Orthopedia Homeobox (OTP) in Pulmonary Neuroendocrine Tumors: The Diagnostic Value and Possible Molecular Interactions
Abstract
1. Introduction
2. OTP in Relation to Prognosis in PC
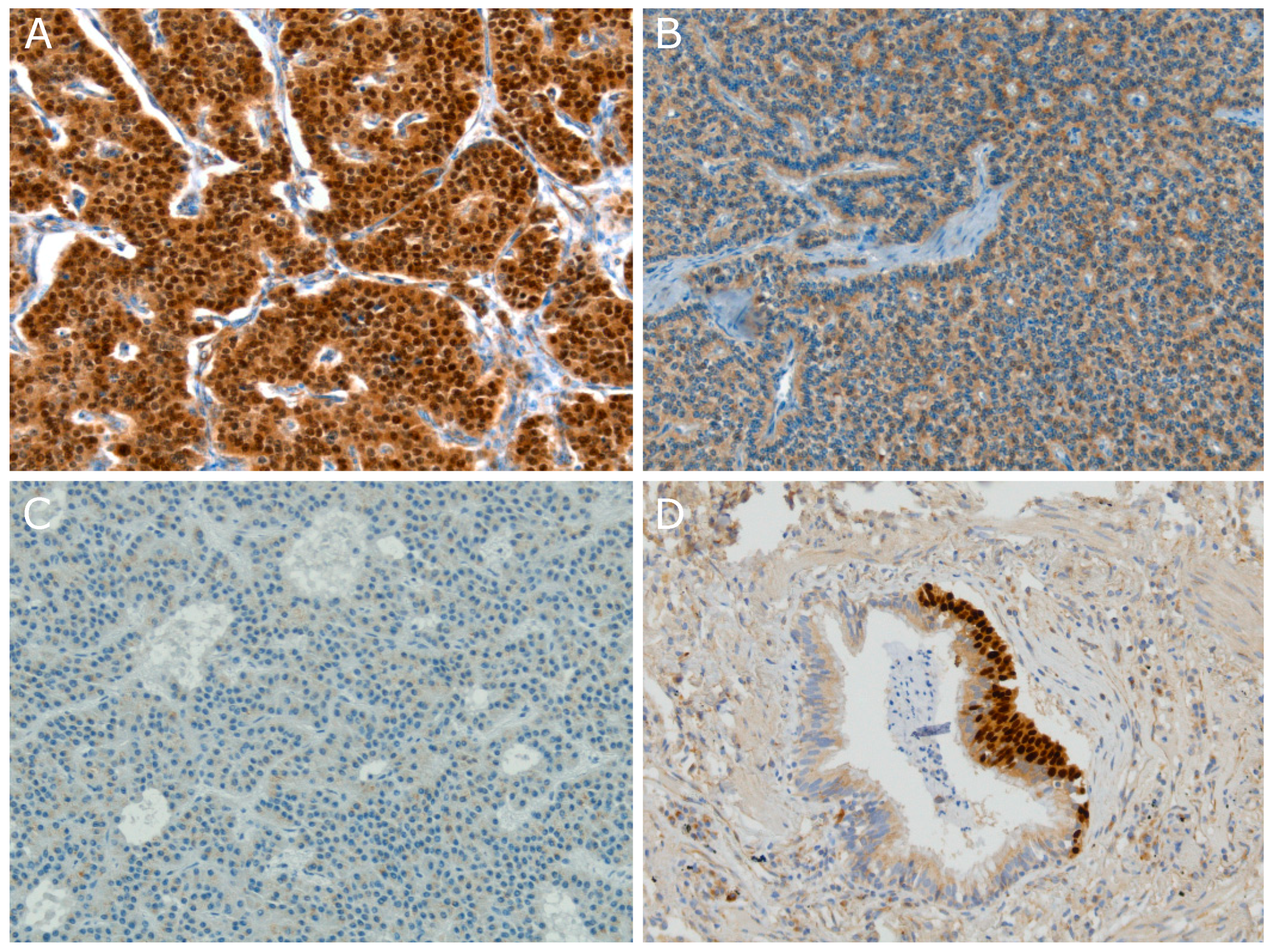
3. OTP Is Specifically Expressed Within Pulmonary Carcinoids
4. OTP in Pulmonary Neuroendocrine Cell Hyperplasia (NECH)
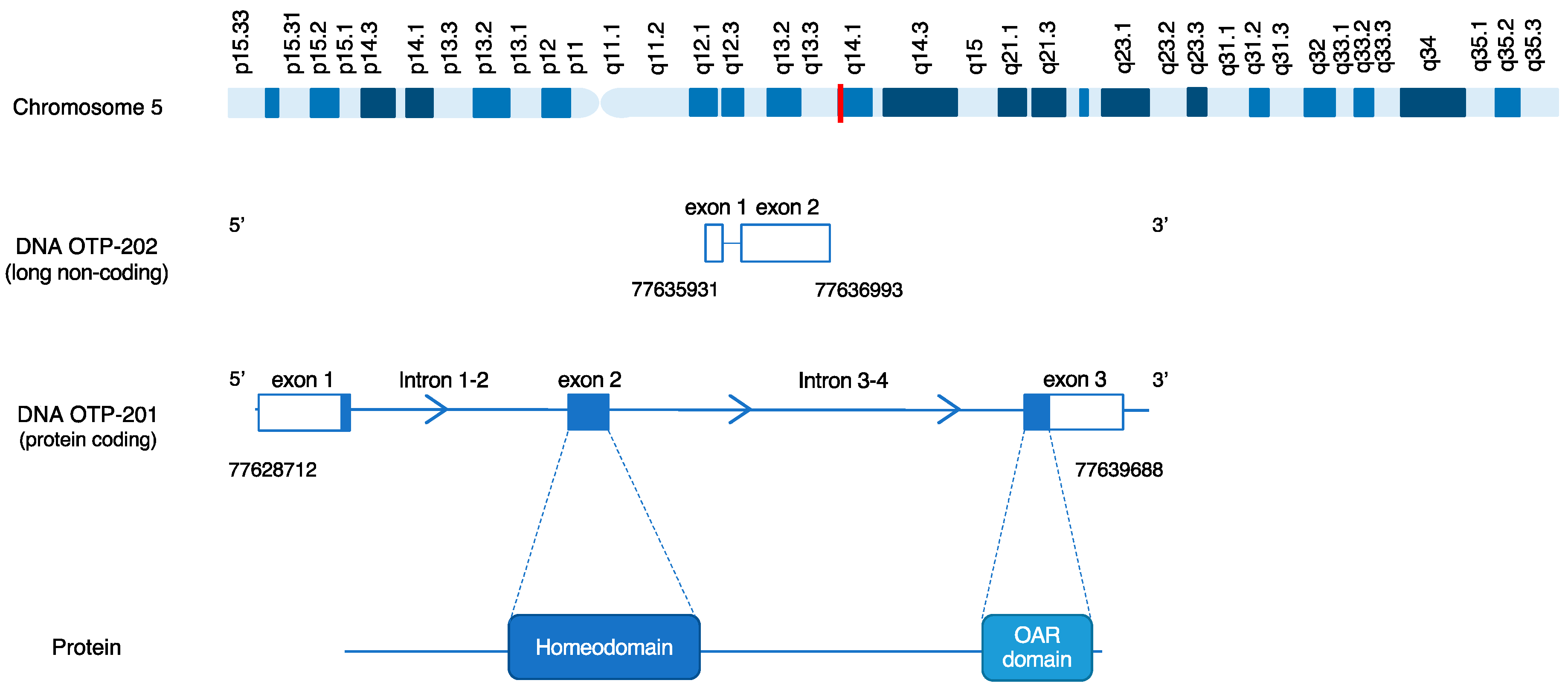
5. OTP Structure
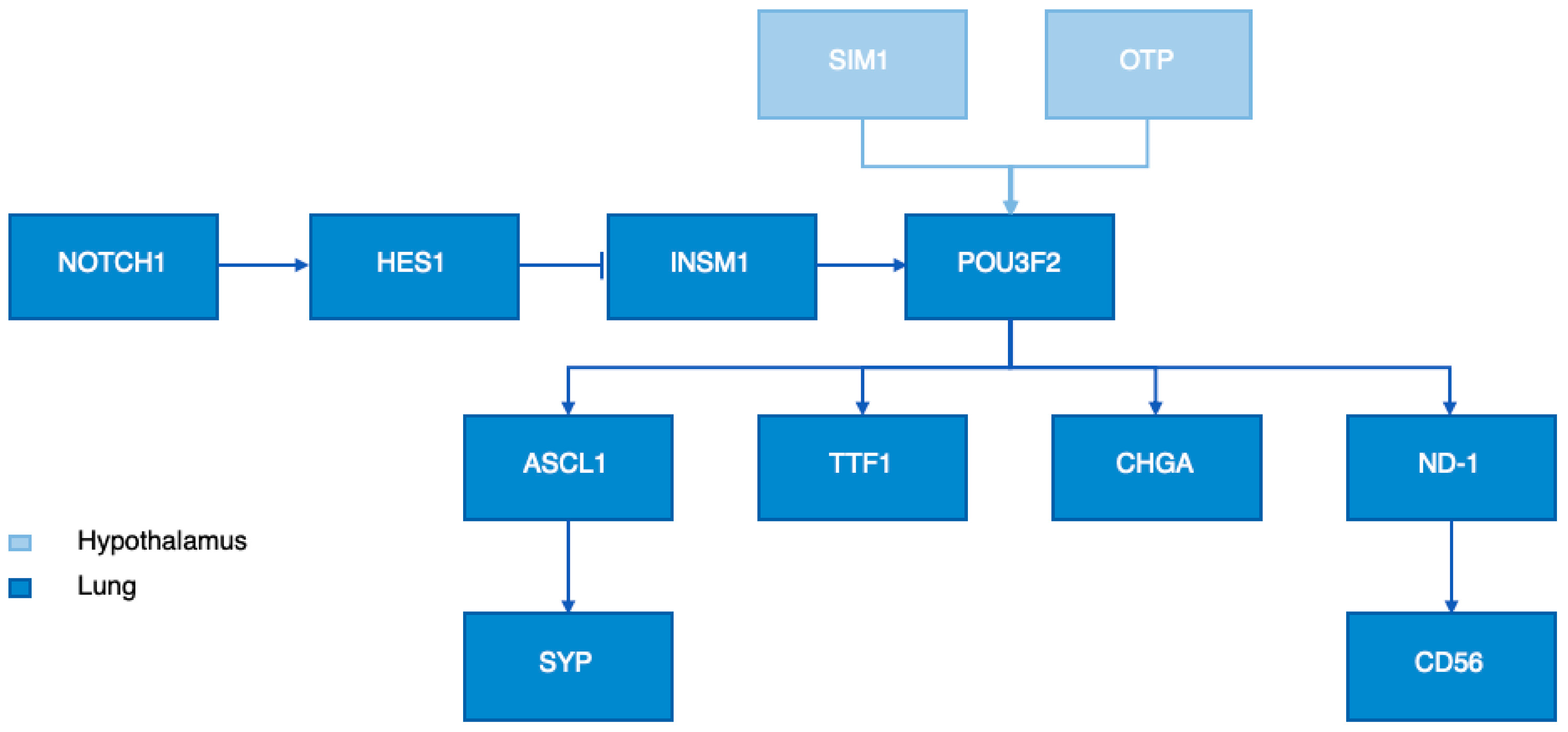
6. OTP Function
6.1. OTP in the Hypothalamus
6.2. OTP in Lung Neuroendocrine Tumors
7. OTP DNA Analysis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef] [PubMed]
- Korse, C.M.; Taal, B.G.; van Velthuysen, M.-L.F.; Visser, O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: Experience of two decades of cancer registry. Eur. J. Cancer 2013, 49, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Hendifar, A.E.; Marchevsky, A.M.; Tuli, R. Neuroendocrine tumors of the lung: Current challenges and advances in the diagnosis and management of well-differentiated disease. J. Thorac. Oncol. 2017, 12, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, WHO Classification of Tumours, 4th ed.; IARC: Lyon, France, 2015; Volume 7. [Google Scholar]
- Oberg, K.; Hellman, P.; Ferolla, P.; Papotti, M. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Rea, F.; Rizzardi, G.; Zuin, A.; Marulli, G.; Nicotra, S.; Bulf, R.; Schiavon, M.; Sartori, F. Outcome and surgical strategy in bronchial carcinoid tumors: Single institution experience with 252 patients. Eur. J. Cardio-Thorac. Surg. 2007, 31, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, G.; Fournel, L.; Strano, S.; Damotte, D.; Charpentier, M.C.; Galia, A.; Terminella, A.; Nicolosi, M.; Regnard, J.F.; Alifano, M. Surgical Resection for Pulmonary Carcinoid: Long-Term Results of Multicentric Study—The Importance of Pathological N Status, More Than We Thought. Lung 2017, 195, 789–798. [Google Scholar] [CrossRef]
