Radiotherapy for T1N0M0 Esophageal Cancer: Analyses of the Predictive Factors and the Role of Endoscopic Submucosal Dissection in the Local Control
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Treatment Outcomes
2.3. Toxicity
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Treatment
4.3. Follow-Up and Evaluation
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, G.Q.; Jiao, G.G.; Chang, F.B.; Fang, W.H.; Song, J.X.; Lu, N.; Lin, D.M.; Xie, Y.Q.; Yang, L. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann. Thorac. Surg. 2004, 77, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Hirahara, N.; Kinugasa, S.; Yoshimura, H. Clinicopathologic Features of Superficial Esophageal Cancer: Results of Consecutive 100 Patients. Ann. Surg. Oncol. 2008, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Igaki, H.; Kato, H.; Tachimori, Y.; Daiko, H.; Fukaya, M.; Yajima, S.; Nakanishi, Y. Clinicopathologic characteristics and survival of patients with clinical Stage I squamous cell carcinomas of the thoracic esophagus treated with three-field lymph node dissection. Eur. J. Cardio Thorac. Surg. 2001, 20, 1089–1094. [Google Scholar] [CrossRef]
- Griffin, M.S.; Shaw, I.H.; Dresner, S.M. Early complications after Ivor Lewis subtotal esophagectomy with two-field lymphadenectomy: Risk factors and management. J. Am. Coll. Surg. 2002, 194, 285–297. [Google Scholar] [CrossRef]
- Tachibana, M.; Kinugasa, S.; Hiroshi, Y.; Shibakita, M.; Tonomoto, Y.; Dhar, D.; Nagasue, N. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. J. Am. Coll. Surg. 2005, 189, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Fujishiro, M.; Niimi, K.; Goto, O.; Kodashima, S.; Yamamichi, N.; Omata, M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest. Endosc. 