1. Introduction
Proton therapy, especially intensity-modulated proton therapy (IMPT) [
1,
2], has the potential to deliver higher doses to the target while keeping the doses to organs at risk (OARs) below tolerance levels [
3]. However, the sharp dose falloff after the pristine Bragg peak of proton beams makes proton dose distribution very sensitive to uncertainties introduced by the anatomic variations in the paths of the proton beams. There are many sources of such uncertainties. Three of the most pertinent ones to proton dose distributions for lung cancer patients are: (1) setup uncertainty (i.e., uncertainty introduced by lack of reproducibility of patient positioning and alignment); (2) range uncertainty (introduced by uncertainty in CT numbers and in the process of converting CT numbers to stopping powers); and (3) uncertainties introduced by physiological movements, which, for lung patients, is predominantly due to respiratory motion.
In intensity modulated photon radiation therapy (IMRT), the planning target volume (PTV) concept is used to account for setup uncertainties and respiratory motion for IMRT. PTV is defined by adding a site-specific empirically-determined margin to the internal target volume (ITV), which is, in turn, defined by adding a margin for target motion to the clinical target volume (CTV). The validity of the PTV concept in photon radiation therapy is based on the general observation that X-ray dose distributions behave essentially like a “dose cloud” around the target and are not perturbed significantly by changes in anatomy along the paths of radiation beams. In this so-called “static dose cloud approximation,” the CTV can be assumed to reside within the PTV and receive the prescribed dose with high probability (e.g., 95–98%) over the course of radiotherapy.
The conventional PTV concept, which is a simple geometrical expansion of the CTV, is not extensible to proton therapy, where anatomical misalignment with respect to the planning CT can lead to significant distortion of the dose distribution not only at the edges of the planning target volume but also inside the planning target [
4,
5]. Shifts in patient anatomy along the beam direction have the minimal effect on proton dose distributions, but there is uncertainty in the range of protons, which depends on the depth of the point of interest. In the current practice of designing treatment plans for passively scattered proton therapy (PSPT), the PTV concept is extended to define beam-specific PTVs in which the lateral margins are the same as the conventional margins assigned to the CTV; however, proximal and distal margins for each beam are set equal to depth-dependent estimates of the range uncertainty. In addition, beam-specific aperture and compensator smearing is used to make the plan robust [
6,
7,
8]. For lack of better methods, PSPT plans are still evaluated for clinical appropriateness based on the conventional PTV concept.
The conventional PTV concept is even less appropriate for IMPT for which dose distributions of individual beams are highly heterogeneous. Park et al. [
9] proposed a ray-tracing and ray-shifting method to design a beam specific PTV (bs-PTV) which works for the single field uniform dose (SFUD) optimization (also called single field optimization or SFO IMPT). This approach leads to larger target volumes and the CTV coverage is still less than satisfactory.
For multi-field optimized intensity modulated proton therapy (MFO-IMPT), the dose distributions from multiple fields are combined to form a uniform dose distribution in the target and to achieve a balanced sparing of normal tissues. Small variations in anatomy can significantly perturb the matched composite dose distribution. Thus, IMPT is highly vulnerable to motion and other uncertainties. Furthermore, because of the highly heterogeneous per-beam dose distributions of MFO-IMPT and the fact each individual beam would not, in general, cover the whole target, the beam-specific PTV-based approach is not applicable to MFO-IMPT. These factors necessitate that IMPT planning employ robust optimization approaches to make its dose distributions more resilient in the face of uncertainties.
A variety of three-dimensional robust optimization (3DRO) methods for IMPT have been proposed. These include probabilistic treatment planning [
10,
11], voxel-wise worst-case robust optimization [
12,
13] and scenario-wise mini-max robust optimization [
14]. The probabilistic optimization method optimizes treatment plans based on a large number of dose distributions produced by randomly sampling setup and range uncertainty scenarios with an assumed probability distribution. An alternative approach is to sample a limited number of worst-case scenarios to model the effect of uncertainties in IMPT planning. A voxel-wise worst-case robust optimization method and a scenario-wise robust optimization method have been proposed to avoid the large-scale computations of the probabilistic approach by recalculating dose distributions for only a limited number of worst-case scenarios. In addition to the advantage of not requiring very large sets of dose calculations, another advantage of the worst-case approach over the probabilistic method is that the knowledge of detailed probability distributions for setup and range uncertainties is not required.
