Pancreatic Cancer Related Health Disparities: A Commentary
Abstract
1. Introduction
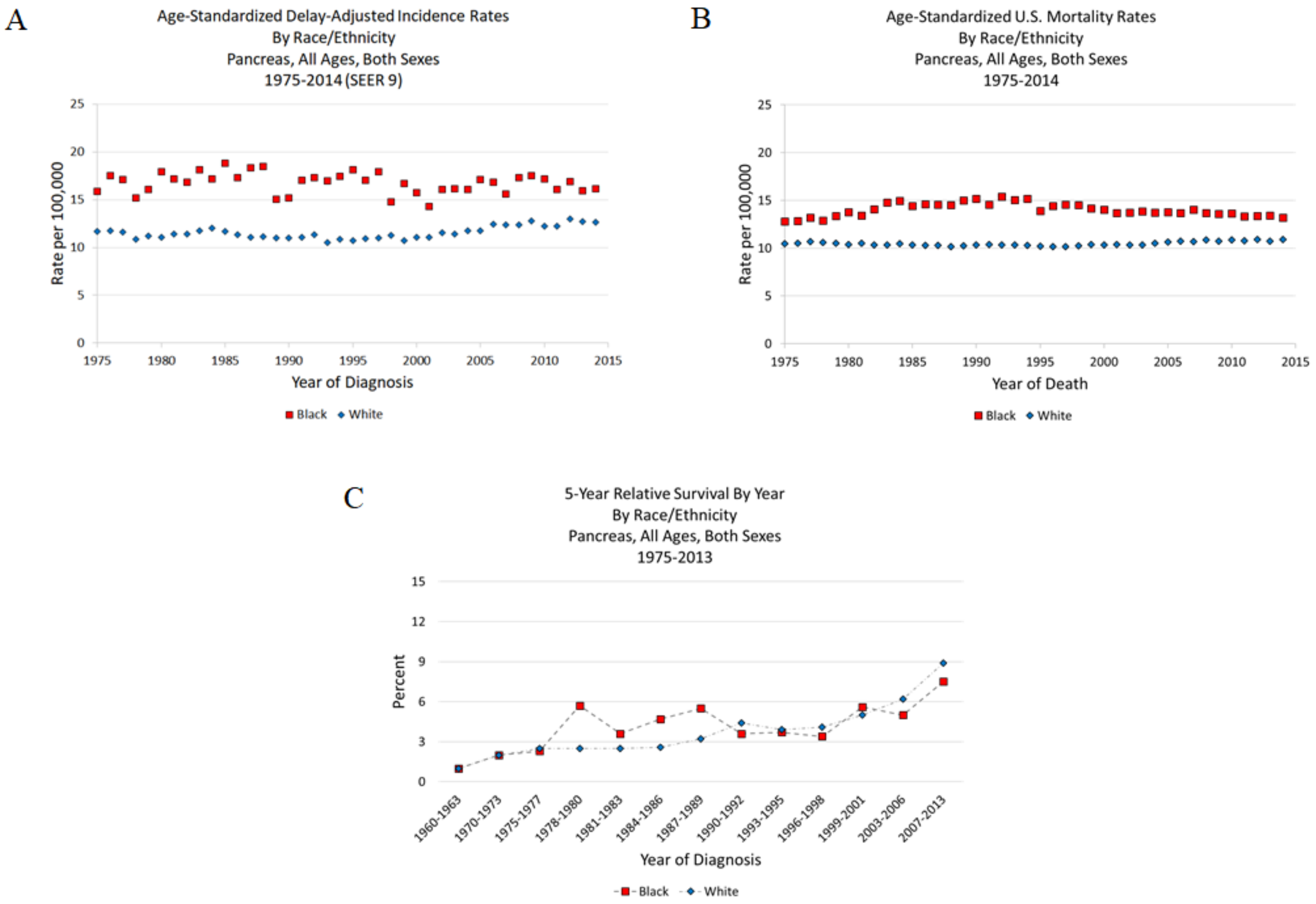
2. Pancreatic Cancer Statistics
3. Socioeconomic and Lifestyle Factors as Contributors to Pancreatic Cancer Disparities
3.1. Socioeconomic Factors
3.2. Lifestyle Factors
4. Biological Factors Potentially Contributing to Disparities
4.1. Zinc Deficiency Factor
4.2. Genomic Factors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenbert, R.; Rosenzweig, A.B.; Fleshman, J.M.; Martrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–2014 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Arnold, L.D.; Patel, A.V.; Yan, Y.; Jacobs, E.J.; Thun, M.J.; Calle, E.E.; Colditz, G.A. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol. Biomark. Prev. 2009, 18, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ma, H.; Hong, G.; Sun, H.; Wang, J. Survival improvement in patients with pancreatic cancer by decade: A period analysis of the SEER database, 1981–2010. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https://seer.cancer.gov/csr/1975_2014/ (accessed on 2 April 2018).