- Cañizares, M.A.; Matilla, J.; Cueto, A.; Algar, J.; Muguruza, I.; Moreno-Mata, N.; Moreno-Balsalobre, R.; Guijarro, R.; Arrabal, R.; Garcia-Fontan, E. Atypical carcinoid tumours of the lung: Prognostic factors and patterns of recurrence. Thorax 2014, 69, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Sarkaria, I.; Pietanza, C.; Travis, W.; Roh, M.S.; Sica, G.; Healy, D.; Rusch, V.; Huang, J. Recurrence of pulmonary carcinoid tumors after resection: Implications for postoperative surveillance. Ann. Thorac. Surg. 2013, 96, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Yuste, M.; Matilla, J.M.; Cañizares, M.A.; Molins, L.; Guijarro, R. Surgical treatment of low and intermediate grade lung net. J. Thorac. Dis. 2017, 9, S1435. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.R.; Claessen, S.M.; Jonkers, Y.M.; Van Suylen, R.J.; Dingemans, A.M.C.; De Herder, W.W.; De Krijger, R.R.; Smit, E.F.; Thunnissen, F.B.; Seldenrijk, C.A.; et al. Deletions of 11q22. 3-q25 are associated with atypical lung carcinoids and poor clinical outcome. Am. J. Pathol. 2011, 179, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Rindi, G.; Travis, W.D.; Papotti, M. Ki-67 antigen in lung neuroendocrine tumors: Unraveling a role in clinical practice. J. Thorac. Oncol. 2014, 9, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.R.; Henfling, M.E.; Van Neste, L.; van Suylen, R.J.; Anne-marie, C.D.; Dinjens, W.N.; Haesevoets, A.; Rudelius, M.; Thunnissen, E.; Volante, M. CD44 and OTP are strong prognostic markers for pulmonary carcinoids. Clin. Cancer Res. 2013, 19, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Papaxoinis, G.; Nonaka, D.; O’Brien, C.; Sanderson, B.; Krysiak, P.; Mansoor, W. Prognostic Significance of CD44 and Orthopedia Homeobox Protein (OTP) Expression in Pulmonary Carcinoid Tumours. Endocr. Pathol. 2017, 28, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.R.; Van Neste, L.; Henfling, M.E.; Eijkenboom, I.; Eijk, P.P.; van Velthuysen, M.-L.; Vink, A.; Volante, M.; Ylstra, B.; Van Criekinge, W.J.C. An exploration of pathways involved in lung carcinoid progression using gene expression profiling. Carcinogenesis 2013, 34, 2726–2737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nonaka, D.; Papaxoinis, G.; Mansoor, W. Diagnostic Utility of Orthopedia Homeobox (OTP) in Pulmonary Carcinoid Tumors. Am. J. Surg. Pathol. 2016, 40, 738–744. [Google Scholar] [CrossRef]