The voxel-wise worst-case robust optimization method [
12,
13] generates a single worst-case dose distribution derived from dose distributions for multiple uncertainty scenarios. For a voxel inside the target volume, the minimum from all scenarios is taken to be the worst-case dose. For a normal tissue voxel, the maximum is assumed to be the worst-case dose. During optimization, only one objective function based on the worst-case dose distribution is evaluated and optimized. In contrast, the mini-max worst-case robust optimization approach [
14] evaluates the objective functions for all uncertainty scenarios, selects the worst objective function score in each optimization iteration and minimizes it.
The robust optimization methods mentioned above consider only the setup and range uncertainties. They do not address the respiratory motion. The lack of optimization methods that explicitly account for respiratory motion may have been one of the inhibiting factors in the use of IMPT for lung cancer patients. One way to mitigate the impact of respiratory motion is to use respiratory gating or breath-hold; however, this introduces concerns related to prolongation of treatment, inter- and intra-fractional reproducibility of internal anatomy and patient tolerance. Another proposed approach to account for respiratory motion is the tracking of the moving target with scanning particle beamlets [
15,
16,
17]. However, online motion tracking and synchronization is technically highly challenging [
18,
19].
In another approach, to explicitly account for the respiratory motion in IMPT, Graeff et al. proposed the idea of 4D optimization to achieve target dose coverage at each respiratory phase for heavy ion treatments [
19,
20]. They calculated water-equivalent path length (WEPL) for each breathing phase and set field-specific margin in WEPL coordinate system to account for range uncertainties introduced by respiratory motion. However, setup uncertainties and uncertainties in range due to CT number statistical fluctuations, the conversion of CT numbers to proton stopping power ratios and the artifacts in the CT image were ignored in their method. The main challenge for their method would be accounting for setup uncertainty in the WEPL coordinate system. If a rigid margin for ITV or CTV in each phase is used to account for setup uncertainties, dose deterioration would be introduced by setup uncertainties, similar to the situation of PTV based 3D IMPT optimization. Graeff et al. provided a useful solution to account for only breathing motion. However, they did not provide a robust way to simultaneously solve the dose deteriorations introduced by breathing motion, range uncertainty and setup uncertainties at the same time, which are fully provided in our current study. Recently, Cummings et al. also conducted a study related to the phase-based optimization, but they did not provide details of their algorithm [
21]. It is unclear how the breathing motion, setup and range uncertainties were simultaneously processed. Furthermore, only dose statistical data for different phases were presented in their study. It is unclear how the dose uncertainties introduced by setup and range uncertainties were evaluated.
Liu et al. have also proposed a 4DRO strategy [
22]. Dose distributions for all respiratory phases were deformed to a reference phase, e.g., the end-exhale phase, and summed together to form the cumulative 4D dose distribution. Such 4D dose distributions for different setup and range uncertainty scenarios were then calculated. A voxel-wise worst-case robust optimization method [
12,
13] was applied to the cumulative 4D dose distributions to achieve conformal target coverage and normal tissue sparing. The approach by Liu et al. can generate the appropriate robustness for cumulative 4D dose distribution. However, since it does not explicitly optimize dose distribution based on individual respiratory phases, it is not clear whether it is able to ensure coverage and robustness in each phase individually. Their approach may be more sensitive to variability in breathing patterns than the approach we have adopted for the present study. In our approach, IMPT dose distributions for each of the individual respiratory phases are simultaneously optimized to account for the respiratory motion while incorporating setup and range uncertainties.
It should be noted that, while the primary focus of our research is multi-field optimized IMPT, the methodology developed is appropriate for single-field optimized IMPT as well. In this paper, from now on, by the term IMPT, we mean multi-field IMPT, in which intensity distributions of all beams are simultaneously optimized to achieve the best approximation of the specified dosimetric objectives.
2. Results
Ten non-small cell lung cancer (NSCLC) patients with wide ranges of tumor sizes, locations and motion magnitudes (
Table 1) were used to evaluate the potential of our 4DRO method. All three optimization methods (PTV-based, 3DRO and 4DRO) were applied to each of the ten patients. For each patient, after optimization, 4D dose distributions were also computed for plans produced by 3DRO and PTV-based optimization. Some of key calculation results are reported in this section.