- Ellis, L.; Woods, L.M.; Esteve, J.; Eloranta, S.; Coleman, M.P.; Rachet, B. Cancer incidence, survival, and mortality: Explaining the concepts. Int. J. Cancer 2014, 135, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Khawja, S.L.; Mohammed, S.; Silberfein, E.J.; Musher, B.L.; Fisher, W.E.; Van Buren, G. Pancreatic cancer disparities in African Americans. Pancreas 2015, 44, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Shavers, V.L.; Harlan, L.C.; Jackson, M.; Robinson, J. Racial/ethnic patterns of care for pancreatic cancer. J. Palliat. Med. 2009, 12, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.; Tramontano, A.C.; Kong, C.Y.; Pandharipande, P.; Dowling, E.C.; Shrag, D.; Hur, C. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med. 2018, 7, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vargas-Bustamante, A.; Mortensen, K.; Ortega, A.N. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med. Care 2016, 54, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Siegel, R.; Jemal, A. Pancreatic cancer death rates by race among US men and women, 1970–2009. J. Natl. Cancer Inst. 2013, 105, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Factors That Contribute to Health Disparities in Cancer. Available online: https://www.cdc.gov/cancer/healthdisparities/basic_info/challenges.htm (accessed on 5 April 2018).
- Singh, G.K.; Jemal, A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: Over six decades of changing patterns and widening inequalities. J. Environ. Public Health 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Hoover, R.N.; Bown, L.M.; Swanson, G.M.; Schiffman, M.; Greenberg, R.S.; Hayes, R.B.; Lillemoe, K.D.; Schoenberg, J.B.; Schwartz, A.G.; et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans. Epidemiology 2013, 14, 45–54. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Bethea, T.N.; Kitahara, C.M.; Sonderman, J.; Patel, A.V.; Harvey, C.; Knutsen, S.F.; Park, Y.; Park, S.Y.; Fraser, G.E.; Jacobs, E.J.; et al. A pooled analysis of body mass index and pancreatic cancer mortality in African Americans. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistic Report, 2017; Centers for Disease Control and Prevention, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2017.
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O.; Thun, M.J.; Jemal, A.; Ward, E.M. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2908–2912. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Y.; Elo, I.T. The contribution of smoking to Black-White differences in U.S. mortality. Demography 2013, 50, 545–568. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.; Naishadham, D.; Jemal, A. Cancer statistics for African Americans, 2013. CA Cancer J. Clin. 2013, 63, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.W. Smoking and the health gap in minorities. Ann. Epidemiol. 1993, 3, 159–164. [Google Scholar] [CrossRef]
- Singal, V.; Singal, A.K.; Kuo, Y.F. Racial disparities in treatment for pancreatic cancer and impact on survival: A population-based analysis. J. Cancer Res. Clin. Oncol. 2012, 138, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, L.; Welton, M.; Robb, S.W. Racial disparities of pancreatic cancer in Georgia: A county-wide comparison of incidence and mortality across the state, 2000–2011. Cancer Med. 2016, 5, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat. Res. 2001, 475, 7–20. [Google Scholar] [CrossRef]
- Ames, B.N.; Wakimoto, P. Are vitamin and mineral deficiencies a major cancer risk? Nat. Rev. Cancer 2002, 2, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.X.; Han, B.; Shaw, D.G.; Nimni, M. Zinc as a possible preventive and therapeutic agent in pancreatic, prostate, and breast cancer. Eur. J. Cancer Prev. 2016, 25, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.-M.; Cousins, R.J.; et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Liu, Y.; Zou, J.; Franklin, R.B. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J. Biol. Chem. 1999, 274, 17499–17504. [Google Scholar] [CrossRef] [PubMed]
