The Endometriotic Tumor Microenvironment in Ovarian Cancer
Abstract
1. Introduction
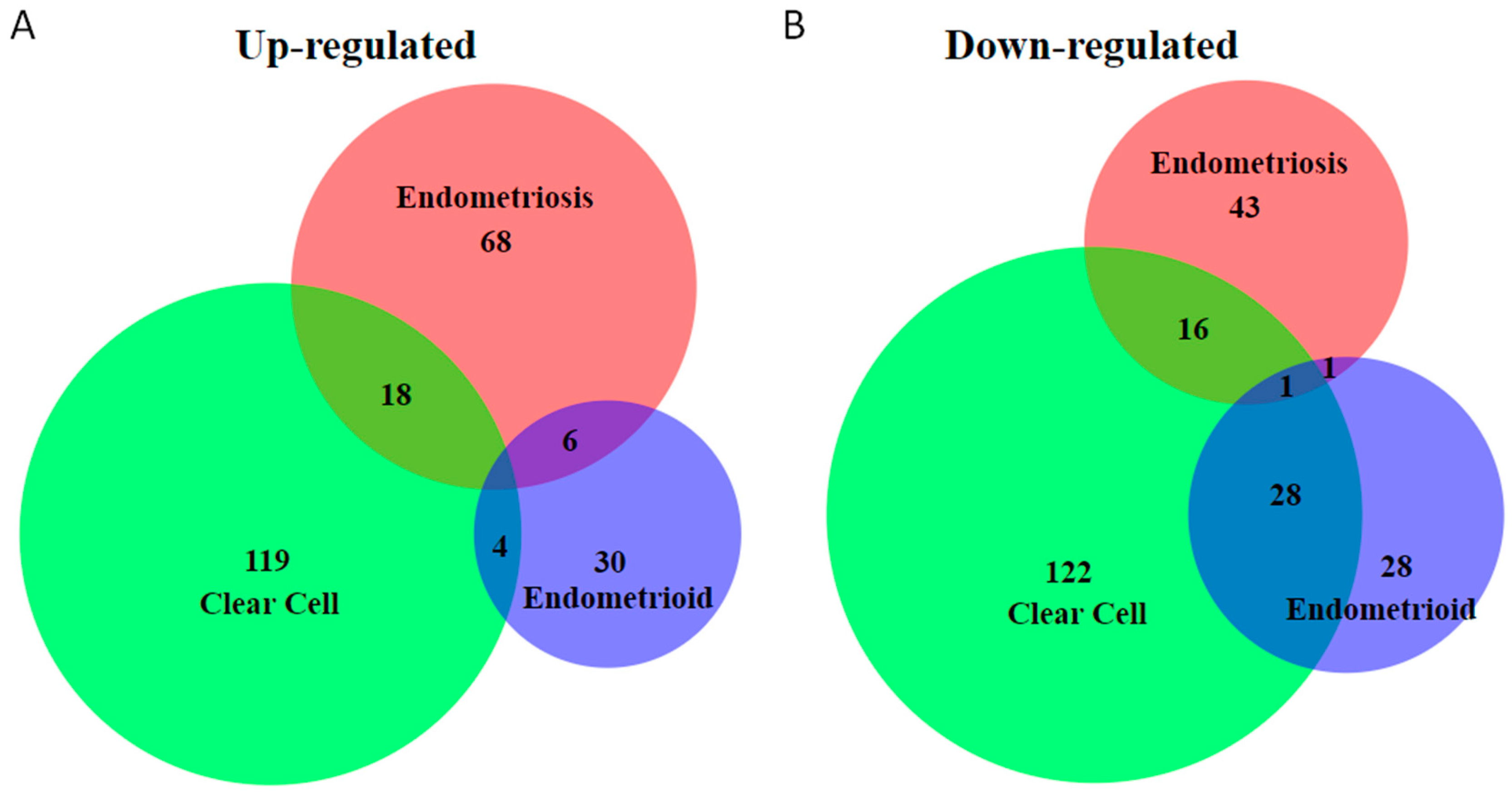
2. Unique Molecular Features of Endometriosis-Associated Ovarian Cancer
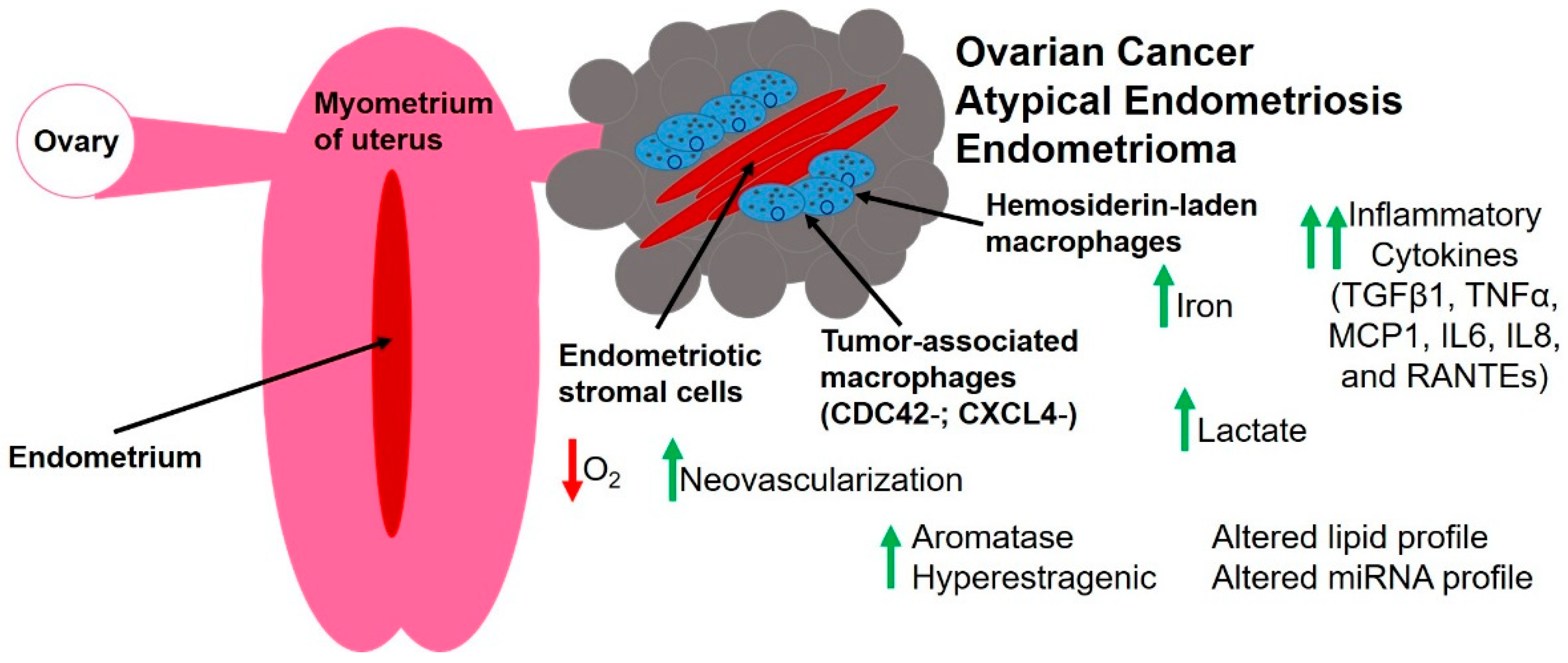
3. The Unique Endometriotic Tumor Microenvironment
3.1. Hypoxia and Endothelial Cells
3.2. Fibroblasts and Extracellular Matrix Components
3.3. Immune Cells and Inflammatory Mediators
3.4. Altered Metabolism
3.5. Steroid Hormones
3.6. Small RNA Molecules
4. Model Systems for Studying Rare Ovarian Cancers
4.1. Genetically Engineered Mouse Models
4.1.1. Candidate Genes in Genetically Engineered Mouse Models
4.1.2. Endometriosis
4.1.3. Clear Cell Ovarian Cancer
4.1.4. Endometrioid
4.1.5. Low-Grade Serous Ovarian Cancer
4.2. Other Models
4.2.1. Immortalized Cell Lines
4.2.2. Xenograft Models
4.2.3. Three Dimensional (3D) and Co-Culture Models
5. Future of Precision Therapy for/or Prevention of Ovarian Cancer
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Hediger, M.L.; Peterson, C.M.; Croughan, M.; Sundaram, R.; Stanford, J.; Chen, Z.; Fujimoto, V.Y.; Varner, M.W.; Trumble, A.; et al. Incidence of endometriosis by study population and diagnostic method: The endo study. Fertil. Steril. 2011, 96, 360–365. [Google Scholar] [CrossRef] [PubMed]
- DiVasta, A.D.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs. adulthood. Am. J. Obstet. Gynecol. 2018, 218, 324.e1–324.e11. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Grangier, L.; Streuli, I.; Dallenbach, P.; Marci, R.; Wenger, J.M.; Pluchino, N. Psychosocial impact of endometriosis: From co-morbidity to intervention. Best Pract. Res. Clin. Obstet. Gynaecol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.L.; Stram, D.O.; Ness, R.B.; Stram, D.A.; Roman, L.D.; Templeman, C.; Lee, A.W.; Menon, U.; Fasching, P.A.; McAlpine, J.N.; et al. Population distribution of lifetime risk of ovarian cancer in the united states. Cancer Epidemiol. Biomark. Prev. 2015, 24, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Nagle, C.M.; Olsen, C.M.; Webb, P.M.; Jordan, S.J.; Whiteman, D.C.; Green, A.C.; Australian Cancer Study Group; Australian Ovarian Cancer Study Group. Endometrioid and clear cell ovarian cancers: A comparative analysis of risk factors. Eur. J. Cancer 2008, 44, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Rossing, M.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; Doherty, J.A.; Weiss, N.S. Risk of epithelial ovarian cancer in relation to benign ovarian conditions and ovarian surgery. Cancer Causes Control 2008, 19, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Gridley, G.; Persson, I.; Baron, J.; Bergqvist, A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am. J. Obstet. Gynecol. 1997, 176, 572–579. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sumimoto, K.; Moniwa, N.; Imai, M.; Takakura, K.; Kuromaki, T.; Morioka, E.; Arisawa, K.; Terao, T. Risk of devloping ovarian cancer among women with ovarian endometrioma: A cohort study in shizuoka, Japan. Int. J. Cancer 2007, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Vigano, P.; Parazzini, F.; Stoppelli, S.; Giambattista, E.; Vercellini, P. Association between endometriosis and cancer: A comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol. Oncol. 2006, 101, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih Ie, M. The dualistic model of ovarian carcinogenesis: Revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed]
- Prat, J. Pathology of borderline and invasive cancers. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tao, X.; Zhou, J.; Lu, Y.; Wang, Z.; Liu, H.; Xu, C. Improved clinical outcomes of patients with ovarian carcinoma arising in endometriosis. Oncotarget 2017, 8, 5843–5852. [Google Scholar] [CrossRef] [PubMed]
- Dinkelspiel, H.E.; Matrai, C.; Pauk, S.; Pierre-Louis, A.; Chiu, Y.L.; Gupta, D.; Caputo, T.; Ellenson, L.H.; Holcomb, K. Does the presence of endometriosis affect prognosis of ovarian cancer? Cancer Investig. 2016, 34, 148–154. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, D.S.; Burger, R.A.; Manetta, A.; DiSaia, P.; Berman, M.L. Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. Gynecol. Oncol. 1995, 59, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Melin, A.; Lundholm, C.; Malki, N.; Swahn, M.L.; Sparen, P.; Bergqvist, A. Endometriosis as a prognostic factor for cancer survival. Int. J. Cancer 2011, 129, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Quirk, J.T.; Natarajan, N.; Mettlin, C.J. Age-specific ovarian cancer incidence rate patterns in the united states. Gynecol. Oncol. 2005, 99, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M. Treatment of ovarian cancer in young women. Clin. Obstet. Gynecol. 2012, 55, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Storey, D.J.; Rush, R.; Stewart, M.; Rye, T.; Al-Nafussi, A.; Williams, A.R.; Smyth, J.F.; Gabra, H. Endometrioid epithelial ovarian cancer: 20 years of prospectively collected data from a single center. Cancer 2008, 112, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Noli, S.; Cipriani, S.; Scarfone, G.; Villa, A.; Grossi, E.; Monti, E.; Vercellini, P.; Parazzini, F. Long term survival of ovarian endometriosis associated clear cell and endometrioid ovarian cancers. Int. J. Gynecol. Cancer 2013, 23, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Becker, C.M.; Bast, R.C., Jr. The origin of ovarian cancer. BJOG 2012, 119, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Tessier-Cloutier, B.; Lawrence, K.M.; Nazeran, T.; Karnezis, A.N.; Salamanca, C.; Cheng, A.S.; McAlpine, J.N.; Hoang, L.N.; Gilks, C.B.; et al. Clear cell and endometrioid carcinomas: Are their differences attributable to distinct cells of origin? J. Pathol. 2017, 243, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Chui, M.H.; Wang, T.L.; Shih, I.M. Endometriosis: Benign, malignant, or something in between? Oncotarget 2017, 8, 78263–78264. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Huntsman, D.G. Distinct developmental trajectories of endometriotic epithelium and stroma: Implications for the origins of endometriosis. J. Pathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. Arid1a mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Tsunoda, H.; Nishida, M.; Morishita, Y.; Takimoto, Y.; Kubo, T.; Noguchi, M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene Pten in benign endometrial cyst of the ovary: Possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000, 60, 7052–7056. [Google Scholar] [PubMed]