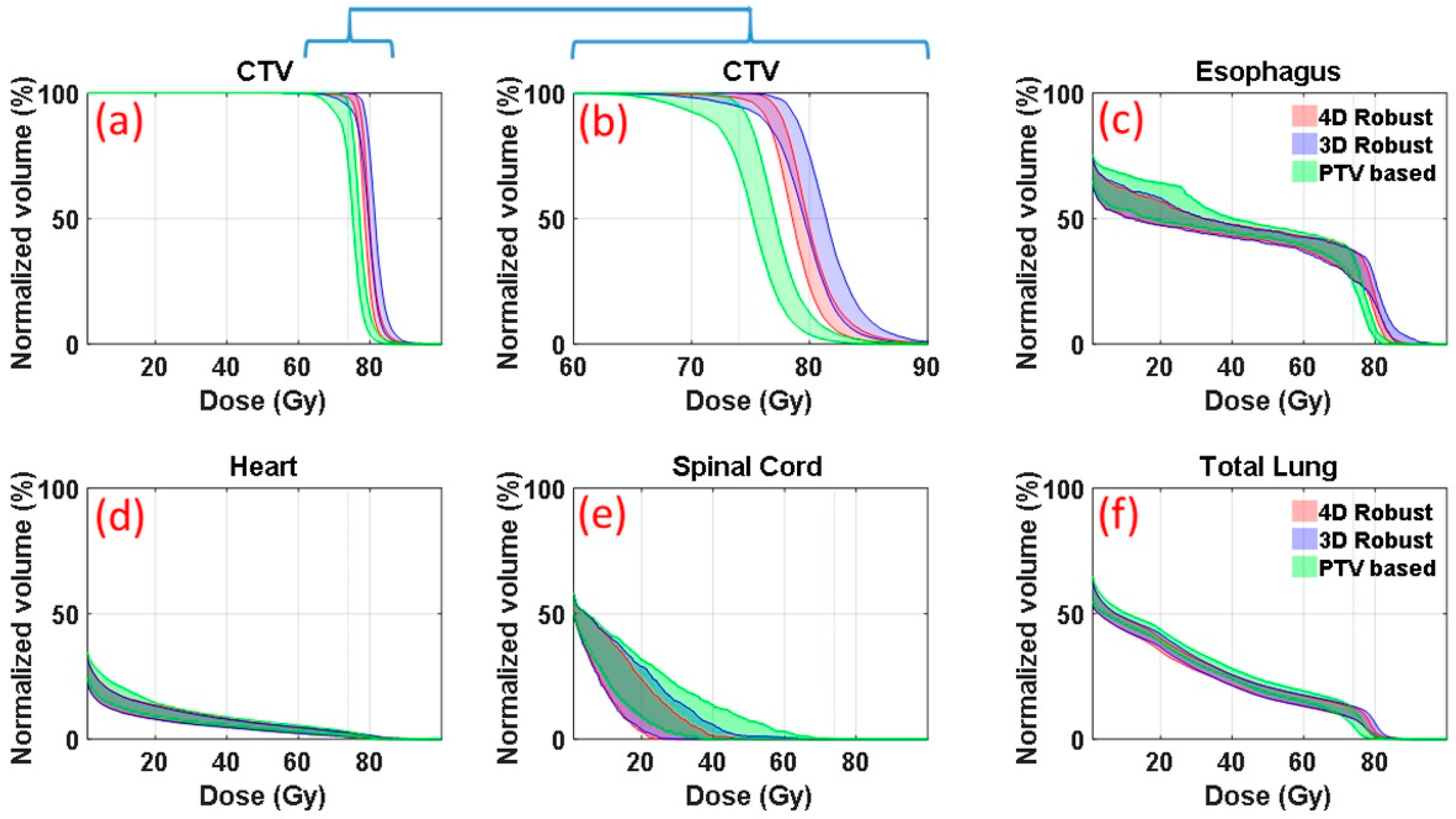
Figure 1 is an illustrative example (patient E in
Table 1 in the section of Materials and Methods, medium motion and medium ITV size) of DVH bands of anatomic structures of interest. DVHs were derived from the 90 dose distributions (nine uncertainty scenarios for each of the ten respiratory phases) for patient’s plans optimized with the 4DRO (red), 3DRO (blue) and PTV-based (green) optimization approaches. For 3DRO and PTV-based optimization approaches, dose distributions were recalculated for each of the ten respiratory phases using the optimized set of beamlet intensities for each of the nine uncertainty scenarios.