- Hanley, K.Z.; Dureau, Z.J.; Cohen, C.; Shin, D.M.; Owonikoko, T.K.; Sica, G.L. Orthopedia homeobox is preferentially expressed in typical carcinoids of the lung. Cancer Cytopathol. 2018, 126, 236–242. [Google Scholar] [CrossRef]
- Papaxoinis, G.; Lamarca, A.; Quinn, A.M.; Mansoor, W.; Nonaka, D. Clinical and Pathologic Characteristics of Pulmonary Carcinoid Tumors in Central and Peripheral Locations. Endocr. Pathol. 2018. [Google Scholar] [CrossRef]
- Yoxtheimer, L.M.; Heymann, J.J.; Cohen, C.; Rao, R.A.; Goyal, A.; Siddiqui, M.T. Immunohistochemical analysis of OTP and NKX6. 1 in neuroendocrine tumors of the lung and pancreas. Diagn. Cytopathol. 2018, 46, 1010–1014. [Google Scholar] [CrossRef]
- Viswanathan, K.; Borczuk, A.C.; Siddiqui, M.T. Orthopedia homeobox protein (OTP) is a sensitive and specific marker for primary pulmonary carcinoid tumors in cytologic and surgical specimens. J. Am. Soc. Cytopathol. 2019, 8, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.R.; van Suylen, R.J.; den Bakker, M.A.; van Oosterhout, M.F.; Thunnissen, F.B.; Volante, M.; Dingemans, A.M.; Scheltinga, M.R.; Bootsma, G.P.; Pouwels, H.M.; et al. Interobserver variability for the WHO classification of pulmonary carcinoids. Am. J. Surg. Pathol. 2014, 38, 1429–1436. [Google Scholar] [CrossRef]
- Roy, M.; Buehler, D.G.; Zhang, R.; Schwalbe, M.L.; Baus, R.M.; Salamat, M.S.; Lloyd, R.V.; Rosenbaum, J.N. Expression of Insulinoma-Associated Protein 1 (INSM1) and Orthopedia Homeobox (OTP) in Tumors with Neuroendocrine Differentiation at Rare Sites. Endocr. Pathol. 2018, 30, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, S.M.; Miller, Y.E.; Waldron, J.A., Jr.; Bogin, R.M.; Sunday, M.E.; Staton, G.W., Jr.; Beam, W.R.; King, T.E., Jr. Brief report: Idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N. Engl. J. Med. 1992, 327, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, C.; Oliveira, R.C.; Saraiva, J.; Bernardo, J.; Carvalho, L. Pulmonary Peripheral Carcinoids after Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia and Tumorlets: Report of 3 Cases. Case Rep. Pulmonol. 2015, 2015, 851046. [Google Scholar] [CrossRef]
