Direct Oral Anticoagulant Drugs: On the Treatment of Cancer-Related Venous Thromboembolism and their Potential Anti-Neoplastic Effect
Abstract
:1. Introduction
2. Treatment of Cancer-Associated Venous Thromboembolism: Current Guidelines
3. Low-Molecular-Weight Heparins (LMWHs)
4. General Aspects of DOACs and Concerns in Cancer Patients
5. Evidence of Utilization of DOACs in Cancer Patients
5.1. Subgroup Analysis
5.1.1. Rivaroxaban
5.1.2. Edoxaban
5.1.3. Dabigatran
5.1.4. Apixaban
5.2. Meta-Analysis
5.3. Observational Studies
5.4. Concluded Prospective Studies
5.4.1. Edoxaban: The Hokusai VTE Cancer Clinical Trial
5.4.2. Rivaroxaban: The SELECT-D Trial
5.4.3. Meta-Analysis of the Prospective Studies
5.5. Ongoing or in 2018 Concluded Clinical Trials
6. Anticancer Effect
6.1. Anticancer Effect of Heparins
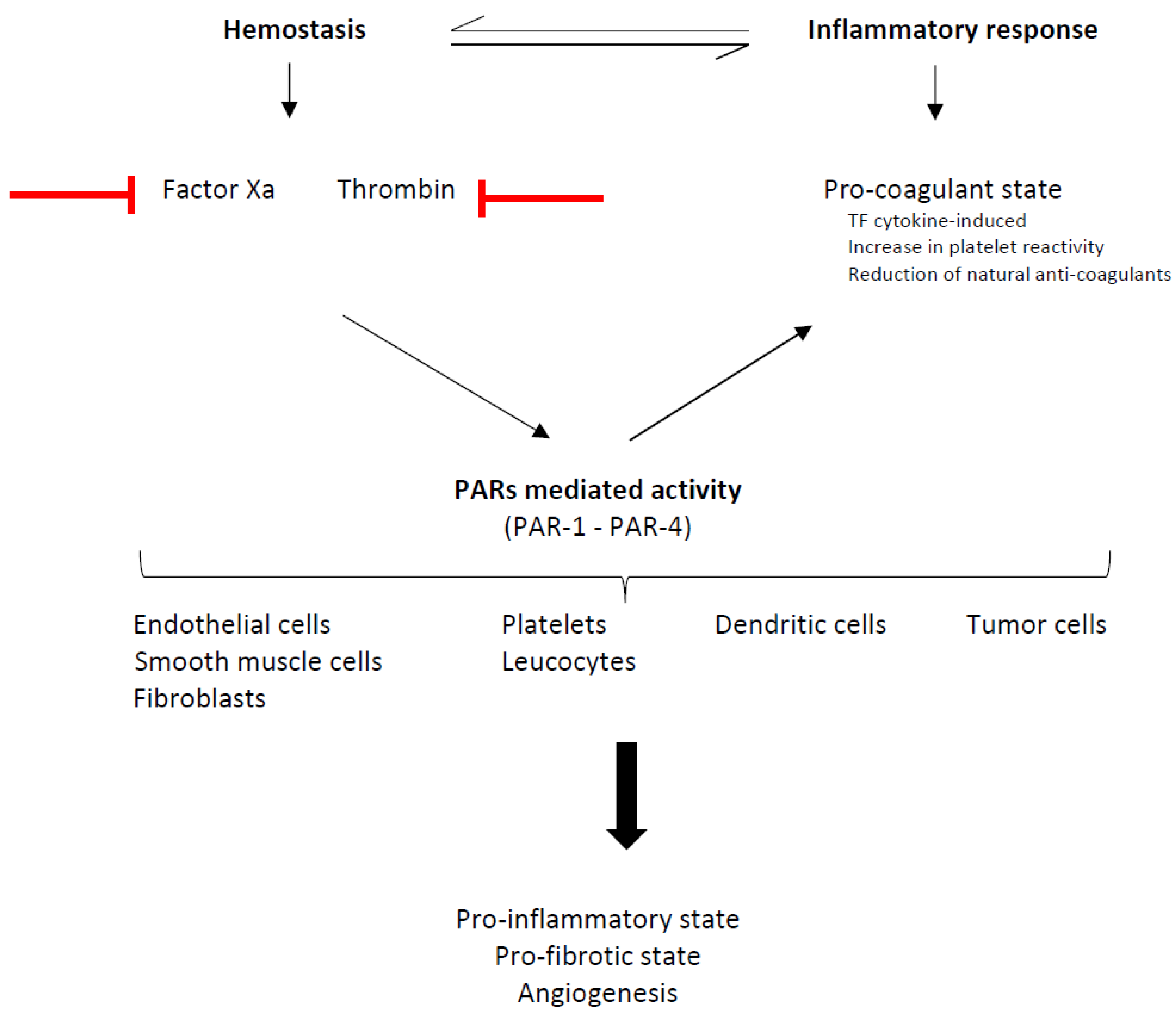
6.2. DOACs Beyond the Anticoagulation: A Potential Antineoplastic Effect?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, A.Y.; Levine, M.N. Venous thromboembolism and cancer: Risks and outcomes. Circulation 2003, 107 (Suppl. 1), I17–I21. [Google Scholar] [CrossRef] [PubMed]
- Horsted, F.; West, J.; Grainge, M.J. Risk of venous thromboembolism in patients with cancer: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001275. [Google Scholar] [CrossRef]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, H.T.; Mellemkjaer, L.; Olsen, J.H.; Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Ageno, W.; Giorgi Pierfranceschi, M.; Imberti, D.; Malato, A.; Nitti, C.; Salvi, A.; Siragusa, S.; Squizzato, A.; Vitale, J.; et al. Prognostic relevance of an asymptomatic venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2011, 9, 1081–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braekkan, S.K.; Borch, K.H.; Mathiesen, E.B.; Njolstad, I.; Wilsgaard, T.; Hansen, J.B. Body height and risk of venous thromboembolism: The Tromso Study. Am. J. Epidemiol. 2010, 171, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Mahe, I.; Chidiac, J.; Helfer, H.; Noble, S. Factors influencing adherence to clinical guidelines in the management of cancer-associated thrombosis. J. Thromb. Haemost. 2016, 14, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; McCrae, K.R.; Milentijevic, D.; Fortier, J.; Nelson, W.W.; Laliberte, F.; Crivera, C.; Lefebvre, P.; Yannicelli, D.; Schein, J. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res. Pract. Thromb. Haemost. 2017, 1, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Lyman, G.H.; Bohlke, K.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J. Clin. Oncol. 2015, 33, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.G.; Keeling, D.M.; Laffan, M.; Tait, R.C.; Makris, M. Guideline on aspects of cancer-related venous thrombosis. Br. J. Haematol. 2015, 170, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Bounameaux, H.; Brenner, B.; Cajfinger, F.; Debourdeau, P.; Khorana, A.A.; Pabinger, I.; Solymoss, S.; Douketis, J.; Kakkar, A. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016, 17, e452–e466. [Google Scholar] [CrossRef] [Green Version]
- Mandala, M.; Falanga, A.; Roila, F.; Group, E.G.W. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011, 22 (Suppl. 6), vi85–vi92. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Cancer-Associated Venous Thromboembolic Disease Version 2.2018. Available online: https://www.nccn.org (accessed on 11 November 2018).
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef]
- Green, D.; Hull, R.D.; Brant, R.; Pineo, G.F. Lower mortality in cancer patients treated with low-molecular-weight versus standard heparin. Lancet 1992, 339, 1476. [Google Scholar] [CrossRef]