Figure 1b shows the same CTV data as in
Figure 1a but on an expanded dose scale. The upper and lower bounds of the bands are highlighted by solid lines. The lower bounds of the CTV bands indicate the worst-case CTV coverage; whereas the upper bounds of the OARs show the worst-case normal tissue sparing. The band widths of CTV DVHs at critical dose and volume points (e.g., at
D95%) quantitatively indicate the robustness of dose distributions—the narrower the band the more robust is the dose distribution. 4DRO is able to provide both superior robustness and superior CTV coverage in the face of respiratory motion compared to 3DRO. For normal structure DVHs (
Figure 1c–f), the lower bounds are not of importance since typical optimizers do not strive to lower the dose any further once the specified criteria have been met. The results in
Figure 1 indicate that both 4DRO and 3DRO are superior with respect to CTV robustness and coverage as well as normal tissue sparing compared to the PTV-based optimization.
It is interesting to point out that, for this patient, 4DRO achieved improvements in the target coverage without compromising normal tissue sparing compared to 3DRO. Compared to PTV-based optimization, both 3DRO and 4DRO show modest improvement in sparing for heart and total lung and substantially increased sparing for spinal cord.
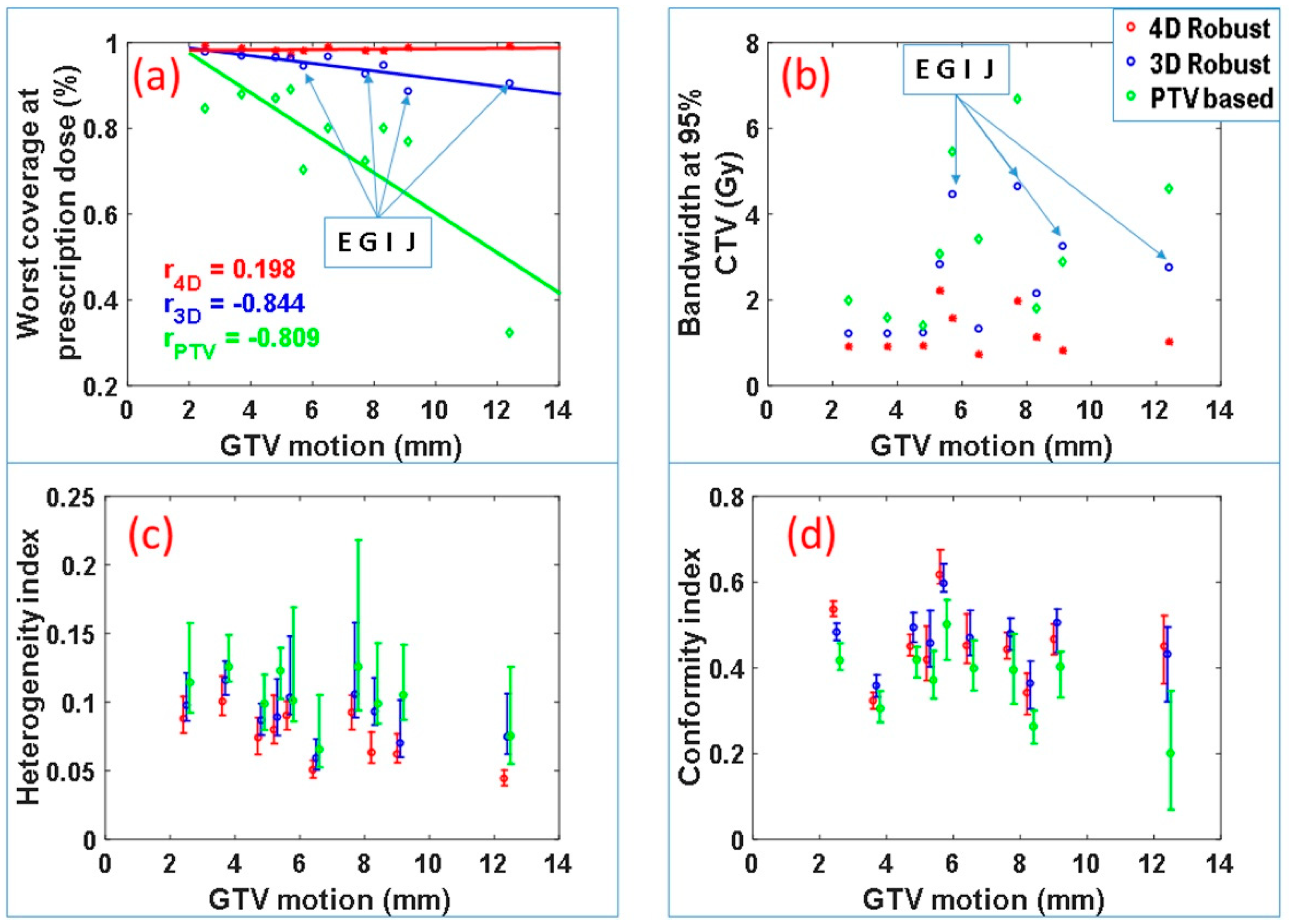
Figure 2 summarizes the CTV coverage and robustness data for all of the ten patients. The worst-case CTV coverage and the bandwidth of DVH bands at 95% of the CTV for the IMPT plans produced by the three optimization approaches are displayed in
Figure 1a,b. The plans produced by the 4DRO approach appear to have the best CTV coverage and narrowest bandwidth. A one-sided paired
t test reveals that, for this patient cohort, 4DRO significantly improves the target coverage compared to 3DRO (
p = 0.0025) as well as PTV-based optimization (
p = 0.0012). The paired
t test also finds that 4DRO significantly improves plan robustness compared to 3DRO (
p = 0.0018) and PTV-based optimization (