2009, 70, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Hassan, C.; Carlino, A.; Pagano, N.; Zullo, A.; Rando, G.; Strangio, G.; Romeo, F.; Nicita, R.; Rosati, R.; et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest. Endosc. 2010, 71, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Sato, A.; Fukuda, H.; Kagami, Y.; Udagawa, H.; Togo, A.; Ando, N.; Tanaka, O.; Shinoda, M.; Yamana, H.; et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn. J. Clin. Oncol. 2009, 39, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Kodaira, T.; Tachibana, H.; Tomita, N.; Makita, C.; Itoh, M.; Abe, T.; Muro, K.; Tajika, M.; Niwa, Y.; et al. Clinical outcome of definitive radiation therapy for superficial esophageal cancer. Jpn. J. Clin. Oncol. 2017, 47, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.Y.; Kountouri, M.; Roth, A.; Jean-Loui, F.; Huber, O.; Mönig, S.; Zilli, T. Endoscopic resection with adjuvant chemo-radiotherapy for superficial esophageal squamous cell carcinoma: A critical review. Crit. Rev. Oncol. Hematol. 2018, 124, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Ishihara, R.; Motoori, M.; Kawaguchi, Y.; Uedo, N.; Takeuchi, Y.; Higashino, K.; Yano, M.; Nakamura, S.; Iishi, H. Comparison Between Definitive Chemoradiotherapy and Esophagectomy in Patients with Clinical Stage I Esophageal Squamous Cell Carcinoma. Am. J. Gastroenterol. 2011, 106, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Motoori, M.; Yano, M.; Ishihara, R.; Yamamoto, S.; Kawaguchi, Y.; Tanaka, K.; Kishi, K.; Miyashiro, I.; Fujiwara, Y.; Shingai, T.; et al. Comparison Between Radical Esophagectomy and Definitive Chemoradiotherapy in Patients with Clinical T1bN0M0 Esophageal Cancer. Ann. Surg. Oncol. 2012, 19, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, G.; Sasamoto, R.; Abe, E.; Ohta, A.; Sato, H.; Tanaka, K.; Maruyama, K.; Kaizu, M.; Ayukawa, F.; Yamana, N.; et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat. Oncol. 