- Davies, S.J.; Gosney, J.R.; Hansell, D.M.; Wells, A.U.; du Bois, R.M.; Burke, M.M.; Sheppard, M.N.; Nicholson, A.G. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: An under-recognised spectrum of disease. Thorax 2007, 62, 248–252. [Google Scholar] [CrossRef]
- Derks, J.L.; Leblay, N.; Lantuejoul, S.; Dingemans, A.C.; Speel, E.M.; Fernandez-Cuesta, L. New Insights into the Molecular Characteristics of Pulmonary Carcinoids and Large Cell Neuroendocrine Carcinomas, and the Impact on Their Clinical Management. J. Thorac. Oncol. 2018, 13, 752–766. [Google Scholar] [CrossRef]
- McGinnis, W.; Hart, C.P.; Gehring, W.J.; Ruddle, F.H. Molecular cloning and chromosome mapping of a mouse DNA sequence homologous to homeotic genes of Drosophila. Cell 1984, 38, 675–680. [Google Scholar] [CrossRef]
- Lin, X.; State, M.W.; Vaccarino, F.M.; Greally, J.; Hass, M.; Leckman, J.F. Identification, chromosomal assignment, and expression analysis of the human homeodomain-containing gene Orthopedia (OTP). Genomics 1999, 60, 96–104. [Google Scholar] [CrossRef]
- Kaji, T.; Nonogaki, K. Role of homeobox genes in the hypothalamic development and energy balance. Front. Biosci. (Landmark Ed.) 2013, 18, 740–747. [Google Scholar]
- Wang, W.; Lufkin, T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev. Biol. 2000, 227, 432–449. [Google Scholar] [CrossRef]
- Acampora, D.; Postiglione, M.P.; Avantaggiato, V.; Di Bonito, M.; Simeone, A. The role of Otx and Otp genes in brain development. Int. J. Dev. Biol. 2000, 44, 669–677. [Google Scholar] [PubMed]
- Biran, J.; Tahor, M.; Wircer, E.; Levkowitz, G. Role of developmental factors in hypothalamic function. Front. Neuroanat. 2015, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Acampora, D.; Postiglione, M.P.; Avantaggiato, V.; Di Bonito, M.; Vaccarino, F.M.; Michaud, J.; Simeone, A. Progressive impairment of development. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999, 13, 2787–2800. [Google Scholar] [CrossRef] [PubMed]