p = 0.0011) based on values of the DVH bandwidth at 95% of CTV. For the 3DRO and PTV-based optimization plans, negative correlations were found between CTV coverage and GTV motion magnitudes (correlation coefficients −0.844 and −0.809 respectively—note that the absolute value of correlation coefficient larger than 0.5 means significant correlation and less than 0.5 means no significant correlation), which implies that the CTV coverage of the plan optimized by 3DRO and PTV-based optimization methods degrades as GTV motion magnitude increases. However, for the plans produced by 4DRO, the correlation coefficient is only 0.19. The small magnitude of correlation coefficient indicates that the plans produced by 4DRO has good CTV coverage regardless of GTV motion magnitude.
We found that, for patients E, G, I and J, the diaphragm overlapped, to varying degrees, with the ITV from one or more beams eye view, which means the diaphragm intrudes in the path of one or more proton beams. It is important to note that, even for these cases, 4DRO is able to improve target coverage and plan robustness to a greater degree compared to 3DRO. Among these four patients, the worst-case CTV coverage of plans produced by 3DRO is relatively low: 94% and 93% for patient E and G, and approximately 90% for patients I and J. The worst-case CTV coverage of plans produced by 4DRO is above 98% for all cases. This important finding is presumably due to the fact that 4DRO is able to explicitly compensate for the dose perturbation caused by the diaphragm incursion.
Figure 2c displays the median values and the ranges of the target dose heterogeneity index (HI) for all 90 scenarios for each patient. The HI for 4DRO is significantly lower (i.e., has superior dose homogeneity) than for 3DRO and PTV-based optimization (
p < 0.001 for minimum, maximum and median HI). Superiority of HI for 4DRO compared to 3DRO implies that 4DRO is able to compensate for perturbations of dose distributions inside the target boundaries.
Figure 2d displays conformity indices for the three optimization methods for the patient cohort. Both 4DRO and 3DRO produced dose distributions with significantly superior conformity indices than the PTV-based method (
p < 0.05). However, the conformity indices of dose distributions produced by 4DRO and 3DRO were not significantly different, which is logical since the ITV used for 3DRO is a composite envelope of CTVs used for 4DRO.