- Levine, M.; Gent, M.; Hirsh, J.; Leclerc, J.; Anderson, D.; Weitz, J.; Ginsberg, J.; Turpie, A.G.; Demers, C.; Kovacs, M. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N. Engl. J. Med. 1996, 334, 677–681. [Google Scholar] [CrossRef]
- Koopman, M.M.; Prandoni, P.; Piovella, F.; Ockelford, P.A.; Brandjes, D.P.; van der Meer, J.; Gallus, A.S.; Simonneau, G.; Chesterman, C.H.; Prins, M.H. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N. Engl. J. Med. 1996, 334, 682–687. [Google Scholar] [CrossRef]
- Columbus, I.; Buller, H.R.; Gent, M.; Gallus, A.S.; Ginsberg, J.; Prins, M.H.; Baildon, R. Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. N. Engl. J. Med. 1997, 337, 657–662. [Google Scholar]
- Simonneau, G.; Sors, H.; Charbonnier, B.; Page, Y.; Laaban, J.P.; Azarian, R.; Laurent, M.; Hirsch, J.L.; Ferrari, E.; Bosson, J.L.; et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l’Embolie Pulmonaire. N. Engl. J. Med. 1997, 337, 663–669. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.D.; Pineo, G.F.; Brant, R.F.; Mah, A.F.; Burke, N.; Dear, R.; Wong, T.; Cook, R.; Solymoss, S.; Poon, M.C.; et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am. J. Med. 2006, 119, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Marjanovic, Z.; Valcke, J.; Lorcerie, B.; Gruel, Y.; Solal-Celigny, P.; Le Maignan, C.; Extra, J.M.; Cottu, P.; Farge, D. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch. Intern. Med. 2002, 162, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Deitcher, S.R.; Kessler, C.M.; Merli, G.; Rigas, J.R.; Lyons, R.M.; Fareed, J.; Oncenox Investigators. Secondary prevention of venous thromboembolic events in patients with active cancer: Enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin. Appl. Thromb. Hemost. 2006, 12, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Kamphuisen, P.W.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Khorana, A.A.; CATCH Investigators. Tinzaparin vs. Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA 2015, 314, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Hakoum, M.B.; Kahale, L.A.; Tsolakian, I.G.; Matar, C.F.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Barba, M.; Schunemann, H.; Akl, E.A. Anticoagulation for the initial treatment of venous thromboembolism in people with cancer. Cochrane Database Syst. Rev. 2018, 1, CD006649. [Google Scholar] [CrossRef]
- Investigators, E.; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar]
- Investigators, E.-P.; Buller, H.R.; Prins, M.H.; Lensin, A.W.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; Wells, P.; et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Masiukiewicz, U.; Pak, R.; Thompson, J.; Raskob, G.E.; et al. Oral apixaban for the treatment of acute venous thromboembolism. N. Engl. J. Med. 2013, 369, 799–808. [Google Scholar] [CrossRef]
- Hokusai, V.T.E.I.; Buller, H.R.; Decousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z.; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kakkar, A.K.; Goldhaber, S.Z.; Schellong, S.; Eriksson, H.; Mismetti, P.; Christiansen, A.V.; Friedman, J.; Le Maulf, F.; Peter, N.; et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014, 129, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z.; Schellong, S.; Kakkar, A.; Eriksson, H.; Feuring, M.; Kreuzer, J.; Fraessdorf, M.; Schulman, S. Treatment of acute pulmonary embolism with dabigatran versus warfarin. A pooled analysis of data from RE-COVER and RE-COVER II. Thromb. Haemost. 2016, 116, 714–721. [Google Scholar] [PubMed]