- Yamamoto, S.; Tsuda, H.; Takano, M.; Tamai, S.; Matsubara, O. Loss of arid1a protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 2012, 25, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Leung, Y.; Walsh, M.D.; Walters, R.J.; Young, J.P.; Buchanan, D.D. Kras mutations in ovarian low-grade endometrioid adenocarcinoma: Association with concurrent endometriosis. Hum. Pathol. 2012, 43, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, J.Y.; Guo, J.B.; Wang, L.Q.; Luo, Y.; Zhang, Z.Y.; Liu, F.Y.; Tan, J.; Wang, F.; Huang, O.P. The presence of kras, ppp2r1a and arid1a mutations in 101 Chinese samples with ovarian endometriosis. Mutat. Res. 2018, 809, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Lee, A.F.; Al-Agha, O.M.; Chow, C.; Kalloger, S.E.; Scott, D.W.; Steidl, C.; Wiseman, S.M.; Gascoyne, R.D.; Gilks, B.; et al. Loss of baf250a (arid1a) is frequent in high-grade endometrial carcinomas. J. Pathol. 2011, 224, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Takano, M.; Tamai, S.; Matsubara, O. Pik3ca mutations and loss of arid1a protein expression are early events in the development of cystic ovarian clear cell adenocarcinoma. Virchows Arch. 2012, 460, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P.; Samartzis, N.; Noske, A.; Fedier, A.; Caduff, R.; Dedes, K.J.; Fink, D.; Imesch, P. Loss of arid1a/baf250a-expression in endometriosis: A biomarker for risk of carcinogenic transformation? Mod. Pathol. 2012, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Mao, T.L.; Seckin, T.; Wu, C.H.; Guan, B.; Ogawa, H.; Futagami, M.; Mizukami, H.; Yokoyama, Y.; Kurman, R.J.; et al. Loss of arid1a expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int. J. Gynecol. Cancer 2012, 22, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Awadallah, A.; Xin, W. Loss of arid1a/baf250a expression in ovarian endometriosis and clear cell carcinoma. Int. J. Clin. Exp. Pathol. 2012, 5, 642–650. [Google Scholar] [PubMed]
- Anglesio, M.S.; Yong, P.J. Endometriosis-associated ovarian cancers. Clin. Obstet. Gynecol. 2017, 60, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Buggio, L.; Makieva, S.; Scarfone, G.; Cribiu, F.M.; Parazzini, F.; Somigliana, E. Perimenopausal management of ovarian endometriosis and associated cancer risk: When is medical or surgical treatment indicated? Best Pract. Res. Clin. Obstet. Gynaecol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.L.; Russell, K.; Millis, S.; Gatalica, Z.; Bender, R.; Voss, A. Molecular profiling of clear cell ovarian cancers: Identifying potential treatment targets for clinical trials. Int. J. Gynecol. Cancer 2016, 26, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Nakayama, K.; Nakamura, K.; Ono, R.; Sanuki, K.; Yamashita, H.; Ishibashi, T.; Minamoto, T.; Iida, K.; Razia, S.; et al. Affinity-purified DNA-based mutation profiles of endometriosis-related ovarian neoplasms in Japanese patients. Oncotarget 2018, 9, 14754–14763. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.L.; Shih Ie, M.; Mao, T.L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A., Jr.; Vogelstein, B.; et al. Frequent mutations of chromatin remodeling gene arid1a in ovarian clear cell carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, Y.; Tokunaga, H.; Saito, S.; Shimokawa, K.; Katsuoka, F.; Bin, L.; Kojima, K.; Nagasaki, M.; Yamamoto, M.; Yaegashi, N.; et al. Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing. Genes Chromosom. Cancer 2018, 57, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Grisham, R.N.; Iyer, G.; Garg, K.; DeLair, D.; Hyman, D.M.; Zhou, Q.; Iasonos, A.; Berger, M.F.; Dao, F.; Spriggs, D.R. Braf mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 2013, 119, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Sieben, N.L.; Macropoulos, P.; Roemen, G.M.; Kolkman-Uljee, S.M.; Jan Fleuren, G.; Houmadi, R.; Diss, T.; Warren, B.; Al Adnani, M.; De Goeij, A.P.; et al. In ovarian neoplasms, braf, but not kras, mutations are restricted to low-grade serous tumours. J. Pathol. 2004, 202, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Oldt, R., 3rd; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih Ie, M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, D.; Ma, Y.; Long, Z. Analysis of the oncogene braf mutation and the correlation of the expression of wild-type BRAF and CREB1 in endometriosis. Int. J. Mol. Med. 2018, 41, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Saare, M.; Krigul, K.L.; Laisk-Podar, T.; Ponandai-Srinivasan, S.; Rahmioglu, N.; Lalit Kumar, P.G.; Zondervan, K.; Salumets, A.; Peters, M. DNA methylation alterations-potential cause of endometriosis pathogenesis or a reflection of tissue heterogeneity? Biol. Reprod. 2018. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chang, W.; Feng, C.; Cui, M.; Xu, T. Endometriosis malignant transformation: Epigenetics as a probable mechanism in ovarian tumorigenesis. Int. J. Genom. 2018, 2018, 1465348. [Google Scholar] [CrossRef] [PubMed]
- Roca, F.J.; Loomans, H.A.; Wittman, A.T.; Creighton, C.J.; Hawkins, S.M. Ten-eleven translocation genes are downregulated in endometriosis. Curr. Mol. Med. 2016, 16, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B. MicroRNAs and endometriosis: Distinguishing drivers from passengers in disease pathogenesis. Semin. Reprod. Med. 2017, 35, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; Hawkins, S.M. Role of microRNAs in cancers of the female reproductive tract: Insights from recent clinical and experimental discovery studies. Clin. Sci. (Lond. Engl. 1979) 2015, 128, 153–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ivan, M.; Hawkins, S.M. The role of microRNA molecules and microRNA-regulating machinery in the pathogenesis and progression of epithelial ovarian cancer. Gynecol. Oncol. 2017, 147, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Machado-Linde, F.; Sanchez-Ferrer, M.L.; Cascales, P.; Torroba, A.; Orozco, R.; Silva Sanchez, Y.; Nieto, A.; Fiol, G. Prevalence of endometriosis in epithelial ovarian cancer. Analysis of the associated clinical features and study on molecular mechanisms involved in the possible causality. Eur. J. Gynaecol. Oncol. 2015, 36, 21–24. [Google Scholar] [PubMed]
- Stamp, J.P.; Gilks, C.B.; Wesseling, M.; Eshragh, S.; Ceballos, K.; Anglesio, M.S.; Kwon, J.S.; Tone, A.; Huntsman, D.G.; Carey, M.S. Baf250a expression in atypical endometriosis and endometriosis-associated ovarian cancer. Int. J. Gynecol. Cancer 2016, 26, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Amant, F.; Neven, P.; Vergote, I.; Moerman, P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Nomura, K.; Ishikawa, E.; Ushigome, S. Ovarian atypical endometriosis: Its close association with malignant epithelial tumours. Histopathology 1997, 30, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Banz, C.; Ungethuem, U.; Kuban, R.J.; Diedrich, K.; Lengyel, E.; Hornung, D. The molecular signature of endometriosis-associated endometrioid ovarian cancer differs significantly from endometriosis-independent endometrioid ovarian cancer. Fertil. Steril. 2010, 94, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Anaya, Y.; Parodi, L.; Chen, L.; Anderson, M.L.; Hawkins, S.M. Distinct molecular pathways in ovarian endometrioid adenocarcinoma with concurrent endometriosis. Int. J. Cancer 2018, in press. [Google Scholar]
- Kolin, D.L.; Dinulescu, D.M.; Crum, C.P. Origin of clear cell carcinoma: Nature or nurture? J. Pathol. 2018, 244, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and cellular pathogenesis of endometriosis. Curr. Womens Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.; Hitchcock, A. Endometriosis: Its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br. J. Obstet. Gynaecol. 1986, 93, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Zhang, Z.; Lv, L.; Xiong, W.; Zhang, L.; Li, N.; He, H.; Li, Q.; Liu, Y. Autophagy contributes to hypoxia-induced epithelial to mesenchymal transition of endometrial epithelial cells in endometriosis. Biol. Reprod. 2018. [Google Scholar] [CrossRef] [PubMed]
- Allavena, G.; Carrarelli, P.; Del Bello, B.; Luisi, S.; Petraglia, F.; Maellaro, E. Autophagy is upregulated in ovarian endometriosis: A possible interplay with p53 and heme oxygenase-1. Fertil. Steril. 2015, 103, 1244–1251.e1. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Xiong, Y.; Li, N.; He, H.; Du, Y.; Liu, Y. Hypoxia-inducible factor-1alpha promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction 2017, 153, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.X.; Zhao, S.Z.; Dong, M.; Yu, X.R. Hypoxia responsive miR-210 promotes cell survival and autophagy of endometriotic cells in hypoxia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 399–406. [Google Scholar] [PubMed]