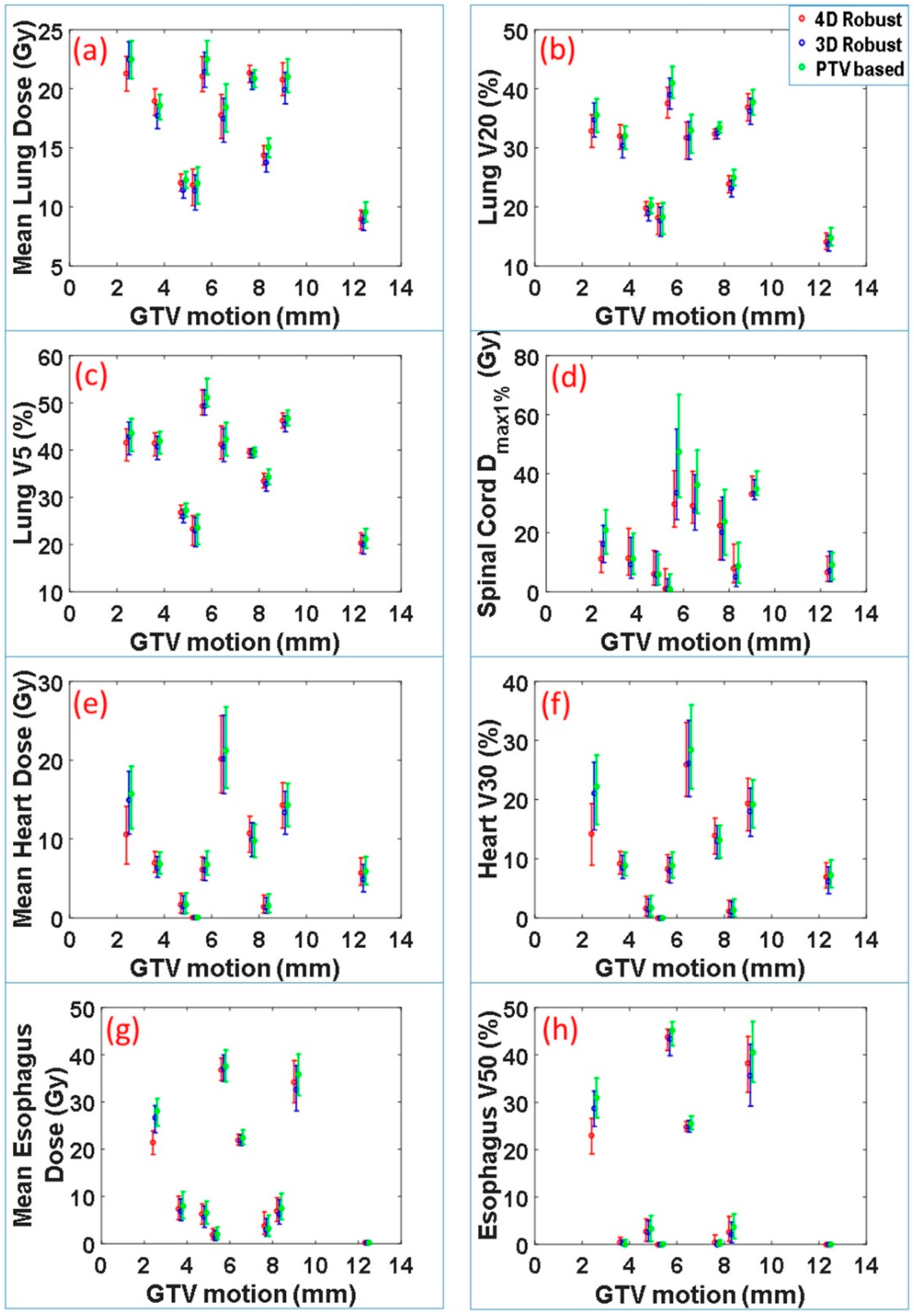
The dose-volume indices for critical organs at risk were also calculated. These included maximum dose received by 1% volume of spinal cord
Dmax1%, mean lung dose, lung V20, lung V5, mean heart dose, heart V30, mean esophagus dose and esophagus V50. The range and median values of these indices for 90 scenarios vs. motion magnitude for each patient are displayed in
Figure 3. Normal tissue sparing was not significantly different between the 4DRO and 3DRO plans. However, both 4DRO and 3DRO plans demonstrated significantly superiority in the sparing of critical organs compared to PTV-based optimization (
p < 0.05). These data indicate that the robust optimization is able to achieve superior target coverage and robustness without compromising normal tissue sparing.