- Lee, A.Y.; Peterson, E.A. Treatment of cancer-associated thrombosis. Blood 2013, 122, 2310–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrier, M.; Khorana, A.A.; Zwicker, J.; Noble, S.; Lee, A.Y. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2013, 11, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Hakeam, H.A.; Al-Sanea, N. Effect of major gastrointestinal tract surgery on the absorption and efficacy of direct acting oral anticoagulants (DOACs). J. Thromb. Thrombolysis 2017, 43, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Crowther, M.A. How I treat with anticoagulants in 2012: New and old anticoagulants, and when and how to switch. Blood 2012, 119, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.; Epps, Q.J. Direct oral anticoagulants: A review of common medication errors. J. Thromb. Thrombolysis 2018. [Google Scholar] [CrossRef] [PubMed]
- Voigtlaender, M.; Langer, F. Direct oral anticoagulants for the treatment of cancer-associated venous thromboembolism. Hamostaseologie 2017, 37, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Kamphuisen, P.W.; Agnelli, G. Antithrombotic therapy for prophylaxis and treatment of venous thromboembolism in patients with cancer: Review of the literature on current practice and emerging options. ESMO Open. 2017, 2, e000188. [Google Scholar] [CrossRef] [PubMed]
- Wharin, C.; Tagalakis, V. Management of venous thromboembolism in cancer patients and the role of the new oral anticoagulants. Blood Rev. 2014, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van Es, N.; Coppens, M.; Schulman, S.; Middeldorp, S.; Buller, H.R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: Evidence from phase 3 trials. Blood 2014, 124, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.H.; Lensing, A.W.A.; Brighton, T.A.; Lyons, R.M.; Rehm, J.; Trajanovic, M.; Davidson, B.L.; Beyer-Westendorf, J.; Pap, Á.F.; Berkowitz, S.D.; et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): A pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014, 1, e37–e46. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Segers, A.; Angchaisuksiri, P.; Oh, D.; Boda, Z.; Lyons, R.M.; Meijer, K.; Gudz, I.; Weitz, J.I.; et al. Edoxaban for venous thromboembolism in patients with cancer: Results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol. 2016, 3, e379–e387. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Schellong, S.; Eriksson, H.; Baanstra, D.; Kvamme, A.M.; Friedman, J.; Mismetti, P.; Goldhaber, S.Z. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N. Engl. J. Med. 2013, 368, 709–718. [Google Scholar] [CrossRef]
- Schulman, S.; Goldhaber, S.Z.; Kearon, C.; Kakkar, A.K.; Schellong, S.; Eriksson, H.; Hantel, S.; Feuring, M.; Kreuzer, J. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb. Haemost. 2015, 114, 150–157. [Google Scholar]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Gallus, A.S.; Lee, T.C.; Pak, R.; Raskob, G.E.; Weitz, J.I.; Yamabe, T. Oral apixaban for the treatment of venous thromboembolism in cancer patients: Results from the AMPLIFY trial. J. Thromb. Haemost. 2015, 13, 2187–2191. [Google Scholar] [CrossRef]
- Van der Hulle, T.; den Exter, P.L.; Kooiman, J.; van der Hoeven, J.J.; Huisman, M.V.; Klok, F.A. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. J. Thromb. Haemost. 2014, 12, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Carrier, M.; Cameron, C.; Delluc, A.; Castellucci, L.; Khorana, A.A.; Lee, A.Y. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: A systematic review and meta-analysis. Thromb. Res. 2014, 134, 1214–1219. [Google Scholar] [CrossRef]