- Lin, X.; Dai, Y.; Xu, W.; Shi, L.; Jin, X.; Li, C.; Zhou, F.; Pan, Y.; Zhang, Y.; Lin, X.; et al. Hypoxia promotes ectopic adhesion ability of endometrial stromal cells via tgf-beta1/smad signaling in endometriosis. Endocrinology 2018, 159, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.W.; Lessey, B.A. Kras activation and over-expression of sirt1/bcl6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci. Rep. 2017, 7, 6765. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.Y.; Chang, N.; Tsai, J.L.; Lin, S.C.; Tsai, S.J.; Wu, M.H. Hypoxia-inhibited DUSP2 expression promotes IL-6/STAT3 signaling in endometriosis. Am. J. Reprod. Immunol. 2017, 78. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Yoo, J.Y.; Kim, T.H.; Shin, J.H.; Langenheim, J.F.; Ferguson, S.D.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Jeong, J.W. Aberrant activation of signal transducer and activator of transcription-3 (stat3) signaling in endometriosis. Hum. Reprod. (Oxf. Engl.) 2015, 30, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Wang, C.C.; Wu, M.H.; Yang, S.H.; Li, Y.H.; Tsai, S.J. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J. Clin. Endocrinol. Metab. 2012, 97, E1515–E1523. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.Y.; Chang, N.; Lin, S.C.; Li, Y.H.; Wu, M.H. Inhibition of dual specificity phosphatase-2 by hypoxia promotes interleukin-8-mediated angiogenesis in endometriosis. Hum. Reprod. (Oxf. Engl.) 2014, 29, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Yamamoto, S.; Takano, M.; Matsubara, O.; Furuya, K. Aberrant expression of the mammalian target of rapamycin, hypoxia-inducible factor-1alpha, and glucose transporter 1 in the development of ovarian clear-cell adenocarcinoma. Int. J. Gynecol. Pathol. 2012, 31, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Garrett, L.; Henretta, M.; Ferriss, J.S.; Lee, L.; Horowitz, N. When ‘never-events’ occur despite adherence to clinical guidelines: The case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol. Oncol. 2010, 116, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.Y.; Lin, S.C.; Wu, M.H.; Tsai, S.J. Pathological functions of hypoxia in endometriosis. Front. Biosci. (Elite Ed.) 2015, 7, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Wang, W.; Zhang, Y.; Song, E.; Fan, Y.; Wei, B. Hypoxia-inducible factor-1alpha: A promising therapeutic target in endometriosis. Biochimie 2016, 123, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Meserve, E.E.; Crum, C.P. Benign conditions of the ovary. In Diagnostic Gynecologic and Obstetric Pathology, 3rd ed.; Crum, C.P., Haefner, H.K., Peters, W.A., III, Eds.; Elsevier, Inc.: Philadephia, PA, USA, 2018; pp. 761–799. [Google Scholar]
- Hantak, A.M.; Bagchi, I.C.; Bagchi, M.K. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int. J. Dev. Biol. 2014, 58, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Medina, R.; Schabel, A.B.; Young, P.; Gama, P.; Parekh, T.V.; Brody, J.; Cunha, G.R.; Osteen, K.G.; Bruner-Tran, K.L.; et al. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation 2005, 73, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Osteen, K.G.; Rodgers, W.H.; Gaire, M.; Hargrove, J.T.; Gorstein, F.; Matrisian, L.M. Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc. Natl. Acad. Sci. USA 1994, 91, 10129–10133. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.; Cook, C.D.; Ahrens, C.C.; Wang, A.J.; Brown, A.; Kumar, M.; Stockdale, L.; Rothenberg, D.; Renggli, K.; Gordon, E.; et al. On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials 2017, 130, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Hamilton, A.; Kwintkiewicz, J.; Vo, K.C.; Giudice, L.C. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol. Reprod. 2009, 80, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C.; Aghajanova, L.; Nezhat, C.R.; Giudice, L.C. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology 2009, 150, 4701–4712. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Horcajadas, J.A.; Weeks, J.L.; Esteban, F.J.; Nezhat, C.N.; Conti, M.; Giudice, L.C. The protein kinase a pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology 2010, 151, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Tatsumi, K.; Horcajadas, J.A.; Zamah, A.M.; Esteban, F.J.; Herndon, C.N.; Conti, M.; Giudice, L.C. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol. Reprod. 2011, 84, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Velarde, M.C.; Giudice, L.C. The progesterone receptor coactivator hic-5 is involved in the pathophysiology of endometriosis. Endocrinology 2009, 150, 3863–3870. [Google Scholar] [CrossRef] [PubMed]
- Barragan, F.; Irwin, J.C.; Balayan, S.; Erikson, D.W.; Chen, J.C.; Houshdaran, S.; Piltonen, T.T.; Spitzer, T.L.; George, A.; Rabban, J.T.; et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol. Reprod. 2016, 94, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, H.; Cai, J.; Zhang, T.; Guo, J.; Feng, D.; Wang, Z. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011, 303, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Zillhardt, M.; Hua, Y.; Tiwari, P.; Murmann, A.E.; Peter, M.E.; Lengyel, E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012, 2, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Creighton, C.J.; Han, D.Y.; Zariff, A.; Anderson, M.L.; Gunaratne, P.H.; Matzuk, M.M. Functional microRNA involved in endometriosis. Mol. Endocrinol. 2011, 25, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Kim, J.J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: It is affected, and let me show you why. Fertil. Steril. 2017, 108, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Rovere-Querini, P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Canet, B.; Pons, C.; Espinosa, I.; Prat, J. Cdc42-positive macrophages may prevent malignant transformation of ovarian endometriosis. Hum. Pathol. 2012, 43, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Tanaka, R.; Miyagi, E.; Kami, D.; Nagahama, K.; Miyagi, Y.; Nagashima, Y.; Hirahara, F.; Inayama, Y.; Aoki, I. Impaired CXCL4 expression in tumor-associated macrophages (tams) of ovarian cancers arising in endometriosis. Cancer Biol. Ther. 2012, 13, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Eisermann, J.; Gast, M.J.; Pineda, J.; Odem, R.R.; Collins, J.L. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil. Steril. 1988, 50, 573–579. [Google Scholar] [CrossRef]
- Calhaz-Jorge, C.; Costa, A.P.; Barata, M.; Santos, M.C.; Melo, A.; Palma-Carlos, M.L. Tumour necrosis factor alpha concentrations in the peritoneal fluid of infertile women with minimal or mild endometriosis are lower in patients with red lesions only than in patients without red lesions. Hum. Reprod. (Oxf. Engl.) 2000, 15, 1256–1260. [Google Scholar] [CrossRef]
- Jin, C.H.; Yi, K.W.; Ha, Y.R.; Shin, J.H.; Park, H.T.; Kim, T.; Hur, J.Y. Chemerin expression in the peritoneal fluid, serum, and ovarian endometrioma of women with endometriosis. Am. J. Reprod. Immunol. 2015, 74, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Brown, J.K.; Saunders, P.T.; Duncan, W.C.; Horne, A.W. The peritoneum is both a source and target of TGF-Beta in women with endometriosis. PLoS ONE 2014, 9, e106773. [Google Scholar] [CrossRef] [PubMed]