- Mawson, C.; Fischer, M. The occurrence of zinc in the human prostate gland. Can. J. Med. Sci. 1952, 30, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V.Y.; Sviridova, T.; Zaichick, S. Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int. Urol. Nephrol. 1997, 29, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Ogunlewe, J.; Osegbe, D. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer 1989, 63, 1388–1392. [Google Scholar] [CrossRef]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Levy, B.A.; Desouki, M.M.; Zou, J.; Bagasra, O.; Johnson, L.A.; Hanna, N.; Franklin, R.B. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Boil. Ther. 2011, 12, 297–303. [Google Scholar] [CrossRef]
- Farzin, L.; Moassesi, M.E.; Sajadi, F.; Faghih, M.A.A. Evaluation of trace elements in pancreatic cancer patients in Iran. Middle East J. Cancer 2013, 4, 79–86. [Google Scholar]
- Rishi, I.; Baidouri, H.; Abbasi, J.A.; Bullard-Dillard, R.; Kajdacsy-Balla, A.; Pestaner, J.P.; Skacel, M.; Tubbs, R.; Bagasra, O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Engelken, J.; Carnero-Montoro, E.; Pybus, M.; Andrews, G.K.; Lalueza-Fox, C.; Comas, D.; Sekler, I.; de la Rasilla, M.; Rosas, A.; Stoneking, M.; et al. Extreme population differences in the human zinc transporter ZIP4 (SLC39A4) are explained by positive selection in Sub-Saharan Africa. PLoS Genet. 2014, 10, e1004128. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.E.; Burch, J.B.; Hussey, J.; Temples, T.; Bolick-Aldrich, S.; Mosley-Broughton, C.; Liu, Y.; Hebert, J.R. Soil zinc content, groundwater usage, and prostate cancer incidence in South Carolina. Cancer Causes Control 2009, 20, 345. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G.; Chaplin, G. Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 8962–8968. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Rizzato, C.; Kraft, P.; Kooperberg, C.; Petersen, G.M.; Wang, Z.; Arslan, A.A.; Beane-Freeman, L.; Bracci, P.M.; Buring, J.; et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat. Genet. 2014, 46, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.K.; Guda, C. Genome-wide DNA methylation analysis reveals molecular subtypes of pancreatic cancer. Oncotarget 2017, 8, 28990–29012. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Rubbi, L.; Dawson, D.W.; Donahue, T.R.; Pellegrini, M. Pancreatic cancer patient survival correlates with DNA methylation of pancreas development genes. PLoS ONE 2015, 10, e0128814. [Google Scholar] [CrossRef] [PubMed]
- Prevot, P.P.; Simion, A.; Grimont, A.; Colletti, M.; Khalaileh, A.; Van den Steen, G.; Sempoux, C.; Xu, X.; Roelants, V.; Hald, J.; et al. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut 2012, 61, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Couzin, J. Cancer research. Probing the roots of race and cancer. Science 2007, 315, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Y.; Ding, Y.B.; Liu, X.Q.; Chen, X.M.; Cheng, S.Q.; Li, L.B.; Ma, M.F.; He, J.L.; Wang, Y.X. Racial/ethnic disparities in human DNA methylation. Biochim. Biophys. Acta 2014, 1846, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Adkins, R.M.; Krushkal, J.; Tylavsky, F.A.; Thomas, F. Racial differences in gene-specific DNA methylation levels are present at birth. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Noone, A.M.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2015; National Cancer Institute: Bethesda, MD, USA, 2018. Available online: https://seer.cancer.gov/csr/1975_2015/ (accessed on 16 April 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarton, L.; Yoon, S.; Oh, S.; Agyare, E.; Trevino, J.; Han, B.; Lee, E.; Setiawan, V.W.; Permuth, J.B.; Schmittgen, T.D.; et al. Pancreatic Cancer Related Health Disparities: A Commentary. Cancers 2018, 10, 235. https://doi.org/10.3390/cancers10070235
Scarton L, Yoon S, Oh S, Agyare E, Trevino J, Han B, Lee E, Setiawan VW, Permuth JB, Schmittgen TD, et al. Pancreatic Cancer Related Health Disparities: A Commentary. Cancers. 2018; 10(7):235. https://doi.org/10.3390/cancers10070235
Chicago/Turabian StyleScarton, Lisa, Saunjoo Yoon, Sungho Oh, Edward Agyare, Jose Trevino, Bo Han, Eunsook Lee, Veronica Wendy Setiawan, Jennifer B. Permuth, Thomas D. Schmittgen, and et al. 2018. "Pancreatic Cancer Related Health Disparities: A Commentary" Cancers 10, no. 7: 235. https://doi.org/10.3390/cancers10070235
APA StyleScarton, L., Yoon, S., Oh, S., Agyare, E., Trevino, J., Han, B., Lee, E., Setiawan, V. W., Permuth, J. B., Schmittgen, T. D., Odedina, F. G., & Wilkie, D. J. (2018). Pancreatic Cancer Related Health Disparities: A Commentary. Cancers, 10(7), 235. https://doi.org/10.3390/cancers10070235