- Larsen, T.B.; Nielsen, P.B.; Skjoth, F.; Rasmussen, L.H.; Lip, G.Y. Non-vitamin K antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: A semi systematic review and meta-analysis of safety and efficacy outcomes. PLoS ONE 2014, 9, e114445. [Google Scholar] [CrossRef] [PubMed]
- Vedovati, M.C.; Germini, F.; Agnelli, G.; Becattini, C. Direct oral anticoagulants in patients with VTE and cancer: A systematic review and meta-analysis. Chest 2015, 147, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Posch, F.; Konigsbrugge, O.; Zielinski, C.; Pabinger, I.; Ay, C. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb. Res. 2015, 136, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Ryu, M.H.; Kang, Y.K.; Kim, K.P.; Chang, H.M.; Ryoo, B.Y.; Kim, S.B.; Lee, J.L.; Park, S.R. Oral rivaroxaban versus subcutaneous low molecular weight heparin treatment for venous thromboembolism in patients with upper gastrointestinal, hepatobiliary and pancreatic cancer. Ann. Oncol. 2016, 27 (Suppl. 6), 695. [Google Scholar] [CrossRef]
- Theberge, I.; Bowdridge, J.; Forgie, M.A.; Carrier, M.; Louzada, M.; Siquiera, L.; Rhodes, M.; Wells, P.S. Rivaroxaban shows promise as effective therapy for cancer patients with venous thromboembolic disease. Thromb. Res. 2017, 152, 4–6. [Google Scholar] [CrossRef]
- Ross, J.A.; Miller, M.M.; Rojas Hernandez, C.M. Comparative effectiveness and safety of direct oral anticoagulants (DOACs) versus conventional anticoagulation for the treatment of cancer-related venous thromboembolism: A retrospective analysis. Thromb. Res. 2017, 150, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Laube, E.; Miao, Y.; Sarasohn, D.M.; Parameswaran, R.; Stefanik, S.; Brar, G.; Samedy, P.; Wills, J.; Harnicar, S.; et al. Safe and effective use of rivaroxaban for treatment of cancer-associated venous thromboembolic disease: A prospective cohort study. J. Thromb. Thrombolysis 2017, 43, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Streiff, M.B.; Milentijevic, D.; McCrae, K.; Yannicelli, D.; Fortier, J.; Nelson, W.W.; Laliberte, F.; Crivera, C.; Lefebvre, P.; Schein, J.; et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am. J. Hematol. 2018, 93, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, L.G.; Haas, S.; Kreutz, R.; Folkerts, K.; Gebel, M.; Monje, D.; Schneider, J.; van Eickels, M.; Sahin, K.; Zell, E.; et al. Healthcare resource use in XALIA: A subgroup analysis of a non-interventional study of rivaroxaban versus standard anticoagulation for deep vein thrombosis. Eur. J. Intern. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2018. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific; Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor with Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef]
- Sobieraj, D.M.; Baker, W.L.; Smith, E.; Sasiela, K.; Trexler, S.E.; Kim, O.; Coleman, C.I. Anticoagulation for the Treatment of Cancer-Associated Thrombosis: A Systematic Review and Network Meta-Analysis of Randomized Trials. Clin. Appl. Thromb. Hemost. 2018. [Google Scholar] [CrossRef]
- Vedovati, M.C.; Giustozzi, M.; Bonitta, G.; Agnelli, G.; Becattini, C. Efficacy and safety of anticoagulant agents in patients with venous thromboembolism and cancer: A network meta-analysis. Thromb. Res. 2018, 170, 175–180. [Google Scholar] [CrossRef]
- Al Yami, M.S.; Badreldin, H.A.; Mohammed, A.H.; Elmubark, A.M.; Alzahrani, M.Y.; Alshehri, A.M. Direct oral anticoagulants for the treatment of venous thromboembolism in patients with active malignancy: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2018, 46, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov (accessed on 13 October 2018).