- Worley, M.J.; Welch, W.R.; Berkowitz, R.S.; Ng, S.W. Endometriosis-associated ovarian cancer: A review of pathogenesis. Int. J. Mol. Sci. 2013, 14, 5367–5379. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen receptor beta modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Altan, Z.M.; Denis, D.; Kagan, D.; Grund, E.M.; Palmer, S.S.; Nataraja, S.G. A long-acting tumor necrosis factor alpha-binding protein demonstrates activity in both in vitro and in vivo models of endometriosis. J. Pharmacol. Exp. Ther. 2010, 334, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Craessaerts, M.; Timmerman, D.; Cornillie, F.; Kennedy, S. Anti-TNF-alpha treatment for deep endometriosis-associated pain: A randomized placebo-controlled trial. Hum. Reprod. (Oxf. Engl.) 2008, 23, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.J.; William, J.; Bulun, S. Endometriosis and ovarian cancer: A review of clinical, pathologic, and molecular aspects. Int. J. Gynecol. Pathol. 2011, 30, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Hamanishi, J.; Konishi, I. Ovarian clear cell carcinoma as a stress-responsive cancer: Influence of the microenvironment on the carcinogenesis and cancer phenotype. Cancer Lett. 2011, 310, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, H.; Higashiura, Y.; Kajihara, H.; Kobayashi, H. A potential link of oxidative stress and cell cycle regulation for development of endometriosis. Gynecol. Endocrinol. 2012, 28, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Crosignani, P.; Somigliana, E.; Vigano, P.; Buggio, L.; Bolis, G.; Fedele, L. The ‘incessant menstruation’ hypothesis: A mechanistic ovarian cancer model with implications for prevention. Hum. Reprod. (Oxf. Engl.) 2011, 26, 2262–2273. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Brown, J.K.; Maybin, J.; Saunders, P.T.; Duncan, W.C.; Horne, A.W. Transforming growth factor-β induced warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, 3450–3459. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Ahmad, S.F.; Brown, J.K.; Duncan, W.C.; Horne, A.W. Id2 mediates the transforming growth factor-β1-induced warburg-like effect seen in the peritoneum of women with endometriosis. MHR Basic Sci. Reprod. Med. 2016, 22, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Ferrando, M.; Diaz-Gimeno, P.; Quintana, F.; Fernandez, G.; Castells, I.; Simon, C. Lipidomic profiling of endometrial fluid in women with ovarian endometriosisdagger. Biol. Reprod. 2017, 96, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Y.; Guan, L.; Zhang, H.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Ma, Z.; Liang, X.; et al. Discovery of phosphatidic acid, phosphatidylcholine, and phosphatidylserine as biomarkers for early diagnosis of endometriosis. Front. Physiol 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Imir, A.; Fenkci, V.; Yilmaz, M.B.; Bulun, S.E. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17beta-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator sp1: A mechanism for defective estradiol metabolism. Am. J. Obstet. Gynecol. 2007, 196, 391.e1–391.e7, discussion 391.e7–391.e8. [Google Scholar] [CrossRef] [PubMed]
- Khorram, O.; Taylor, R.N.; Ryan, I.P.; Schall, T.J.; Landers, D.V. Peritoneal fluid concentrations of the cytokine rantes correlate with the severity of endometriosis. Am. J. Obstet. Gynecol. 1993, 169, 1545–1549. [Google Scholar] [CrossRef]
- Tsai, S.J.; Wu, M.H.; Lin, C.C.; Sun, H.S.; Chen, H.M. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J. Clin. Endocrinol. Metab. 2001, 86, 5765–5773. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Lee, J.; Balasubbramanian, D.; Stanley, J.A.; Long, C.R.; Meagher, M.W.; Osteen, K.G.; Bruner-Tran, K.L.; Burghardt, R.C.; Starzinski-Powitz, A.; et al. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc. Natl. Acad. Sci. USA 2015, 112, 9716–9721. [Google Scholar] [CrossRef] [PubMed]
- Modugno, F.; Ness, R.B.; Allen, G.O.; Schildkraut, J.M.; Davis, F.G.; Goodman, M.T. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am. J. Obstet. Gynecol. 2004, 191, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Buchold, G.M.; Matzuk, M.M. Minireview: The roles of small rna pathways in reproductive medicine. Mol. Endocrinol. 2011, 25, 1257–1279. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P.; Chen, C.Z. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat. Rev. 2004, 5, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Yanaihara, N.; Noguchi, Y.; Saito, M.; Takenaka, M.; Takakura, S.; Yamada, K.; Okamoto, A. microRNA gene expression signature driven by miR-9 overexpression in ovarian clear cell carcinoma. PLoS ONE 2016, 11, e0162584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, G.; Wang, G.; Zhao, J.; Wang, B.; Yu, X.; Ding, Y. Profile of differentially expressed miRNAs in high-grade serous carcinoma and clear cell ovarian carcinoma, and the expression of miR-510 in ovarian carcinoma. Mol. Med. Rep. 2015, 12, 8021–8031. [Google Scholar] [CrossRef] [PubMed]
- Vilming Elgaaen, B.; Olstad, O.K.; Haug, K.B.; Brusletto, B.; Sandvik, L.; Staff, A.C.; Gautvik, K.M.; Davidson, B. Global miRNA expression analysis of serous and clear cell ovarian carcinomas identifies differentially expressed miRNAs including miR-200c-3p as a prognostic marker. BMC Cancer 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Wyman, S.K.; Parkin, R.K.; Mitchell, P.S.; Fritz, B.R.; O’Briant, K.; Godwin, A.K.; Urban, N.; Drescher, C.W.; Knudsen, B.S.; Tewari, M. Repertoire of microRNAs in epithelial ovarian cancer as determined by next generation sequencing of small RNA cDNA libraries. PLoS ONE 2009, 4, e5311. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.L.; Ali, S.; Bandyopadhyay, S.; Alosh, B.; Hayek, K.; Daaboul, M.F.; Winer, I.; Sarkar, F.H.; Ali-Fehmi, R. Comparative analysis of differentially expressed miRNAs and their downstream mRNAs in ovarian cancer and its associated endometriosis. J Cancer Sci. Ther. 2015, 7, 258–265. [Google Scholar] [PubMed]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.G.; Alder, H.; et al. microRNA signatures in human ovarian cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef] [PubMed]
- Braicu, O.L.; Budisan, L.; Buiga, R.; Jurj, A.; Achimas-Cadariu, P.; Pop, L.A.; Braicu, C.; Irimie, A.; Berindan-Neagoe, I. miRNA expression profiling in formalin-fixed paraffin-embedded endometriosis and ovarian cancer samples. Onco Targets Ther. 2017, 10, 4225–4238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ding, Y.; Tie, B.; Sun, Z.F.; Jiang, J.Y.; Zhao, J.; Lin, X.; Cui, S. miRNA expression pattern associated with prognosis in elderly patients with advanced OPSC and OCC. Int. J. Oncol. 2013, 43, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Calura, E.; Fruscio, R.; Paracchini, L.; Bignotti, E.; Ravaggi, A.; Martini, P.; Sales, G.; Beltrame, L.; Clivio, L.; Ceppi, L.; et al. miRNA landscape in stage i epithelial ovarian cancer defines the histotype specificities. Clin. Cancer Res. 2013, 19, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Vlad, A.M.; Lin, H.M.; Mantia-Smaldone, G.; Laskey, R.; Lee, M.; Lin, Y.; Donnellan, N.; Klein-Patel, M.; Lee, T.; et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin. Cancer Res. 2013, 19, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, S.; Wang, X.Y.; Wang, D.B. Expression of miR-126 and Crk in endometriosis: miR-126 may affect the progression of endometriosis by regulating Crk expression. Arch. Gynecol. Obstet. 