases = 50; higher PDGF plasma levels observed in HCC vs. healthy controls (p < 0.001). |
| 2015 | Alkozai EM [37] | HBV/HCV with or without HCC = 38, healthy volunteers = 20; intraplatelet and plasma levels of PDGF comparable between patients and controls. |
| 2016 | Lewandowski RJ [38] | Unresectable HCC treated with TARE alone = 12 or TARE + sorafenib = 11; In TARE/sorafenib group, PDGF decreased, in TARE only PDGF increased respect to baseline (p = 0.03). |
| 2017 | Hayashi T [39] | Hepatic arterial infusion chemotherapy = 104 vs. sorafenib = 39; patients treated with sorafenib with higher serum PDGF-BB (>300 pg/mL) achieved longer survival. |
| 2018 | Chen B [40] | HCC after hepatectomy = 90; higher PDGF-B expression correlated with tumor size (p = 0.02), TNM stage (p = 0.047), and portal vein emboli and metastases (p = 0.04). Higher PDGF-B is associated with worse survival (p = 0.002). |
| 2019 | Aryal B [41] | HCC patients undergoing resection = 40; lower serum PDGF-BB independent predictor of HCC recurrence after hepatic resection (HR = 5.64, p < 0.01). |
| Year | Author | Results |
|---|---|---|
| Serotonin | ||
| 2015 | Fatima S [43] | HCC cell lines and 33 pairs of HCC and corresponding adjacent non-tumor tissues. Receptors 5-HT1D (21/33, 63.6%), 5-HT2B (12/33, 36.4%), and 5-HT7 (15/33, 45.4%) were overexpressed. Serotonin increased total b-catenin and active b-catenin, and decreased phosphorylated b-catenin protein levels. |
| 2016 | Abdel-Razik A [44] | HCV–cirrhosis + HCC (n = 82), HCV–cirrhosis (n = 80), chronic HCV (n = 100), healthy controls (n = 60). Serotonin levels higher in cirrhotic vs. chronic HCV cases (p < 0.001) and in HCC vs. only cirrhosis cases (p < 0.001). HCC diagnosis better using serotonin vs. AFP or PIVKA (AUC 0.94 vs. 0.82 and 0.92). |
| 2016 | Abdel-Hamid NM [45] | HCC rat models. Significant increase in serotonin. Only serotonin exhibited a significant increase in early histological stage HCC development. |
| 2017 | Aryal B [46] | 40 HCC patients undergoing partial hepatectomy. Intra-platelet serotonin levels predicted HCC recurrence (HR = 0.1, 95%CI = 0.01–0.89). Disease-free interval significantly worse in patients with low intra-platelet serotonin (p = 0.029). |
| 2017 | Chan HL [47] | Taiwan’s National Health Insurance Research Database included 59,859 HCC cases vs. 285,124 matched controls. SSRIs associated with lower HCC risk, and the findings were dose-dependent (p < 0.001). |
| 2017 | Chang CM [48] | Taiwan’s National Health Insurance Research Database 9070 HCC vs. non-HCC subjects analyzed after matching for age and sex. HR for HCC in patients with SSRI use was 0.28 (95%CI = 0.12–0.64; p = 0.003). For SSRI users with a cumulative defined daily dose of 28–89, 90–364, and ≥365, HRs 0.51, 0.22, and 0.12. |
| 2017 | Liu S [49] | Human HCC cell lines. Yes-associated protein promoted by serotonin, favoring cell proliferation, invasion, and metastasis. |
| 2017 | Yang Q [50] | Zebrafish HCC model. Serotonin-activated human stellate cells promote HCC carcinogenesis and increase serotonin synthesis via transforming growth factor TGFb1 expression, hence causing a sex disparity in HCC (more tumor cases in male fishes). |
| 2019 | Zuo X [51] | 96 pairs of HCC and peritumor samples from resected patients. Serotonin 1D expression level significantly up-regulated in HCC tissues and cell lines, closely correlating with unfavorable clinicopathological characteristics. |
| EGF | ||