- Blechman, J.; Borodovsky, N.; Eisenberg, M.; Nabel-Rosen, H.; Grimm, J.; Levkowitz, G. Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development 2007, 134, 4417–4426. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G. Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: A review and update. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Sato, H.; Sakaeda, M.; Shishido-Hara, Y.; Hiramatsu, C.; Kamma, H.; Shimoyamada, H.; Fujiwara, M.; Endo, T.; Aoki, I.; et al. POU domain transcription factor BRN2 is crucial for expression of ASCL1, ND1 and neuroendocrine marker molecules and cell growth in small cell lung cancer. Pathol. Int. 2013, 63, 158–168. [Google Scholar] [CrossRef]
- Sakaeda, M.; Sato, H.; Ishii, J.; Miyata, C.; Kamma, H.; Shishido-Hara, Y.; Shimoyamada, H.; Fujiwara, M.; Endo, T.; Tanaka, R.; et al. Neural lineage-specific homeoprotein BRN2 is directly involved in TTF1 expression in small-cell lung cancer. Lab. Investig. 2013, 93, 408–421. [Google Scholar] [CrossRef][Green Version]
- Fujino, K.; Motooka, Y.; Hassan, W.A.; Ali Abdalla, M.O.; Sato, Y.; Kudoh, S.; Hasegawa, K.; Niimori-Kita, K.; Kobayashi, H.; Kubota, I.; et al. Insulinoma-Associated Protein 1 is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer. Am. J. Pathol. 2015, 185, 3164–3177. [Google Scholar] [CrossRef]
- Crabtree, J.S.; Singleton, C.S.; Miele, L. Notch Signaling in Neuroendocrine Tumors. Front. Oncol. 2016, 6, 94. [Google Scholar] [CrossRef]
- Kunnimalaiyaan, M.; Chen, H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 2007, 12, 535–542. [Google Scholar] [CrossRef]
- Kunnimalaiyaan, M.; Yan, S.; Wong, F.; Zhang, Y.W.; Chen, H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery 2005, 138, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, M.; Traeger, K.; Chen, H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G636–G642. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, M.; Vaccaro, A.M.; Ndiaye, M.A.; Chen, H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J. Biol. Chem. 2006, 281, 39819–39830. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cuesta, L.; Peifer, M.; Lu, X.; Sun, R.; Ozretić, L.; Seidel, D.; Zander, T.; Leenders, F.; George, J.; Müller, C.; et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat. Commun. 2014, 5, 3518. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.K.; Thomas, C.F.; Dong, J.; Schulte, S.C.; Khadka, P.; Sun, Z.; Kosari, F.; Jen, J.; Molina, J.; Vasmatzis, G.; et al. Pathways impacted by genomic alterations in pulmonary carcinoid tumors. Clin. Cancer Res. 2018, 24, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Mafficini, A.; Sikora, K.O.; Fassan, M.; Barbi, S.; Corbo, V.; Mastracci, L.; Rusev, B.; Grillo, F.; Vicentini, C.; et al. Lung neuroendocrine tumours: Deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J. Pathol. 2017, 241, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Armengol, G.; Sarhadi, V.K.; Rönty, M.; Tikkanen, M.; Knuuttila, A.; Knuutila, S. Driver gene mutations of non-small-cell lung cancer are rare in primary carcinoids of the lung: NGS study by ion Torrent. Lung 2015, 193, 303–308. [Google Scholar] [CrossRef]
- Vollbrecht, C.; Werner, R.; Walter, R.F.H.; Christoph, D.C.; Heukamp, L.C.; Peifer, M.; Hirsch, B.; Burbat, L.; Mairinger, T.; Schmid, K.W.; et al. Mutational analysis of pulmonary tumours with neuroendocrine features using targeted massive parallel sequencing: A comparison of a neglected tumour group. Br. J. Cancer 2015, 113, 1704. [Google Scholar] [CrossRef]
- Alcala, N.; Leblay, N.; Gabriel, A.; Mangiante, L.; Hervas, D.; Giffon, T.; Sertier, A.-S.; Ferrari, A.; Derks, J.; Ghantous, A.; et al. Integrative and comparative genomic analyses identify clinically-relevant groups of pulmonary carcinoids and unveil the supra-carcinoids. Nat. Commun. 2019, 10, 23. [Google Scholar] [CrossRef]
- Swarts, D.R.; Scarpa, A.; Corbo, V.; Van Criekinge, W.; van Engeland, M.; Gatti, G.; Henfling, M.E.; Papotti, M.; Perren, A.; Ramaekers, F.C.; et al. MEN1 gene mutation and reduced expression are associated with poor prognosis in pulmonary carcinoids. J. Clin. Endocrinol. Metab. 2014, 99, E374–E378. [Google Scholar] [CrossRef]
- Wajed, S.A.; Laird, P.W.; DeMeester, T.R. DNA methylation: An alternative pathway to cancer. Ann. Surg. 2001, 234, 10. [Google Scholar] [CrossRef] [PubMed]
| Study Population | Histology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Year [Ref] | Initial Cohort | Included in Analysis | Age (years) | Gender (n) | DIPNECH | TC | AC | HGNECs | WHO Classification (year) | Normal Tissue Included (n) | Other (NE) Tissues Included (n) |