2015, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Kumashiro, R.; Hisamatsu, Y.; Nakanishi, R.; Egashira, A.; Saeki, H.; Oki, E.; Ohga, T.; Kakeji, Y.; Tsujitani, S.; et al. Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J. Gastroenterol. 2011, 46, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Ishihara, R.; Yamasaki, Y.; Hanaoka, N.; Yamamoto, S.; Arao, M.; Suzuki, S.; Iwatsubo, T.; Kato, M.; Tonai, Y.; et al. Efficacy and Safety of Endoscopic Resection Followed by Chemoradiotherapy for Superficial Esophageal Squamous Cell Carcinoma: A Retrospective Study. Clin. Transl. Gastroenterol. 2017, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Uchinami, Y.; Myojin, M.; Takahashi, H.; Harada, K.; Shimizu, S.; Hosokawa, M. Prognostic factors in clinical T1N0M0 thoracic esophageal squamous cell carcinoma invading the muscularis mucosa or submucosa. Radiat. Oncol. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Muto, M.; Minashi, K.; Boku, N.; Fukuda, H. A phase II trial of combined treatment of endoscopic mucosal resection and chemoradiotherapy for clinical stage I esophageal carcinoma: Japan clinical oncology group study (JCOG0508). Jpn. J. Clin. Oncol. 2009, 39, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Fujishiro, M.; Niimi, K.; Goto, O.; Kodashima, S.; Yamamichi, N.; Omata, M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009, 41, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Hisano, O.; Nonoshita, T.; Hirata, H.; Sasaki, T.; Watanabe, H.; Wakiyama, H.; Ono, M.; Ohga, S.; Honda, H. Additional radiotherapy following endoscopic submucosal dissection for T1a-MM/T1b-SM esophageal squamous cell carcinoma improves locoregional control. Radiat. Oncol. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Takenaka, R.; Omori, M.; Imae, T.; Okuma, K.; Ohtomo, K.; Nakagawa, K. Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: A single institutional retrospective study. Radiat. Oncol. 2015, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, M.; Nihei, K.; Ishikura, S.; Minashi, K.; Yano, T.; Muto, M.; Ohtsu, A.; Ogino, T. Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother. Oncol. 2009, 92, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Ma, J.B.; Liu, G.; Wu, K.; Shi, X.; Jiang, G.L. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, H.; Nishimura, Y.; Oyama, T.; Kato, H.; Kitagawa, Y.; Kusano, M.; Shimada, H.; Takiuchi, H.; Toh, Y.; Doki, Y.; et al. Guidelines for diagnosis and treatment of carcinoma of the Esophagus April 2012 edited by the Japan esophageal society. Esophagus 2015, 12, 1–30. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Cancer. Common Terminology Criteria for Adverse Events (CTCAE); National Institute of Cancer: Rockville, MD, USA, 2010. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
| ESD 2 Group | Non-ESD Group | p Value | |
|---|---|---|---|
| Characteristic | (n = 29) | (n = 21) | |
| Age (years) | |||
| Median (range) | 68 (50–82) | 75 (59–87) | 0.018 |
| Sex, n | |||
| Male | 24 | 17 | 0.99 |
| Female | 5 | 4 | |
| Performance status, n | |||
| 0 | 25 | 16 | 0.59 |
| ≥1 | 4 | 5 | |
| Main tumor location, n | |||
| Upper thorax | 4 | 4 | 0.44 |
| Middle thorax | 13 | 10 | |
| Lower thorax | 12 | 7 | |
| T Stage, n | |||
| T1a | 8 | 3 | 0.44 |
| T1b | 21 | 18 | |
| Tumor length (cm) | |||
| Median (range) | 2.3 (1–10) | 5 (1–20) | 0.017 |
| Tumor number, n | |||
| 1 | 5 | 6 | 0.34 |
| ≥2 | 24 | 15 | |
| Concurrent chemotherapy, n | |||
| yes | 28 | 18 | 0.39 |
| no | 1 | 3 | |
| Radiation field, n | |||
| ENI 1 | 13 | 4 | 0.11 |
| Non-ENI | 16 | 17 | |
| Total radiation dose, n | |||
| ≤50.4 Gy | 26 | 12 | 0.02 |
| >50.4 Gy | 3 | 9 |
| Number (%) | Depth of Invasion | ||||