| 2014 | Huang P [52] | Cell bio-behaviors of HCC with low or high metastasis detected by live cell monitoring system. EGF significantly induced cell proliferation in HepG2 cells. EGF prompted cell movement in both HepG2 and HCCLM3 and regulated the production of CXCL5 and CXCL8 from HCC, which were inhibited by EGFR inhibitor, Erk inhibitor (U0126), or PI3K inhibitors. |
| 2014 | Fuchs BC [53] | Three different HCC animal models: rat model induced by diethylnitrosamine, mouse model induced by carbon tetrachloride, and a rat model induced by bile duct ligation. Erlotinib reduced EGFR phosphorylation in hepatic stellate cells, also decreasing hepatocyte proliferation and liver injury. Erlotinib also blocked the development of HCC. |
| 2014 | D’Alessandro R [54] | Human HCC cell lines with or without Sorafenib/Regorafenib. Drug-mediated inhibition of cell growth, migration, and invasion were all antagonized by platelet lysates. EGF and insulin-like growth factor-I able to antagonize Sorafenib in a proliferation assay, particularly in combination. |
| 2015 | Badawy AA [55] | 40 core liver biopsies from patients with HCV, 20 liver specimens from HCC cases with HCV, and 5 normal controls. EGFR and TGF-α were overexpressed in HCC and cirrhotic cases compared to HCV cases without cirrhosis. EGFR was detected in 33.3% of the examined HCC cases. |
| VEGF | ||
| 2004 | Kim SJ [56] | 52 HCC, 26 liver cirrhosis patients and 30 healthy controls. Serum VEGF per platelet count was higher in HCC than in liver cirrhosis patients and healthy controls (p < 0.01). Statistically significant correlation between serum VEGF and platelet count in HCC patients. Serum VEGF per platelet count higher in patients with advanced-stage and portal-vein thrombosis (p < 0.01). Patients with high serum VEGF per platelet count had poor response to treatment and shorter overall survival (p < 0.01). Serum VEGF per platelet count independent prognostic factor for the presence of portal vein thrombosis (p < 0.01). |
| 2009 | Hu J [57] | 162 AFP-negative HCC patients undergoing curative resection. Positive rates of VEGF and PD-ECGF in tumor tissues were 59.9% and 62.3%. At multivariate analysis, VEGF/PD-ECGF index independent prognostic factor for overall survival and relapse-free survival (p = 0.002 and p < 0.001). |
| 2009 | Corradini SG [58] | 24 patients undergoing liver transplant. VEGF-A more expressed in HCC than in non-cirrhotic tissue (p < 0.05). |
| 2011 | Ferroni P [59] | HCC (n = 70), cirrhosis (n = 45), and control subjects (n = 70). Median concentrations of plasma VEGF/platelet higher in HCC or cirrhotic patients compared to controls (p = 0.002). VEGF/platelet-load correlated with tumor diameter (p < 0.05). |
| 2012 | Guo JH [60] | 60 HCC patients undergoing TACE or transarterial infusion for unresectable tumor vs. 12 healthy volunteers. Median serum VEGF level in the HCC patients significantly higher than that of healthy controls (p = 0.021). Serum VEGF levels significantly correlated with platelet counts. Patients with serum VEGF level >285 pg/mL had worse overall survival (p = 0.002). By multivariate analysis, the serum VEGF level was a significant prognostic factor. |
| 2013 | Zhan P [61] | Meta-analysis of 11 studies evaluating the correlation between serum VEGF level and survival in patients with HCC. Combined hazard ratios suggested that serum VEGF level had an unfavorable impact on overall survival (HR = 1.88, 95%CI: 1.46–2.30), and disease-free survival (HR = 2.27, 95%CI: 1.55–2.98) in patients with HCC. |