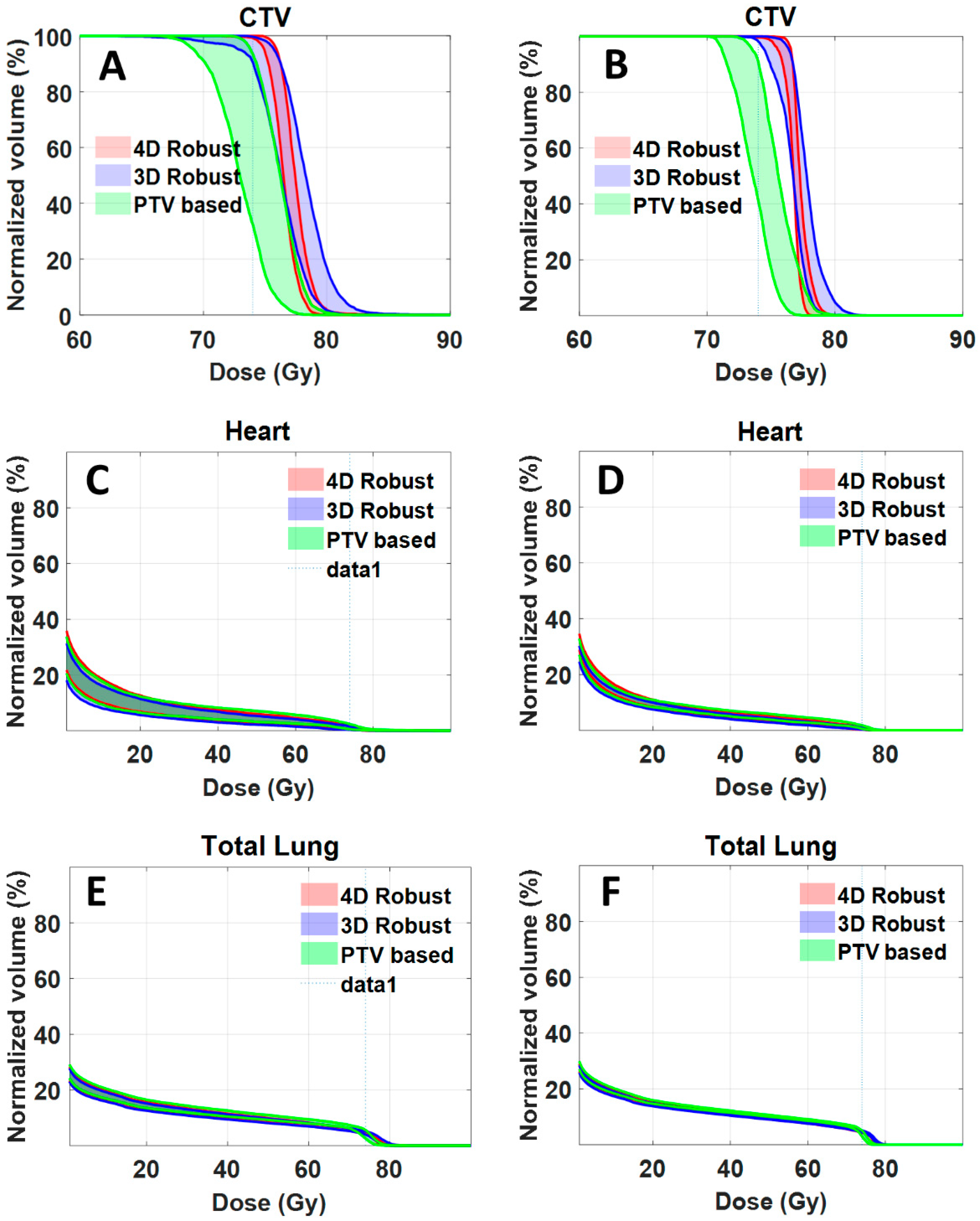
For clinical implementation, it would be necessary to compute and evaluate dose distributions accumulated over all respiratory phases. As an illustrative example, we calculated accumulated dose distribution for one of the patients (patient J).
Figure 4A,C,E shows the DVH bands of 90 dose distributions for CTV, heart and total lung. The bandwidths at 95% CTV for 4DRO, 3DRO and PTV-based optimization was 1.017, 2.756 and 4.6 Gy respectively. The worst-case CTV coverage at prescription dose for 4DRO, 3DRO and PTV-based optimization was 99.2%, 90.5% and 32.5%.
Figure 4B,D,F shows the DVH bands of accumulated dose distributions for the nine setup and range uncertainty scenarios for CTV, heart and total lung. The bandwidths at 95% CTV for 4DRO, 3DRO and PTV-based optimization was 1.017, 1.837 and 2.4 Gy respectively, whereas the worst-case CTV coverage at prescription dose 99.7%, 98.1% and 41.0% respectively. It is interesting that the bandwidth of dose distributions gets narrower and the worst-case dose coverage of CTV improves for all three optimization approaches when dose is accumulated over all respiratory phases. This is presumably due to the averaging effect of dose accumulation. Note that the bandwidth of dose distributions of OARs (heart and total lung) is also narrower. It is obvious that, compared to accumulated dose distributions, the worst case dose distribution from all respiratory phases is more conservative.