- Albert-Weil, J.; Nehorias, J. [Two cases of neoplasms not justified for classical therapy, treated with intravenous injections of heparin and intramuscular injections of leech extracts]. Rev. Pathol. Gen. Physiol. Clin. 1954, 54, 1014–1020. [Google Scholar] [PubMed]
- Zacharski, L.R.; Ornstein, D.L. Heparin and cancer. Thromb. Haemost. 1998, 80, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, B.; Chastang, C.; Brechot, J.M.; Capron, F.; Dautzenberg, B.; Delaisements, C.; Mornet, M.; Brun, J.; Hurdebourcq, J.P.; Lemarie, E. Subcutaneous heparin treatment increases survival in small cell lung cancer. “Petites Cellules” Group. Cancer 1994, 74, 38–45. [Google Scholar] [CrossRef]
- Smorenburg, S.M.; Hettiarachchi, R.J.; Vink, R.; Buller, H.R. The effects of unfractionated heparin on survival in patients with malignancy—A systematic review. Thromb. Haemost. 1999, 82, 1600–1604. [Google Scholar] [PubMed]
- Hettiarachchi, R.J.; Smorenburg, S.M.; Ginsberg, J.; Levine, M.; Prins, M.H.; Buller, H.R. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb. Haemost. 1999, 82, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Lensing, A.W.; Buller, H.R.; Carta, M.; Cogo, A.; Vigo, M.; Casara, D.; Ruol, A.; ten Cate, J.W. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet 1992, 339, 441–445. [Google Scholar] [CrossRef]
- Lazo-Langner, A.; Goss, G.D.; Spaans, J.N.; Rodger, M.A. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J. Thromb. Haemost. 2007, 5, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuderer, N.M.; Khorana, A.A.; Lyman, G.H.; Francis, C.W. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: Impact on survival and bleeding complications. Cancer 2007, 110, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Gunukula, S.; Barba, M.; Yosuico, V.E.; van Doormaal, F.F.; Kuipers, S.; Middeldorp, S.; Dickinson, H.O.; Bryant, A.; Schunemann, H. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011, CD006652. [Google Scholar]
- Lee, A.Y.; Rickles, F.R.; Julian, J.A.; Gent, M.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Levine, M.N. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J. Clin. Oncol. 2005, 23, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Smorenburg, S.M.; Van Noorden, C.J. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol. Rev. 2001, 53, 93–105. [Google Scholar] [PubMed]
- Mousa, S.A.; Petersen, L.J. Anti-cancer properties of low-molecular-weight heparin: Preclinical evidence. Thromb Haemost. 2009, 102, 258–267. [Google Scholar] [PubMed] [Green Version]
- Hu, L.; Lee, M.; Campbell, W.; Perez-Soler, R.; Karpatkin, S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood 2004, 104, 2746–2751. [Google Scholar] [CrossRef] [Green Version]
- Falanga, A. Biological and clinical aspects of anticancer effects of antithrombotics. Pathophysiol. Haemost. Thromb. 2003, 33, 389–392. [Google Scholar] [CrossRef]
- Lee, A.Y. The effects of low molecular weight heparins on venous thromboembolism and survival in patients with cancer. Thromb. Res. 2007, 120 (Suppl. 2), S121–S127. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yamamoto, Y.; Munesue, S.; Harashima, A.; Watanabe, T.; Yonekura, H.; Yamamoto, H.; Tsuchiya, H. Low molecular weight heparin suppresses receptor for advanced glycation end products-mediated expression of malignant phenotype in human fibrosarcoma cells. Cancer Sci. 2013, 104, 740–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzarotti, M.; Fontana, F.; Marras, C.; Boiardi, A.; Croci, D.; Ciusani, E.; Salmaggi, A. In vitro study of low molecular weight heparin effect on cell growth and cell invasion in primary cell cultures of high-grade gliomas. Oncol. Res. 2006, 16, 245–250. [Google Scholar] [CrossRef]