| Hanley et al., 2018 [18] | 63 | 59 | 26–91 | M(24), F(39) | 0 | 9 | 6 | 1 | Yes (2015) | No | Yes (51) |
| Nonaka et al., 2016 * [17] | 159 | 159 | 21–83 | M(62), F(97) | 7 | 123 | 21 | 104 | Yes (2015) | Yes (n/a) | Yes (758) |
| Papaxoinis et al., 2018 * [19] | 166 | 166 | 16–83 | M(62), F(104) | 16 | 132 | 34 | 0 | Yes (2015) | No | No |
| Papaxoinis et al., 2017 * [15] | 108 | 86 | 21–83 | M(44), F(64) | 8 | 69 | 17 | 0 | Yes (2015) | No | No |
| Swarts et al., 2013 [14] | 352 | 348 | 16–83 | M(130), F(159) | 0 | 225 | 63 | 59 | Yes (2003) | Yes (4) | Yes (9) |
| Yoxtheimer et al., 2018 [20] | 50 | 50 | 21–87 | M(30), F(20) | 0 | 8 | 6 | 16 | Yes (2015) | No | Yes (20) |
| Viswanathan et al., 2019 [21] | 60 | 57 | 32–86 | M(31), F(29) | 0 | 11 | 12 | 19 | Yes (2015) | No | Yes (18) |
| Study Year [Ref] | Immunohistochemistry | Outcome (n OTP Positive/Total (%)) | Staining Scoring | |||||
|---|---|---|---|---|---|---|---|---|
| Antibody Supplier # | Dilution | DIPNECH | TC | AC | HGNECs | Considered Positive If | Overall Conclusion | |
| Hanley et al., 2018 [18] | Sigma | (1:800) | - | 9/9 (100%) | 1/6 (17%) | - | Any percentage or intensity of nuclear OTP expression | OTP is a highly sensitive and specific marker for lung carcinoids |
| Nonaka et al., 2016 * [17] | Atlas | (1:150) | 7/7 (100%) | 105/123 (85.4%) | 10/21 (47.6%) | 2/104 (1.9%) | 1 + (1–25%), 2 + (25–50%), 3 + (50–75%), 4 + (>75%) | OTP may serve as a useful diagnostic marker for lung carcinoid tumors |
| Papaxoinis et al., 2018 * [19] | Atlas | (1:150) | 16/16 (100%) | 117/132 (88.6%) | 21/34 (61.8%) | - | More than 5% of the tumor expressed a positive reaction | OTP and TTF1 expression can be used to classify carcinoids into different clusters |
| Papaxoinis et al., 2017 * [15] | Atlas | (1:150) | - | nOTP < 150 14/69 (20.3%) | nOTP <150 8/17 (47%) | - | H-score (ranging from 0–300) | CD44/nOTP expression is an independent predictor of RFS in patients with radically operated PCs |
| cOTP < 150 59/69 (86%) | cOTP < 150 14/17 (82%) | - | ||||||
| nOTP > 150 55/69 (80%) | nOTP > 150 9/17 (53%) | - | ||||||
| cOTP > 150 10/69 (14.5%) | cOTP > 150 3/17 (18%) | - | ||||||