2012, 285, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Gopalan, V.; Smith, R.A.; Lam, A.K. miR-126 in human cancers: Clinical roles and current perspectives. Exp. Mol. Pathol. 2014, 96, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Sestito, R.; Cianfrocca, R.; Rosano, L.; Tocci, P.; Semprucci, E.; Di Castro, V.; Caprara, V.; Ferrandina, G.; Sacconi, A.; Blandino, G.; et al. miR-30a inhibits endothelin a receptor and chemoresistance in ovarian carcinoma. Oncotarget 2016, 7, 4009–4023. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, H.; Wang, A.H.; Zhang, L.Y.; Bai, J. microRNA-532 and microRNA-3064 inhibit cell proliferation and invasion by acting as direct regulators of human telomerase reverse transcriptase in ovarian cancer. PLoS ONE 2017, 12, e0173912. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.; Yang, C. The microRNA-200 family: Small molecules with novel roles in cancer development, progression and therapy. Oncotarget 2015, 6, 6472–6498. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Critchley, H.O.D.; Horne, A.W.; Saunders, P.T.K. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta. Obstet. Gynecol. Scand. 2017, 96, 644–658. [Google Scholar] [CrossRef] [PubMed]
- King, C.M.; Barbara, C.; Prentice, A.; Brenton, J.D.; Charnock-Jones, D.S. Models of endometriosis and their utility in studying progression to ovarian clear cell carcinoma. J. Pathol. 2016, 238, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.; Kitamura, M.; HarrisStansil, T.; Dai, Y.M.; Phipps, M.L. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997, 71, 1842–1849. [Google Scholar] [PubMed]
- Mullany, L.K.; Fan, H.Y.; Liu, Z.; White, L.D.; Marshall, A.; Gunaratne, P.; Anderson, M.L.; Creighton, C.J.; Xin, L.; Deavers, M.; et al. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene 2011, 30, 3522–3536. [Google Scholar] [CrossRef] [PubMed]
- Jamin, S.P.; Arango, N.A.; Mishina, Y.; Hanks, M.C.; Behringer, R.R. Requirement of bmpr1a for mullerian duct regression during male sexual development. Nat. Genet. 2002, 32, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Shimada, M.; Liu, Z.; Cahill, N.; Noma, N.; Wu, Y.; Gossen, J.; Richards, J.S. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 2008, 135, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhai, Y.; Kuick, R.; Karnezis, A.N.; Garcia, P.; Naseem, A.; Hu, T.C.; Fearon, E.R.; Cho, K.R. Impact of oviductal versus ovarian epithelial cell of origin on ovarian endometrioid carcinoma phenotype in the mouse. J. Pathol. 2016, 240, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Czarnecki, A.A.; Dean, M.; Modi, D.A.; Lantvit, D.D.; Hardy, L.; Baligod, S.; Davis, D.A.; Wei, J.J.; Burdette, J.E. Pten loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene 2018, 37, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Soyal, S.M.; Mukherjee, A.; Lee, K.Y.S.; Li, J.; Li, H.; DeMayo, F.J.; Lydon, J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005, 41, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhai, Y.; Fearon, E.R.; Cho, K.R. Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001, 61, 8247–8255. [Google Scholar] [PubMed]
- Saegusa, M.; Okayasu, I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J. Pathol. 2001, 194, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Gamallo, C. Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998, 58, 1344–1347. [Google Scholar] [PubMed]
- Gamallo, C.; Palacios, J.; Moreno, G.; Calvo de Mora, J.; Suarez, A.; Armas, A. Beta-catenin expression pattern in stage I and II ovarian carcinomas: Relationship with beta-catenin gene mutations, clinicopathological features, and clinical outcome. Am. J. Pathol. 1999, 155, 527–536. [Google Scholar] [CrossRef]
- Wright, K.; Wilson, P.; Morland, S.; Campbell, I.; Walsh, M.; Hurst, T.; Ward, B.; Cummings, M.; Chenevix-Trench, G. Beta-catenin mutation and expression analysis in ovarian cancer: Exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int. J. Cancer 1999, 82, 625–629. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Chapela, P.J.; Morgado, M.; Hawkins, S.M.; Lessey, B.A.; Young, S.L.; Carson, D.D. Expression of the transmembrane mucins, MUC1, MUC4 and MUC16, in normal endometrium and in endometriosis. Hum. Reprod. (Oxf. Engl.) 2014, 29, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.G.; Russell, S.E.; Choong, D.Y.; Montgomery, K.G.; Ciavarella, M.L.; Hooi, C.S.; Cristiano, B.E.; Pearson, R.B.; Phillips, W.A. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004, 64, 7678–7681. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.L.; Damrauer, J.S.; Raab, J.R.; Schisler, J.C.; Wilkerson, M.D.; Didion, J.P.; Starmer, J.; Serber, D.; Yee, D.; Xiong, J. Coexistent ARID1A–PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015, 6, 6118. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Rahmanto, Y.S.; Wu, R.C.; Wang, Y.; Wang, Z.; Wang, T.L.; Shih Ie, M. Roles of deletion of ARID1A, a tumor suppressor, in mouse ovarian tumorigenesis. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khatri, S.; Broaddus, R.; Wang, Z.; Hawkins, S.M. Deletion of ARID1A in reproductive tract mesenchymal cells reduces fertility in female mice. Biol. Reprod. 2016, 94, 93. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yoo, J.Y.; Wang, Z.; Lydon, J.P.; Khatri, S.; Hawkins, S.M.; Leach, R.E.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; et al. Arid1a is essential for endometrial function during early pregnancy. PLoS Genet. 2015, 11, e1005537. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Hendrix-Lucas, N.; Kuick, R.; Zhai, Y.; Schwartz, D.R.; Akyol, A.; Hanash, S.; Misek, D.E.; Katabuchi, H.; Williams, B.O.; et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell 2007, 11, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Dinulescu, D.M.; Ince, T.A.; Quade, B.J.; Shafer, S.A.; Crowley, D.; Jacks, T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med. 2005, 11, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-Y.; Liu, Z.; Paquet, M.; Wang, J.; Lydon, J.P.; DeMayo, F.J.; Richards, J.S. Cell type–specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009, 69, 6463–6472. [Google Scholar] [CrossRef] [PubMed]
- Lague, M.N.; Paquet, M.; Fan, H.Y.; Kaartinen, M.J.; Chu, S.; Jamin, S.P.; Behringer, R.R.; Fuller, P.J.; Mitchell, A.; Dore, M.; et al. Synergistic effects of Pten loss and Wnt/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis 2008, 29, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, P.S.; Zhang, L.; Kaneko-Tarui, T.; Curley, M.D.; Taketo, M.M.; Rani, P.; Roberts, D.J.; Teixeira, J.M. Mammalian target of rapamycin is a therapeutic target for murine ovarian endometrioid adenocarcinomas with dysregulated Wnt/β-catenin and Pten. PLoS ONE 2011, 6, e20715. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, P.H.; van der Zee, M.; Heijmans-Antonissen, C.; Jia, Y.; DeMayo, F.J.; Lydon, J.P.; van Deurzen, C.H.; Ewing, P.C.; Burger, C.W.; Blok, L.J. A mouse model for endometrioid ovarian cancer arising from the distal oviduct. Int. J. Cancer 2014, 135, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Baker, S.J.; Hu, T.C.; Norman, K.M.; Fearon, E.R.; Cho, K.R. Type I to type II ovarian carcinoma progression: Mutant Trp53 or PIK3CA confers a more aggressive tumor phenotype in a mouse model of ovarian cancer. Am. J. Pathol. 2013, 182, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Budiu, R.A.; Diaconu, I.; Chrissluis, R.; Dricu, A.; Edwards, R.P.; Vlad, A.M. A conditional mouse model for human muc1-positive endometriosis shows the presence of anti-muc1 antibodies and foxp3+ regulatory t cells. Dis. Model Mech. 2009, 2, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Cousins, F.L.; Murray, A.; Esnal, A.; Gibson, D.A.; Critchley, H.O.; Saunders, P.T. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE 2014, 9, e86378. [Google Scholar] [CrossRef] [PubMed]