| 2014 | Talaat RM [36] | 135 HCC patients (57 Child-Pugh A, 24 Child-Pugh B, and 54 Child-Pugh C stage) and 50 healthy subjects. Significant increase in plasma levels of VEGF (p < 0.001), PDGF (p < 0.001), TNF-α (p < 0.01) in HCC patients. Maximum production of VEGF and TNF-α was present in Child-Pugh C patients. |
| 2014 | Suh YG [62] | 50 HCC patients treated with radiotherapy. Patients with recurrence outside the radiation field had higher VEGF-A/platelet levels before and after radiotherapy (p = 0.04). On multivariate analysis, high level of VEGF/platelet before radiotherapy significant independent prognostic factor for a worse progression-free survival (p = 0.04). |
| 2015 | Cao G [63] | Meta-analysis based on 9 studies evaluating the relationship between VEGF level and clinical outcome in advanced HCC patients treated with sorafenib. Pooled estimates suggested that high level of VEGF was associated with poor overall survival (HR = 1.85; 95%CI: 1.24–2.77; p = 0.003) and poor progression-free survival (HR = 2.09; 95%CI: 1.43–3.05; p < 0.01). |
| 2016 | Aryal B [64] | 37 HCC resected patients. Serum and intra-platelet VEGF-A significantly elevated at four weeks of resection. Preoperative intra-platelet VEGF-A higher in patients with advanced cancer and vascular invasion. Postoperative intra-platelet VEGF-A higher after major liver resection. |
| Year | Author | Survivals |
|---|---|---|
| Resection | ||
| 2014 | Shen SL | 5-year tumor-free survival: APRI < 0.62: 32% 5-year tumor-free survival: APRI ≥ 0.62: 19% |
| 2015 | Ni XC | 2-year survival: PLR < 150: 90% 2-year survival: PLR ≥ 150: 77% |
| 2016 | Ji F | 5-year tumor-free survival: APRI < 1.68: 38% 5-year tumor-free survival: APRI ≥ 1.68: 21% |
| 2016 | Goh BK | 1-year mortality: PLR < 290: 13% 1-year mortality: PLR ≥ 290: 34% |
| Liver Transplantation | ||
| 2013 | Lai Q | 5-year tumor free survival: PLR < 150: 89% 5-year tumor free survival: PLR ≥ 150: 50% |
| 2015 | Xia W | 5-year tumor free survival: PLR < 150: 92% 5-year tumor free survival: PLR ≥ 150: 81% |
| 2016 | Harimoto N | 5-year tumor free survival: PLR< 150: 52% 5-year tumor free survival: PLR ≥ 150: 25% |
| 2017 | Nicolini D | 5-year tumor free survival: PLR < 150: 95% 5-year tumor free survival: PLR ≥ 150: 76% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Q.; Vitale, A.; Manzia, T.M.; Foschi, F.G.; Levi Sandri, G.B.; Gambato, M.; Melandro, F.; Russo, F.P.; Miele, L.; Viganò, L.; et al. Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics. Cancers 2019, 11, 1568. https://doi.org/10.3390/cancers11101568
Lai Q, Vitale A, Manzia TM, Foschi FG, Levi Sandri GB, Gambato M, Melandro F, Russo FP, Miele L, Viganò L, et al. Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics. Cancers. 2019; 11(10):1568. https://doi.org/10.3390/cancers11101568
Chicago/Turabian StyleLai, Quirino, Alessandro Vitale, Tommaso M. Manzia, Francesco G. Foschi, Giovanni B. Levi Sandri, Martina Gambato, Fabio Melandro, Francesco P. Russo, Luca Miele, Luca Viganò, and et al. 2019. "Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics" Cancers 11, no. 10: 1568. https://doi.org/10.3390/cancers11101568
APA StyleLai, Q., Vitale, A., Manzia, T. M., Foschi, F. G., Levi Sandri, G. B., Gambato, M., Melandro, F., Russo, F. P., Miele, L., Viganò, L., Burra, P., Giannini, E. G., & on behalf of the Associazione Italiana per lo Studio del Fegato (AISF) HCC Special Interest Group. (2019). Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics. Cancers, 11(10), 1568. https://doi.org/10.3390/cancers11101568