- Kakkar, A.K.; Levine, M.N.; Kadziola, Z.; Lemoine, N.R.; Low, V.; Patel, H.K.; Rustin, G.; Thomas, M.; Quigley, M.; Williamson, R.C. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS). J. Clin. Oncol. 2004, 22, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Altinbas, M.; Coskun, H.S.; Er, O.; Ozkan, M.; Eser, B.; Unal, A.; Cetin, M.; Soyuer, S. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J. Thromb. Haemost. 2004, 2, 1266–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klerk, C.P.; Smorenburg, S.M.; Otten, H.M.; Lensing, A.W.; Prins, M.H.; Piovella, F.; Prandoni, P.; Bos, M.M.; Richel, D.J.; van Tienhoven, G.; et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. 2005, 23, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Sideras, K.; Schaefer, P.L.; Okuno, S.H.; Sloan, J.A.; Kutteh, L.; Fitch, T.R.; Dakhil, S.R.; Levitt, R.; Alberts, S.R.; Morton, R.F.; et al. Low-molecular-weight heparin in patients with advanced cancer: A phase 3 clinical trial. Mayo Clin. Proc. 2006, 81, 758–767. [Google Scholar] [CrossRef]
- Agnelli, G.; Gussoni, G.; Bianchini, C.; Verso, M.; Mandala, M.; Cavanna, L.; Barni, S.; Labianca, R.; Buzzi, F.; Scambia, G.; et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009, 10, 943–949. [Google Scholar] [CrossRef]
- Perry, J.R.; Julian, J.A.; Laperriere, N.J.; Geerts, W.; Agnelli, G.; Rogers, L.R.; Malkin, M.G.; Sawaya, R.; Baker, R.; Falanga, A.; et al. PRODIGE: A randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J. Thromb. Haemost. 2010, 8, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Van Doormaal, F.F.; Di Nisio, M.; Otten, H.M.; Richel, D.J.; Prins, M.; Buller, H.R. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J. Clin. Oncol. 2011, 29, 2071–2076. [Google Scholar] [CrossRef]
- Agnelli, G.; George, D.J.; Kakkar, A.K.; Fisher, W.; Lassen, M.R.; Mismetti, P.; Mouret, P.; Chaudhari, U.; Lawson, F.; Turpie, A.G.; et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N. Engl. J. Med. 2012, 366, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.K.; Freund, M.; Heigener, D.; Heilmann, L.; Kemkes-Matthes, B.; von Tempelhoff, G.F.; Melzer, N.; Kakkar, A.K.; Investigators, T. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin. Appl. Thromb. Hemost. 2012, 18, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Maraveyas, A.; Waters, J.; Roy, R.; Fyfe, D.; Propper, D.; Lofts, F.; Sgouros, J.; Gardiner, E.; Wedgwood, K.; Ettelaie, C.; et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur. J. Cancer 2012, 48, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, R.; Lopez Vivanco, G.; Font, A.; Gonzalez Billalabeitia, E.; Gurpide, A.; Gomez Codina, J.; Isla, D.; Galan, A.; Bover, I.; Domine, M.; et al. Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: Results from the ABEL study. Thromb. Res. 2013, 132, 666–670. [Google Scholar] [CrossRef]
- Macbeth, F.; Noble, S.; Evans, J.; Ahmed, S.; Cohen, D.; Hood, K.; Knoyle, D.; Linnane, S.; Longo, M.; Moore, B.; et al. Randomized Phase III Trial of Standard Therapy Plus Low Molecular Weight Heparin in Patients With Lung Cancer: FRAGMATIC Trial. J. Clin. Oncol. 2016, 34, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Meyer, G.; Besse, B.; Doubre, H.; Charles-Nelson, A.; Aquilanti, S.; Izadifar, A.; Azarian, R.; Monnet, I.; Lamour, C.; Descourt, R.; et al. Anti-tumour effect of low molecular weight heparin in localised lung cancer: A phase III clinical trial. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef]
- Sanford, D.; Naidu, A.; Alizadeh, N.; Lazo-Langner, A. The effect of low molecular weight heparin on survival in cancer patients: An updated systematic review and meta-analysis of randomized trials. J. Thromb. Haemost. 2014, 12, 1076–1085. [Google Scholar] [CrossRef]
- Che, D.H.; Cao, J.Y.; Shang, L.H.; Man, Y.C.; Yu, Y. The efficacy and safety of low-molecular-weight heparin use for cancer treatment: A meta-analysis. Eur. J. Intern. Med. 2013, 24, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Kahale, L.A.; Ballout, R.A.; Barba, M.; Yosuico, V.E.; van Doormaal, F.F.; Middeldorp, S.; Bryant, A.; Schunemann, H. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst. Rev. 2014, CD006652. [Google Scholar] [CrossRef]