- Brasted, M.; White, C.A.; Kennedy, T.G.; Salamonsen, L.A. Mimicking the events of menstruation in the murine uterus. Biol. Reprod. 2003, 69, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Bielby, H.; Licence, D.; Smith, S.K.; Print, C.G.; Charnock-Jones, D.S. Quantitative cellular and molecular analysis of the effect of progesterone withdrawal in a murine model of decidualization. Biol. Reprod. 2007, 76, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Licence, D.; Cook, E.; Luo, F.; Arends, M.J.; Smith, S.K.; Print, C.G.; Charnock-Jones, D.S. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. J. Pathol. 2011, 224, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Cousins, F.L.; Murray, A.; Esnal-Zufiaurre, A.; Fassbender, A.; Horne, A.W.; Saunders, P.T. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 2014, 184, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.L.; Raab, J.R.; Vernon, M.; Magnuson, T.; Schisler, J.C. Global gene expression profiling of a mouse model of ovarian clear cell carcinoma caused by ARID1A and PIK3CA mutations implicates a role for inflammatory cytokine signaling. Genom. Data 2015, 5, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Tamai, Y.; Ishikawa, T.; Sauer, B.; Takaku, K.; Oshima, M.; Taketo, M.M. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999, 18, 5931–5942. [Google Scholar] [CrossRef] [PubMed]
- Uehara, S.; Abe, H.; Hoshiai, H.; Yajima, A.; Suzuki, M. Establishment and characterization of ovarian endometrioid carcinoma cell line. Gynecol. Oncol. 1984, 17, 314–325. [Google Scholar] [CrossRef]
- Gorai, I.; Nakazawa, T.; Miyagi, E.; Hirahara, F.; Nagashima, Y.; Minaguchi, H. Establishment and characterization of two human ovarian clear cell adenocarcinoma lines from metastatic lesions with different properties. Gynecol. Oncol. 1995, 57, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Wiegand, K.C.; Melnyk, N.; Chow, C.; Salamanca, C.; Prentice, L.M.; Senz, J.; Yang, W.; Spillman, M.A.; Cochrane, D.R.; et al. Type-specific cell line models for type-specific ovarian cancer research. PLoS ONE 2013, 8, e72162. [Google Scholar] [CrossRef]
- Beaufort, C.M.; Helmijr, J.C.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van, I.W.F.; Heine, A.A.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef] [PubMed]
- Ince, T.A.; Sousa, A.D.; Jones, M.A.; Harrell, J.C.; Agoston, E.S.; Krohn, M.; Selfors, L.M.; Liu, W.; Chen, K.; Yong, M.; et al. Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nat. Commun. 2015, 6, 7419. [Google Scholar] [CrossRef] [PubMed]
- Blayney, J.K.; Davison, T.; McCabe, N.; Walker, S.; Keating, K.; Delaney, T.; Greenan, C.; Williams, A.R.; McCluggage, W.G.; Capes-Davis, A.; et al. Prior knowledge transfer across transcriptional data sets and technologies using compositional statistics yields new mislabelled ovarian cell line. Nucleic Acids Res. 2016, 44, e137. [Google Scholar] [CrossRef] [PubMed]
- Zeitvogel, A.; Baumann, R.; Starzinski-Powitz, A. Identification of an invasive, n-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am. J. Pathol. 2001, 159, 1839–1852. [Google Scholar] [CrossRef]
- Brueggmann, D.; Templeman, C.; Starzinski-Powitz, A.; Rao, N.P.; Gayther, S.A.; Lawrenson, K. Novel three-dimensional in vitro models of ovarian endometriosis. J. Ovarian Res. 2014, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Bono, Y.; Kyo, S.; Takakura, M.; Maida, Y.; Mizumoto, Y.; Nakamura, M.; Nomura, K.; Kiyono, T.; Inoue, M. Creation of immortalised epithelial cells from ovarian endometrioma. Br. J. Cancer 2012, 106, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Ohta, I.; Gorai, I.; Miyamoto, Y.; Yang, J.; Zheng, J.H.; Kawata, N.; Hirahara, F.; Shirotake, S. Cyclophosphamide and 5-fluorouracil act synergistically in ovarian clear cell adenocarcinoma cells. Cancer Lett. 2001, 162, 39–48. [Google Scholar] [CrossRef]
- Provencher, D.M.; Lounis, H.; Champoux, L.; Tetrault, M.; Manderson, E.N.; Wang, J.C.; Eydoux, P.; Savoie, R.; Tonin, P.N.; Mes-Masson, A.M. Characterization of four novel epithelial ovarian cancer cell lines. In Vitro Cell Dev. Biol. Anim. 2000, 36, 357–361. [Google Scholar] [CrossRef]
- Nozawa, S.; Tsukazaki, K.; Sakayori, M.; Jeng, C.H.; Iizuka, R. Establishment of a human ovarian clear cell carcinoma cell line (RMG-I) and its single cell cloning-with special reference to the stem cell of the tumor. Hum. Cell 1988, 1, 426–435. [Google Scholar] [PubMed]
- Wong, W.S.; Wong, Y.F.; Ng, Y.T.; Huang, P.D.; Chew, E.C.; Ho, T.H.; Chang, M.Z. Establishment and characterization of a new human cell line derived from ovarian clear cell carcinoma. Gynecol. Oncol. 1990, 38, 37–45. [Google Scholar] [CrossRef]
- Yamada, K.; Tachibana, T.; Hashimoto, H.; Suzuki, K.; Yanagida, S.; Endoh, H.; Kimura, E.; Yasuda, M.; Tanaka, T.; Ishikawa, H. Establishment and characterization of cell lines derived from serous adenocarcinoma (JHOS-2) and clear cell adenocarcinoma (JHOC-5, JHOC-6) of human ovary. Hum. Cell 1999, 12, 131–138. [Google Scholar] [PubMed]
- Lau, D.H.; Lewis, A.D.; Ehsan, M.N.; Sikic, B.I. Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res. 1991, 51, 5181–5187. [Google Scholar] [PubMed]
- Yanagibashi, T.; Gorai, I.; Nakazawa, T.; Miyagi, E.; Hirahara, F.; Kitamura, H.; Minaguchi, H. Complexity of expression of the intermediate filaments of six new human ovarian carcinoma cell lines: New expression of cytokeratin 20. Br. J. Cancer 1997, 76, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Stordal, B.; Timms, K.; Farrelly, A.; Gallagher, D.; Busschots, S.; Renaud, M.; Thery, J.; Williams, D.; Potter, J.; Tran, T.; et al. Brca1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious brca1 mutation. Mol. Oncol. 2013, 7, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Benard, J.; Da Silva, J.; De Blois, M.C.; Boyer, P.; Duvillard, P.; Chiric, E.; Riou, G. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985, 45, 4970–4979. [Google Scholar] [PubMed]
- Hills, C.A.; Kelland, L.R.; Abel, G.; Siracky, J.; Wilson, A.P.; Harrap, K.R. Biological properties of ten human ovarian carcinoma cell lines: Calibration in vitro against four platinum complexes. Br. J. Cancer 1989, 59, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg-Bakker, C.A.; Hagemeijer, A.; Franken-Postma, E.M.; Smit, V.T.; Kuppen, P.J.; van Ravenswaay Claasen, H.H.; Cornelisse, C.J.; Schrier, P.I. Establishment and characterization of 7 ovarian carcinoma cell lines and one granulosa tumor cell line: Growth features and cytogenetics. Int. J. Cancer 1993, 53, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Eva, A.; Robbins, K.C.; Andersen, P.R.; Srinivasan, A.; Tronick, S.R.; Reddy, E.P.; Ellmore, N.W.; Galen, A.T.; Lautenberger, J.A.; Papas, T.S.; et al. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature 1982, 295, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Buick, R.N.; Pullano, R.; Trent, J.M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985, 45, 3668–3676. [Google Scholar] [PubMed]
- Fogh, J. MSKCC. Available online: http://www.mskcc.org (accessed on 14 June 2018).