3. Discussion
It is important to point out that, regardless of the optimization approach (4D robust, 3D robust or PTV-based), the resulting dose distributions for CTVs and organs at risk must be evaluated and compared using the same process, which, in the present work, was in terms of bands of DVHs derived from families of dose distributions for multiple setup and range uncertainty scenarios for each of the ten phases of the respiratory cycle. For all of the ten patients examined, IMPT plans using the 4DRO method provided superior target dose robustness (band widths) and worst-case coverage compared with those using 3DRO. Both 4DRO and 3DRO plans had superior target coverage and plan robustness compared to PTV-based optimization plans. The degree of difference was found to depend on the magnitude of tumor motion and the location of the tumor relative to the diaphragm. Target coverage decreased with an increase in tumor motion for 3DRO and PTV-based optimization. An interesting finding was that, for the patients for whom the diaphragm encroaches into the proton beam paths, 4DRO is especially important. The reason for the superiority of 4D over 3D for such cases is presumably due to the fact that every phase of the respiratory motion is explicitly considered in the 4DRO process. The target coverage and normal tissue sparing is achieved for each phase independently. In our 4DRO approach, since the dose distributions for all uncertainty scenarios were computed for all phases of respiratory motion, the dose perturbation caused by motion-induced anatomy changes is explicitly considered by the optimization process. Thus, regardless of the magnitude of motion and even with the intrusion of the diaphragm into the proton beam path, our 4DRO approach is able to maintain the required target coverage, robustness and dose homogeneity.
As indicated above, plans generated using the traditional PTV-based optimization method do not provide optimal target coverage, robustness and normal tissue sparing. This observation can be attributed to: (1) the patient anatomy is approximated by the average CT image, (2) setup uncertainty is approximated by simply adding a safety margin to ITV to create the PTV, and (3) respiratory motion is accounted for by defining an ITV as a static composite of CTVs on all respiratory phases. It is well known that changes in the spatial density in the beam path can cause a proton beam to undershoot or overshoot and, thus, degrade target coverage and/or deposit excessive dose outside the target. Nevertheless, this may not be apparent unless the dose distributions are examined for each respiratory phase under multiple uncertainty scenarios.
Compared with the PTV-based optimization, 3DRO improves plan robustness by explicitly including setup and range uncertainties. In this mode, the patient anatomy information is approximated with average CT images, and tumor motion is incorporated in the same manner as for the PTV-based method, i.e., by defining a static ITV. Thus, anatomy changes during respiratory motion are ignored. This could, in actuality, lead to under-dosing of the target and an increase in the heterogeneity of the target dose distribution.
In our 4DRO approach, since the dose distribution at each respiratory phase of 4D CT datasets is optimized simultaneously (see Equations (3) and (4) in the section of Materials and Methods), instead of being optimized with the use of the cumulative dose distribution as proposed by Liu, et al. [
22], the dose distribution of the final plan in the current study is independent of the patient’s breathing pattern. Furthermore, our method is robust even if the patient does not breathe consistently from fraction to fraction over the treatment course. The per-phase dose distributions in our method follow the tumor motion in each respiratory phase during optimization (see Equation (3) below). When the patient’s breathing pattern changes (i.e., the time that the tumor stays in certain phases of respiratory motion changes), the final dose distributions in our method can continue to cover the target as long as the patient’s breathing amplitude does not change substantially. This assertion, however, needs to be tested with further studies, the results of which will be reported in future publications.
Another important finding is that the 4DRO is able to reduce the intra-target dose heterogeneities compared to 3DRO. The 4DRO explicitly compensates for perturbations caused by the respiratory motion. This is indicated by the observations that the heterogeneity index for 4DRO is superior compared to the 3DRO while the conformity indices are similar in these two approaches.
One of the limitations of our method is that we have not considered the effect of the interplay of spot scanning with respiratory motion, which is often cited as a concern for IMPT delivery. Studies have shown that the interplay effect does not significantly affect cumulative dose distribution over multiple fractions; although it causes deterioration of single fraction dose distribution [
23,
24,
25,
26,
27]. For large spot sizes, the delivered 4D dose of the multi-fraction IMPT treatment plan may be reliable despite the presence of the interplay effect [
24,
25]. It should be noted that, for the majority of previously reported studies involving the interplay effect, the plans were optimized using traditional ITV and PTV-based optimization methods. Dose errors discussed in these studies arise from two sources: (1) introduced by the pure interplay effect [
23], and (2) introduced by the respiratory motion. Our 4DRO approach described here does not address the dose error due to the pure interplay effect, only that introduced by respiratory motion. However, our yet to be tested hypothesis is that the interplay effect may be incidentally reduced due to the reduction in dose gradients inherent in 4DRO. It may also be reduced by the application of spot pattern repainting [
25,
28] or spot delivery sequence optimization [
29].