- Borsig, L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb. Res. 2010, 125 (Suppl. 2), S66–S71. [Google Scholar] [CrossRef] [Green Version]
- Alberio, L. The new direct oral anticoagulants in special indications: Rationale and preliminary data in cancer, mechanical heart valves, anti-phospholipid syndrome, and heparin-induced thrombocytopenia and beyond. Semin. Hematol. 2014, 51, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, M. The coagulant response in sepsis and inflammation. Hamostaseologie 2010, 30, 4–6. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Cedervall, J. NETosis in Cancer—Platelet-Neutrophil Crosstalk Promotes Tumor-Associated Pathology. Front. Immunol. 2016, 7, 373. [Google Scholar] [CrossRef]
- Rasmussen, U.B.; Vouret-Craviari, V.; Jallat, S.; Schlesinger, Y.; Pages, G.; Pavirani, A.; Lecocq, J.P.; Pouyssegur, J.; Van Obberghen-Schilling, E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991, 288, 123–128. [Google Scholar] [CrossRef]
- Vu, T.K.; Hung, D.T.; Wheaton, V.I.; Coughlin, S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64, 1057–1068. [Google Scholar] [CrossRef]
- Coughlin, S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005, 3, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, K.; Thomas, S.N. Cancer cells in transit: The vascular interactions of tumor cells. Annu. Rev. Biomed. Eng. 2009, 11, 177–202. [Google Scholar] [CrossRef]
- Tsopanoglou, N.E.; Maragoudakis, M.E. Thrombin’s central role in angiogenesis and pathophysiological processes. Eur. Cytokine Netw. 2009, 20, 171–179. [Google Scholar]
- Borensztajn, K.; Peppelenbosch, M.P.; Spek, C.A. Factor Xa: At the crossroads between coagulation and signaling in physiology and disease. Trends Mol. Med. 2008, 14, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Hempel, D.; Sierko, E.; Tucker, S.C.; Honn, K.V. Protease-activated receptors (PARs)—Biology and role in cancer invasion and metastasis. Cancer Metast. Rev. 2015, 34, 775–796. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Hempel, D.; Sierko, E.; Tucker, S.C.; Honn, K.V. Antiplatelet agents for cancer treatment: A real perspective or just an echo from the past? Cancer Metast. Rev. 2017, 36, 305–329. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, C.; Caliskan, A.; Karahan, O.; Yazici, S.; Guclu, O.; Demirtas, S.; Mavitas, B. Investigation of the antiangiogenic behaviors of rivaroxaban and low molecular weight heparins. Blood Coagul. Fibrinol. 2014, 25, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Targeting factor Xa and thrombin: Impact on coagulation and beyond. Thromb. Haemost. 2014, 111, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Pearlstein, E.; Ambrogio, C.; Gasic, G.; Karpatkin, S. Inhibition of the platelet-aggregating activity of two human adenocarcinomas of the colon and an anaplastic murine tumor with a specific thrombin inhibitor: Dansylarginine N-(3-ethyl-1, 5-pentanediyl)amide. Prog. Clin. Biol. Res. 1982, 89, 479–502. [Google Scholar] [PubMed]
- Nieman, M.T.; LaRusch, G.; Fang, C.; Zhou, Y.; Schmaier, A.H. Oral thrombostatin FM19 inhibits prostate cancer. Thromb. Haemost. 2010, 104, 1044–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFeo, K.; Hayes, C.; Chernick, M.; Ryn, J.V.; Gilmour, S.K. Use of dabigatran etexilate to reduce breast cancer progression. Cancer Biol. Ther. 2010, 10, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Vianello, F.; Sambado, L.; Goss, A.; Fabris, F.; Prandoni, P. Dabigatran antagonizes growth, cell-cycle progression, migration, and endothelial tube formation induced by thrombin in breast and glioblastoma. Cancer Med. 2016, 5, 2886–2898. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.T.; Minton, A.R.; Hayes, C.S.; Goss, A.; Van Ryn, J.; Gilmour, S.K. Thrombin inhibition and cyclophosphamide synergistically block tumor progression and metastasis. Cancer Biol. Ther. 2015, 16, 1802–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, A.; Marquis, K.; et al. Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