- Han, S.J.; Hawkins, S.M.; Begum, K.; Jung, S.Y.; Kovanci, E.; Qin, J.; Lydon, J.P.; DeMayo, F.J.; O’Malley, B.W. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat. Med. 2012, 18, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, S.; Aoki, D.; Katsuki, Y.; Nozawa, S. Proliferative activity of early ovarian clear cell adenocarcinoma depends on association with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 127, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [PubMed]
- Lal-Nag, M.; McGee, L.; Guha, R.; Lengyel, E.; Kenny, H.A.; Ferrer, M. A high-throughput screening model of the tumor microenvironment for ovarian cancer cell growth. SLAS Discov. 2017, 22, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Messerli, S.M.; Miller, D.H.; Medina, J.E.; Hamilton, J.W.; Messerli, M.A.; Brodsky, A.S. Cisplatin resistant spheroids model clinically relevant survival mechanisms in ovarian tumors. PLoS ONE 2016, 11, e0151089. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, L.M.; Mantzavinou, A.; Subramanyam, K.S.; Del Carmen, M.G.; Cima, M.J. Ovarian cancer spheroid shrinkage following continuous exposure to cisplatin is a function of spheroid diameter. Gynecol. Oncol. 2017, 146, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.T.; Kaufman, D.G.; Seppala, M.; Lessey, B.A. Endometrial stromal cells regulate epithelial cell growth in vitro: A new co-culture model. Hum. Reprod. (Oxf. Engl.) 2001, 16, 836–845. [Google Scholar] [CrossRef]
- Mori, M.; Ito, F.; Shi, L.; Wang, Y.; Ishida, C.; Hattori, Y.; Niwa, M.; Hirayama, T.; Nagasawa, H.; Iwase, A.; et al. Ovarian endometriosis-associated stromal cells reveal persistently high affinity for iron. Redox Biol. 2015, 6, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.S.; Lee, C.L.; Ng, E.H.Y.; Yeung, W.S.B. Co-culture with macrophages enhances the clonogenic and invasion activity of endometriotic stromal cells. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Practice bulletin No. 114: Management of endometriosis. Obstet. Gynecol. 2010, 116, 223–236.
- Shakiba, K.; Bena, J.F.; McGill, K.M.; Minger, J.; Falcone, T. Surgical treatment of endometriosis: A 7-year follow-up on the requirement for further surgery. Obstet. Gynecol. 2008, 111, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.L.; Sheng, J.; Leung, C.S.; Li, F.; Kim, J.; Ho, S.Y.; Matzuk, M.M.; Lu, K.H.; Wong, S.T.C.; Mok, S.C. Systematic identification of druggable epithelial-stromal crosstalk signaling networks in ovarian cancer. J. Natl. Cancer Inst. 2018. [Google Scholar] [CrossRef] [PubMed]
| Cre | Gene Promoter | Location of Expression | Ref. |
|---|---|---|---|
| Adenovirus (Ad) | Cytomegalovirus | Injection site | [142] |
| Amhr2 | Anti-Mullërian hormone receptor type 2 | Oviduct: stroma Uterus: stroma and smooth muscle cells Ovary: granulosa cells and ovarian surface epithelium | [143,144] |
| Cyp19 | Cytochrome P450 family 19 | Granulosa cells of antral follicles and luteal cells | [145] |
| Ovgp1 | Oviductal glycoprotein 1 | Non-ciliated oviductal epithelial cells | [146] |
| Pax8 | Paired box gene 8 | Fallopian tube, cervix, uterus, and endometrium | [147] |
| Pgr | Progesterone receptor | Oviduct: epithelium Uterus: epithelium, stroma, myometrium Ovary: time-limited granulosa cells | [148] |
| Mouse Allele | Gene Name and Mouse Ref | Effect of Cre Recombination | Endometriosis-Associated Ovarian Cancer Implications and Ref. |
|---|---|---|---|
| Arid1af/f | AT-rich interactive domain 1A | ARID1A loss | 46–95% of clear-cell and 30% of endometrioid tumors have loss of ARID1A [30,43,44,45] |
| Apcf/f | Adenomatous polyposis coli | Overexpression of β-catenin | Mutations in APC lead to activation of β-catenin which is frequently activated in endometrioisis-associated ovarian cancers [149] |
| Ctnnb1f/f | Catenin beta-1 | Overexpression of β-catenin | 16–54% of endometriod ovarian cancers have mutations in β-catenin, leading to nuclear localization, and activation of wingless integration site (WNT) signaling [150,151,152,153] |
| Kraslsl-G12D | Kirsten rat sarcoma | Expression of oncogenic Kras | 29% of low-grade endometrioid ovarian tumors with concurrent endometriosis [33] |
| MUC1+/− | Mucin 1 | Expression human MUC1 in mouse | Expressed in endometrium and endometriosis; potential biomarker for endometriosis or ovarian cancer [154] |
| Pik3caH1047R | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha | Mutation in Pik3ca kinase domain | 20% of clear-cell and 20% of endometrioid ovarian cancers with mutations [155] |
| Ptenf/f | Phosphatase and tensin homolog | PTEN loss and activation of AKT | 20% of clear-cell and 20% of endometrioid cancers [156] |
| Genotype | Phenotype | Penetrance | Details | Ref. |
|---|---|---|---|---|
| Arid1af/f;AdCre (Ovarian bursa) | No cancer | 0/29 with adnexal masses 0/42 with adnexal masses | No endometriosis | [157,158] |
| Arid1af/f;Amhr2Cre | No cancer | 0/20 with adnexal masses | No endometriosis | [159] |
| Arid1af/f;PgrCre | No cancer | 0/20 with adnexal masses | No endometriosis | [160] |
| Ptenf/f;AdCre (Ovarian bursa) | No cancer | 0/5 with adnexal masses 0/63 with adnexal masses | No endometriosis | [158,161] |
| Ptenf/f;AdCre (Infundibulum to ovarian bursa) | Low penetrance endometrioid ovarian cancer at 26 weeks | 8/13 with ovarian endometriosis like lesions 1/13 with ovarian cancer by 26 weeks | Endometriosis-like lesions of ovary (lacked stromal component) | [162] |
| Ptenf/f;Cyp19Cre | No cancer | 0/4 with adnexal masses | No endometriosis | [163] |
| Ptenf/f;Amhr2Cre | Granulosa cell tumor | 5/70 with ovarian cancers by 7 months | No endometriosis | [164] |
| Ptenf/f;Apcf/f;Ovgp1Cre | Endometrioid ovarian carcinoma | 10/15 with ovarian cancers | Metastatic lesions | [146] |
| Ptenf/f;Pax8Cre | Endometrioid oviductal adenocarcinoma | 3/4 with oviductal cancers by 7 months | Oviductal tumors metastasized to ovary | [147] |
| Pik3caH1047R;AdCre (Ovarian bursa) | No cancer | 0/6 with adnexal masses | 4/5 ovarian surface epithelium hyperplasia (microscopic) | [157] |
| KrasG12D;AdCre (Infundibulum to ovarian bursa) | 15/15 endometriosis-like lesions of ovary | 15/15 with endometriosis-like lesions of ovary | Endometriosis-like lesions of ovary (lacked stromal component) | [162] |