|---|---|---|---|---|---|
| All Patients | M3 | SM1 | SM2 | SM3 | |
| Number | 29 | 8 | 4 | 16 | 1 |
| Resection status | |||||
| R0 1 resection | 19 (66%) | 6 | 2 | 11 | 0 |
| R1 2 resection | 10 (34%) | 2 | 2 | 5 | 1 |
| Lymphovascular invasion | |||||
| Positive | 20 (69%) | 5 | 3 | 11 | 1 |
| Negative | 9 (31%) | 3 | 1 | 5 | 0 |
| Poorly differentiated histology | |||||
| Yes | 4 (14%) | 1 | 0 | 3 | 0 |
| No | 25 (86%) | 7 | 4 | 13 | 1 |
| Age | Sex | PS 12 | T Stage | Tumor Length (cm) | RT 13 Field | RT Dose (Gy) | ESD 8 | Resection Status | Lymphovasucular Invasion | Depth of Invasion | Poorly Differentiated Histology | CCRT 4 | Months to Disease Recurrence | Recurrence Site | Salvage Therapy | Status at Last Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 68 | Male | 0 | T1b | 2 | ENI 7 | 40 | + | R0 14 | + | SM1 | – | + | 11 | Metachronous esohageal lesion (IF 9) | ESD | ANED 1 20 m 11 |
| 60 | Male | 0 | T1b | 8 | ENI | 50 | + | R1 15 | + | SM3 | – | + | 24 | Distance | chemo | DID 5 33 m |
| 69 | Male | 1 | T1b | 2.1 | ENI | 40 | + | R0 | + | SM2 | – | + | 10 | Metachronous esohageal lesion (OF 10) | ESD | ANED 45 m |
| 59 | Male | 0 | T1a | 3.4 | Non-ENI | 50.4 | + | R0 | – | M3 | + | + | 50 | Metachronous esohageal lesion (OF) | ESD | ANED 79 m |
| 61 | Male | 0 | T1b | 1 | Non-ENI | 50 | + | R0 | + | SM2 | – | + | 40 | Regional (OF) | CRT | ANED 98 m |
| 75 | Male | 1 | T1b | 2.5 | Non-ENI | 60 | + | R1 | + | SM2 | + | + | 9 | Regional (OF) | chemo | AWD 3 21 m |
| 65 | Male | 0 | T1b | 10 | Non-ENI | 40 | + | R0 | – | SM2 | – | + | 27 | Local | APC 2 | ANED 112 m |
| 57 | Male | 0 | T1b | 1 | Non-ENI | 60 | + | R1 | + | SM2 | – | – | 24 | Regional (IF) | chemo | DID 34 m |
| 86 | Male | 0 | T1b | 6 | Non-ENI | 42 | – | – | 2 | Local | chemo | DID 31 m | ||||