| DOACs vs. LMWHs | Diagnosis for Inclusion | Design, Phase, Estimated Number of Included Patients | Name of the Study | NCT, State, Estimated Completion Date |
|---|---|---|---|---|
| Rivaroxaban | ||||
| vs. low molecular weight heparins (LMWHs) | Venous Thromboembolism (VTE) | RCT phase III 450 | CONKO_011/AIO-SUP-0115/Ass.: Rivaroxaban in the Treatment of Venous Thromboembolism (VTE) in Cancer Patients—a Randomized Phase III Study | 02583191 Recruiting December 2018 |
| Rivaroxaban only | Pulmonary embolism (PE) and Deep Vein Thrombosis (DVT) | Prospective phase III 500 | A Non-interventional Study on Xarelto for Treatment of Venous Thromboembolism (VTE) and Prevention of Recurrent VTE in Patients with Active Cancer (COSIMO) | 02742623 Active, not recruiting 15.03.2019 |
| Rivaroxaban only | PE and DVT of upper and lower extremities | Retrospective NA 375 | Rivaroxaban Utilization for Treatment and Prevention of Thromboembolism in Cancer Patients: Experience at a Comprehensive Cancer Center | 02502396 Active, not recruiting September 2020 |
| vs. LMWHs | VTE | RCT 200 | Cancer Associated Thrombosis, a Pilot Treatment Study Using Rivaroxaban (CASTA-DIVA) | 02746185 Completed 25.04.2018 |
| vs. Dalteparin | VTE | RCT phase II 176 | A Randomized Phase II Study to Compare the Safety and Efficacy of Dalteparin vs. Rivaroxaban for Cancer-associated Venous Thromboembolism (PRIORITY) | 03139487 Recruiting May 2020 |
| Apixaban | ||||
| vs. LMWHs | VTE | RCT phase III 1168 | Apixaban for the Treatment of Venous Thromboembolism in Patients with Cancer (CARAVAGGIO) | 03045406 Recruiting June 2019 |
| vs. Dalteparin | VTE | RCT Phase III 315 | Apixaban or Dalteparin in Reducing Blood Clots in Patients with Cancer Related Venous Thromboembolism (ADAM VTE) | 02585713 Completed November 2018 |
| Apixaban only | Venous Thrombosis | Single Group Assignment 300 | Apixaban as Treatment of Venous Thrombosis in Patients with Cancer: The CAP Study (CAP) | 02581176 Completed May 2018 |
| Dabigatran | ||||
| vs. tinzaparin | VTE | Phase III 99 | A Study of Dabigatran Etexilate as Primary Treatment of Malignancy Associated Venous Thromboembolism | 03240120 Recruiting 31.12.2021 |
| All DOACS (apixaban, edoxaban, dabigatran, rivaroxaban) | ||||
| vs. LMWHS alone or with warfarin | VTE (cumulative recurrence) | RCT NA 940 | Direct oral anticoagulants (DOACs) vs. LMWH+/−warfarin for VTE in cancer: a randomized effectiveness trial (CANVAS TriaL) | 02744092 Recruiting September 2019 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandoni, F.; Alberio, L. Direct Oral Anticoagulant Drugs: On the Treatment of Cancer-Related Venous Thromboembolism and their Potential Anti-Neoplastic Effect. Cancers 2019, 11, 46. https://doi.org/10.3390/cancers11010046
Grandoni F, Alberio L. Direct Oral Anticoagulant Drugs: On the Treatment of Cancer-Related Venous Thromboembolism and their Potential Anti-Neoplastic Effect. Cancers. 2019; 11(1):46. https://doi.org/10.3390/cancers11010046
Chicago/Turabian StyleGrandoni, Francesco, and Lorenzo Alberio. 2019. "Direct Oral Anticoagulant Drugs: On the Treatment of Cancer-Related Venous Thromboembolism and their Potential Anti-Neoplastic Effect" Cancers 11, no. 1: 46. https://doi.org/10.3390/cancers11010046
APA StyleGrandoni, F., & Alberio, L. (2019). Direct Oral Anticoagulant Drugs: On the Treatment of Cancer-Related Venous Thromboembolism and their Potential Anti-Neoplastic Effect. Cancers, 11(1), 46. https://doi.org/10.3390/cancers11010046