| KrasG12D;AdCre (Uterotubal injection to ovarian bursa) | 7/15 with peritoneal endometriosis | 7/15 with peritoneal endometriosis | Peritoneal endometriosis | [162] |
| KrasG12D;AdCre (IP injection) | No cancer | 0/13 with adnexal masses | No endometriosis | [162] |
| KrasG12D;Amhr2Cre | No cancer | 0/4 with adnexal masses | No endometriosis Abnormal follicles | [145,163] |
| KrasG12D;Cyp19Cre | No cancer | 0/4 with adnexal masses | No endometriosis Abnormal follicles | [145,163] |
| KrasG12D;PgrCre | No cancer | 0/3 with adnexal masses | No endometriosis | [163] |
| Ctnnb1f/+;Amhr2Cre | Endometrioid ovarian carcinoma | 5/6 with ovarian cancer by 6 months | No endometriosis | [165] |
| Arid1af/f;Pik3caH1047R;AdCre (Ovarian bursa) | Poorly differentiated clear-cell ovarian carcinoma | 23/30 with ovarian cancer by 7 weeks | 77% penetrance No endometriosis Aggressive metastatic tumors | [157] |
| Arid1af/f;Ptenf/f;AdCre (Ovarian bursa) | 5/13 endometrioid ovarian carcinoma 8/13 undifferentiated adenocarcinoma | 13/22 with ovarian cancer by 9 months | 59% penetrance No endometriosis Aggressive undifferentiated tumors | [158] |
| Apcf/f;PgrCre | Endometrioid ovarian carcinoma | 12/43 with ovarian cancer | No endometriosis 16% endometrioid ovarian cysts | [166] |
| Ptenf/f;Apcf/f;AdCre (Ovarian bursa) | Endometrioid ovarian carcinoma | 29/29 with ovarian cancer | 100% penetrance No endometriosis Aggressive metastatic tumors | [161] |
| Ptenf/f;Apcf/f;Pik3caH1047R;AdCre (Ovarian bursa) | Endometrioid ovarian carcinoma | 11/11 with ovarian cancer | No endometriosis Aggressive metastatic tumors | [167] |
| KrasG12D;Ptenf/f;AdCre (Infundibulum to ovarian bursa) | Endometrioid ovarian carcinoma | 9/9 with ovarian cancer by 12 weeks | 100% penetrance Aggressive metastatic disease No endometriosis | [162] |
| MUC1+/-;KrasG12D;AdCre (Ovarian bursa) | Endometriosis-like lesions of ovary | No ovarian cancer | endometriosis-like lesions of ovary | [168] |
| Ctnnb1f/+;Ptenf/f;Amhr2Cre | Endometrioid ovarian carcinoma | 5/5 with ovarian cancer by 6 weeks | No endometriosis | [165] |
| KrasG12D;Ptenf/f;Amhr2Cre | Low grade ovarian serous papillary adenocarcinomas | 100% with ovarian tumors by 10 weeks | No endometriosis | [143,163] |
| KrasG12D;Ptenf/f;PgrCre | No cancer | 0/3 with adnexal masses | No endometriosis | [163] |
| KrasG12D;Ptenf/f;Cyp19Cre | No cancer | 0/3 with adnexal masses | No endometriosis | [163] |
| Cell Line | Original Derivation | Putative Histotype by Molecular Studies | Genetic Mutations | Genetic Gains | Ref. |
|---|---|---|---|---|---|
| 11Z | Red peritoneal endometriotic lesion | Benign | Unknown | Unknown | [183] |
| 12Z | Red peritoneal endometriotic lesion | Benign (epithelial-like) | Unknown | Unknown | [183] |
| EEC16 | Benign endometriotic lesion (epithelial-like) | Benign | Unknown | Unknown | [184] |
| EMosis-CC/TERT | Benign endometriotic lesion (epithelial-like) | Benign | Unknown | Unknown | [185] |
| 22B | Red peritoneal endometriotic lesion (Stromal/fibroblast-like) | Benign | Unknown | Unknown | [183] |
| Hs 832(C).T (CRL-7566) | Benign endometriotic ovarian cyst | Benign | Unknown | Unknown | ATCC |
| OVTOKO | Clear-cell (spleen metastasis) | Clear-cell | None | ERRB2, HNF1B, MET, PPM1D, STAT3, TP53, YAP1, ZNF217, CDKN2A, CDKN2B | [177,178,179,182] |
| OVMANA | Clear-cell (primary tumor) | Clear-cell | BRCA2, PIK3CA, ARID1A | ARID1A, MET, PPM1D, TP53, ZNF217 | [178,179,182,186] |
| TOV21G | Clear-cell (primary tumor) | Clear-cell | KRAS, PTEN, PIK3CA, CTNNB1, ARID1A, TPX2 | [178,179,180,181,182,187] | |
| RMG-1 | Clear-cell (ascites) | Clear-cell | TP53 * | ERBB2 | [178,179,182,188] |
| RMG-2 | Clear-cell | Clear-cell | PPP2R1A, ARID1A | ERBB2, HNF1B, MET, PIK3CA, PPM1D, STAT3, ZNF217, CDKN2A, CDKN2B | [179] |
| OCC1 | Clear-cell | Clear-cell | [189] | ||
| JHOC-5 | Clear-cell (pelvic metastasis) | Clear-cell | ARID1A, ERBB2, HNF1B, MET, PIK3CA, PPM1D, STAT2, ZNF217, CDKN2A, CDKN2B | [178,179,182,190] | |
| JHOC-7 | Clear-cell | Clear-cell | PIK3CA | ARID1A, HNF1B, PIK3CA, PPM1D, STAT3, ZNF217 | [179] |
| JHOC-9 | Clear-cell | Clear-cell | PTEN, ARID1A | HNF1B, ZNF217 | [179] |
| ES2 | Poorly differentiated clear-cell (primary tumor) | Endometrioid/Clear-cell | BRAF, TP53, APC, MYC | [178,179,180,181,182,191] | |
| OVISE | Clear-cell (pelvic metastasis) | Endometrioid/Clear-cell | ARID1A | [177,178,179,182] | |
| OVSAYO | Clear-cell | Serous | TP53 | [179] | |
| TOV112D | Endometrioid (primary tumor) | Endometrioid | CTNNB1, TP53 | [179,180,181,182,187] | |
| OVK18 | Endometrioid (ascites) | Endometrioid | TP53, PTEN, KRAS, ARID1A | [178,182,192] | |
| SNU-251 | Endometrioid | Endometrioid | BRCA1 | [193] | |
| 2008 | Endometrioid | Atypical non-serous | TP53 | [179] | |
| IGROV1 | Endometrioid with serous/clear cell (primary tumor) | Endometrioid/Clear-cell | PTEN, TP53, ARID1A, BRCA1, BRCA2, PIK3CA, TPX2 | [178,179,180,182,194] | |
| 59M | Endometrioid with clear cell (ascites) | Endometrioid/Clear-cell | TP53 | MYC | [178,180,182,193,195] |
| COV362 | Endometrioid (pleural effusion) | Serous | TP53, BRCA1, RB1 *, EGFR, APC | MYC | [178,180,182,196] |
| A2780 | Unknown adenocarcinoma | Endometrioid | PTEN, ARID1A, PIK3CA, BRAF | [178,179,180,181,182,197] | |
| HEYA8 | Moderately differentiated papillary serous (peritoneal metastasis) | Unlikely serous | KRAS, BRAF | [178,179,182,198] | |
| SKOV3 | Well differentiated, adenocarcinoma (ascites) | Endometrioid/Clear-cell | PIK3CA, ARID1A | ERBB2 | [178,179,180,181,182,199] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendel, J.R.H.; Wang, X.; Hawkins, S.M. The Endometriotic Tumor Microenvironment in Ovarian Cancer. Cancers 2018, 10, 261. https://doi.org/10.3390/cancers10080261
Wendel JRH, Wang X, Hawkins SM. The Endometriotic Tumor Microenvironment in Ovarian Cancer. Cancers. 2018; 10(8):261. https://doi.org/10.3390/cancers10080261
Chicago/Turabian StyleWendel, Jillian R. Hufgard, Xiyin Wang, and Shannon M. Hawkins. 2018. "The Endometriotic Tumor Microenvironment in Ovarian Cancer" Cancers 10, no. 8: 261. https://doi.org/10.3390/cancers10080261
APA StyleWendel, J. R. H., Wang, X., & Hawkins, S. M. (2018). The Endometriotic Tumor Microenvironment in Ovarian Cancer. Cancers, 10(8), 261. https://doi.org/10.3390/cancers10080261