| Swarts et al., 2013 [14] | Atlas | (1:800) | - | nOTP 10/225 (4%) | nOTP 3/63 (5%) | nOTP 1/59 (2%) | 0 = no staining, 1 = very weak diffuse staining [cytoplasm] or staining in single or very few nuclei, 2 = weak to moderate staining, for nuclear staining in >40% of nuclei, 3–4 = strong to very strong staining in most or all tumor cells, respectively. | OTP and CD44 are powerful prognostic markers for pulmonary carcinoids |
| nOTP + cOTP 165/225 (73%) | nOTP + cOTP 28/63 (44%) | nOTP + cOTP 4/59 (7%) | ||||||
| cOTP 17/225 (8%) | cOTP 15/63 (24%) | cOTP 8/59 (14%) | ||||||
| Yoxtheimer et al., 2018 [20] | Sigma | (1:800) | - | 4/8 (50%) | 1/6 (17%) | 1/16 (6.3%) | Min. 5% of tumor cell positivity of 3 + staining intensity | OTP may be used to grade pulmonary NETs and differentiate them from low-grade NETs originating in other sites |
| Viswanathan et al., 2019 [21] | Sigma | (1:800) | - | 9/11 (82%) | 8/12 (80%) | 0/19 (0%) | Tumor showed >1 + OTP staining in >5% of the tumor within the specimen | OTP is a promising highly sensitive and specific marker for primary pulmonary carcinoid tumors |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moonen, L.; Derks, J.; Dingemans, A.-M.; Speel, E.-J. Orthopedia Homeobox (OTP) in Pulmonary Neuroendocrine Tumors: The Diagnostic Value and Possible Molecular Interactions. Cancers 2019, 11, 1508. https://doi.org/10.3390/cancers11101508
Moonen L, Derks J, Dingemans A-M, Speel E-J. Orthopedia Homeobox (OTP) in Pulmonary Neuroendocrine Tumors: The Diagnostic Value and Possible Molecular Interactions. Cancers. 2019; 11(10):1508. https://doi.org/10.3390/cancers11101508
Chicago/Turabian StyleMoonen, Laura, Jules Derks, Anne-Marie Dingemans, and Ernst-Jan Speel. 2019. "Orthopedia Homeobox (OTP) in Pulmonary Neuroendocrine Tumors: The Diagnostic Value and Possible Molecular Interactions" Cancers 11, no. 10: 1508. https://doi.org/10.3390/cancers11101508
APA StyleMoonen, L., Derks, J., Dingemans, A.-M., & Speel, E.-J. (2019). Orthopedia Homeobox (OTP) in Pulmonary Neuroendocrine Tumors: The Diagnostic Value and Possible Molecular Interactions. Cancers, 11(10), 1508. https://doi.org/10.3390/cancers11101508