| 61 | Male | 0 | T1b | 5 | ENI | 50.4 | – | + | 4 | Metachronous esohageal lesion (OF) | ESD | ANED 65 m | ||||
| 71 | Male | 0 | T1b | 6 | Non-ENI | 50.4 | – | + | 12 | Local | chemo | DID 34 m | ||||
| 75 | Male | 0 | T1b | 1.5 | Non-ENI | 60 | – | + | 20 | Metachronous esohageal lesion (OF) | ESD | ANED 92 m | ||||
| 83 | Female | 2 | T1b | 3 | Non-ENI | 50 | – | + | 2 | Local | no | AWD 39 m | ||||
| 80 | Male | 1 | T1b | 8 | Non-ENI | 54 | – | + | 22 | Regional (OF) | no | DOD 6 23 m | ||||
| 70 | Male | 0 | T1b | 1.5 | Non-ENI | 60 | – | + | 28 | Distance | chemo | DID 38 m |
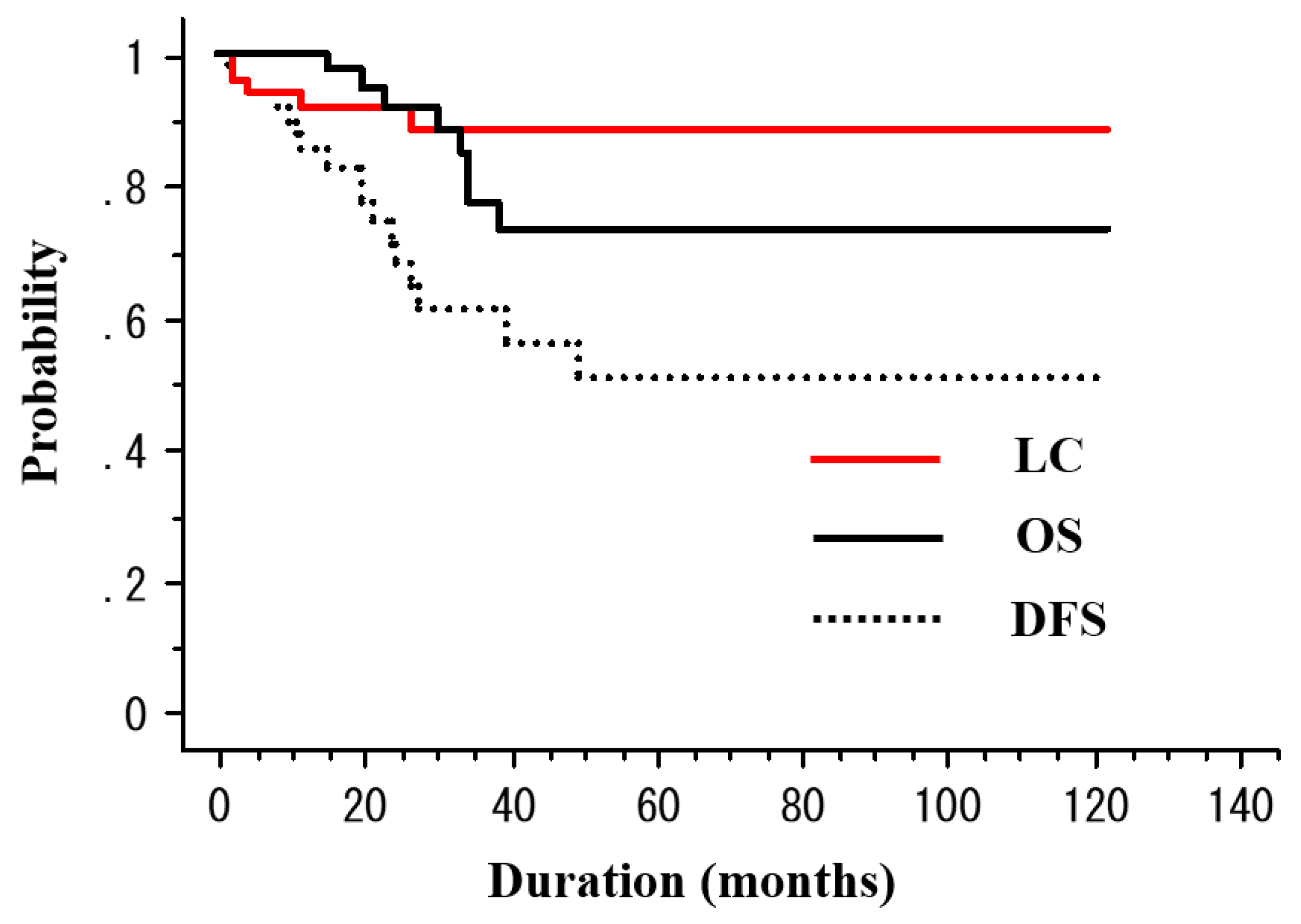
| N | 3-Year DFS 1 (%) | p Value | 3-Year LC 4 (%) | p Value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≤70 | 24 | 62.2 | 0.99 | 87.9 | 0.63 |
| >70 | 26 | 61.1 | 89 | ||
| Sex | |||||
| Male | 41 | 57.8 | 0.38 | 88.3 | 0.82 |
| Female | 9 | 90 | 90 | ||
| PS 5 | |||||
| 0 | 41 | 66.1 | 0.08 | 88.1 | 0.86 |
| ≥1 | 9 | 38.1 | 90 | ||
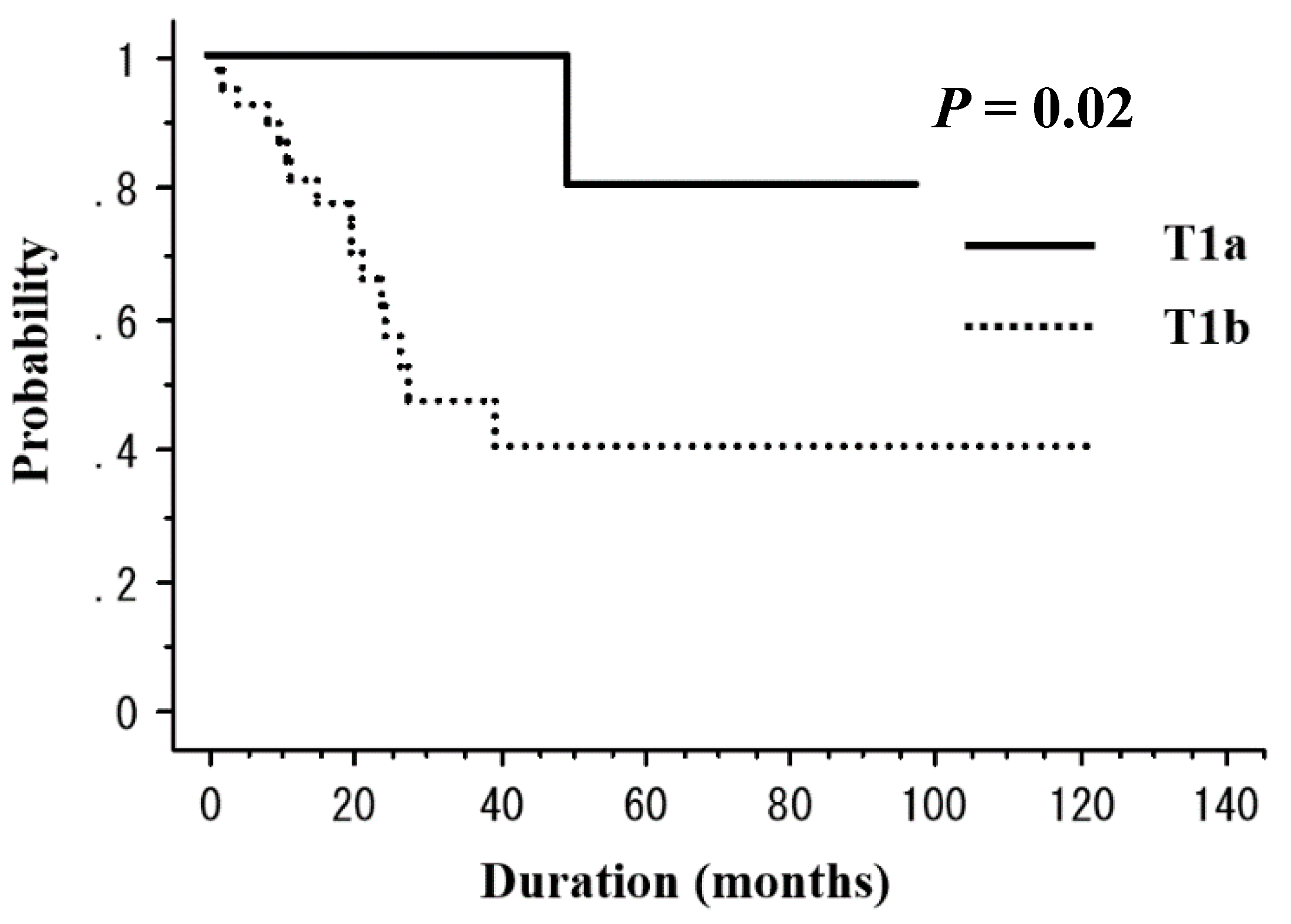
| T stage | |||||
| T1a | 11 | 100 | 0.02 | 100 | 0.18 |
| T1b | 39 | 47.1 | 83.8 | ||
| Tumor number | |||||
| 1 | 39 | 67.7 | 0.43 | 87.7 | 0.89 |
| ≥2 | 11 | 39 | 100 | ||
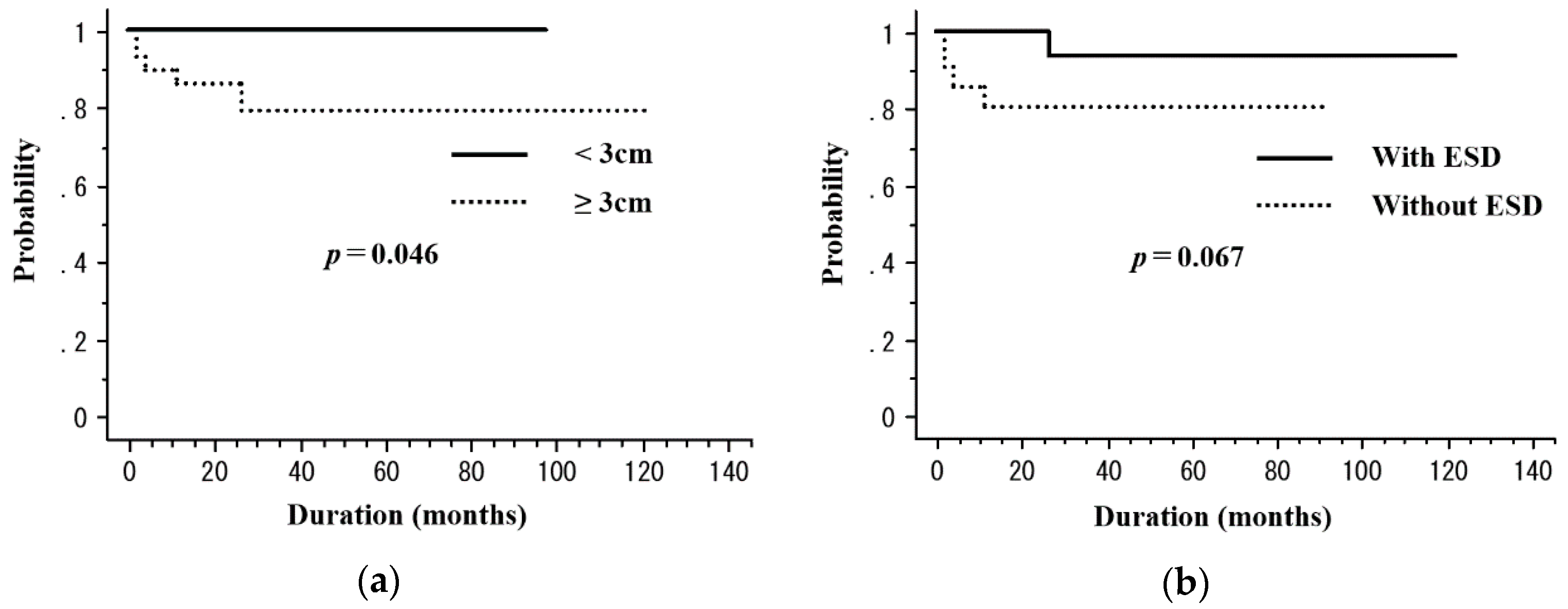
| Tumor length | |||||
| <3 cm | 21 | 55.1 | 0.55 | 100 | 0.046 |
| ≥3 cm | 29 | 65.1 | 79.3 | ||
| Radiation dose | |||||
| ≤50.4 Gy | 38 | 63.9 | 0.94 | 84.5 | 0.19 |
| >50.4 Gy | 12 | 53.9 | 100 | ||
| Radiation field | |||||
| ENI 2 | 17 | 69.9 | 0.65 | 100 | 0.53 |
| Non-ENI | 33 | 58.5 | 85.6 | ||
| ESD 3 | |||||
| Yes | 29 | 70 | 0.37 | 93.3 | 0.067 |
| No | 21 | 52.4 | 80.4 |
| Age (Years) | Sex | Adverse Event | Grade | RT Field | ESD 3 | CRT 1 | Tumor Length (cm) | Tumor Circumferential Extension | RT 4 Dose (Gy) |
|---|---|---|---|---|---|---|---|---|---|
| 80 | Female | Pericardial effusion | 2 | Non-ENI 2 | − | + | 20 | Entire | 50 |
| 70 | Female | Pericardial effusion | 2 | Non-ENI | − | + | 15 | Entire | 60 |
| 54 | Female | Esophageal strictures | 2 | ENI | + | + | 5 | 1/2 | 40 |
| 65 | Male | Esophageal strictures | 2 | ENI | + | + | 7.4 | 2/3 | 40 |
| 60 | Male | Esophageal strictures | 2 | ENI | + | + | 8 | 2/3 | 50 |
| 59 | Male | Esophageal strictures | 2 | Non-ENI | − | + | 14 | Entire | 50.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, G.; Yamazaki, H.; Aibe, N.; Masui, K.; Shimizu, D.; Kimoto, T.; Nishimura, T.; Nakashima, A.; Takenaka, T.; Dohi, O.; et al. Radiotherapy for T1N0M0 Esophageal Cancer: Analyses of the Predictive Factors and the Role of Endoscopic Submucosal Dissection in the Local Control. Cancers 2018, 10, 259. https://doi.org/10.3390/cancers10080259
Suzuki G, Yamazaki H, Aibe N, Masui K, Shimizu D, Kimoto T, Nishimura T, Nakashima A, Takenaka T, Dohi O, et al. Radiotherapy for T1N0M0 Esophageal Cancer: Analyses of the Predictive Factors and the Role of Endoscopic Submucosal Dissection in the Local Control. Cancers. 2018; 10(8):259. https://doi.org/10.3390/cancers10080259
Chicago/Turabian StyleSuzuki, Gen, Hideya Yamazaki, Norihiro Aibe, Koji Masui, Daisuke Shimizu, Takuya Kimoto, Takeshi Nishimura, Akihiro Nakashima, Tadashi Takenaka, Osamu Dohi, and et al. 2018. "Radiotherapy for T1N0M0 Esophageal Cancer: Analyses of the Predictive Factors and the Role of Endoscopic Submucosal Dissection in the Local Control" Cancers 10, no. 8: 259. https://doi.org/10.3390/cancers10080259
APA StyleSuzuki, G., Yamazaki, H., Aibe, N., Masui, K., Shimizu, D., Kimoto, T., Nishimura, T., Nakashima, A., Takenaka, T., Dohi, O., Ishikawa, T., & Yamada, K. (2018). Radiotherapy for T1N0M0 Esophageal Cancer: Analyses of the Predictive Factors and the Role of Endoscopic Submucosal Dissection in the Local Control. Cancers, 10(8), 259. https://doi.org/10.3390/cancers10080259







