Translation Control by p53
Abstract
1. Introduction
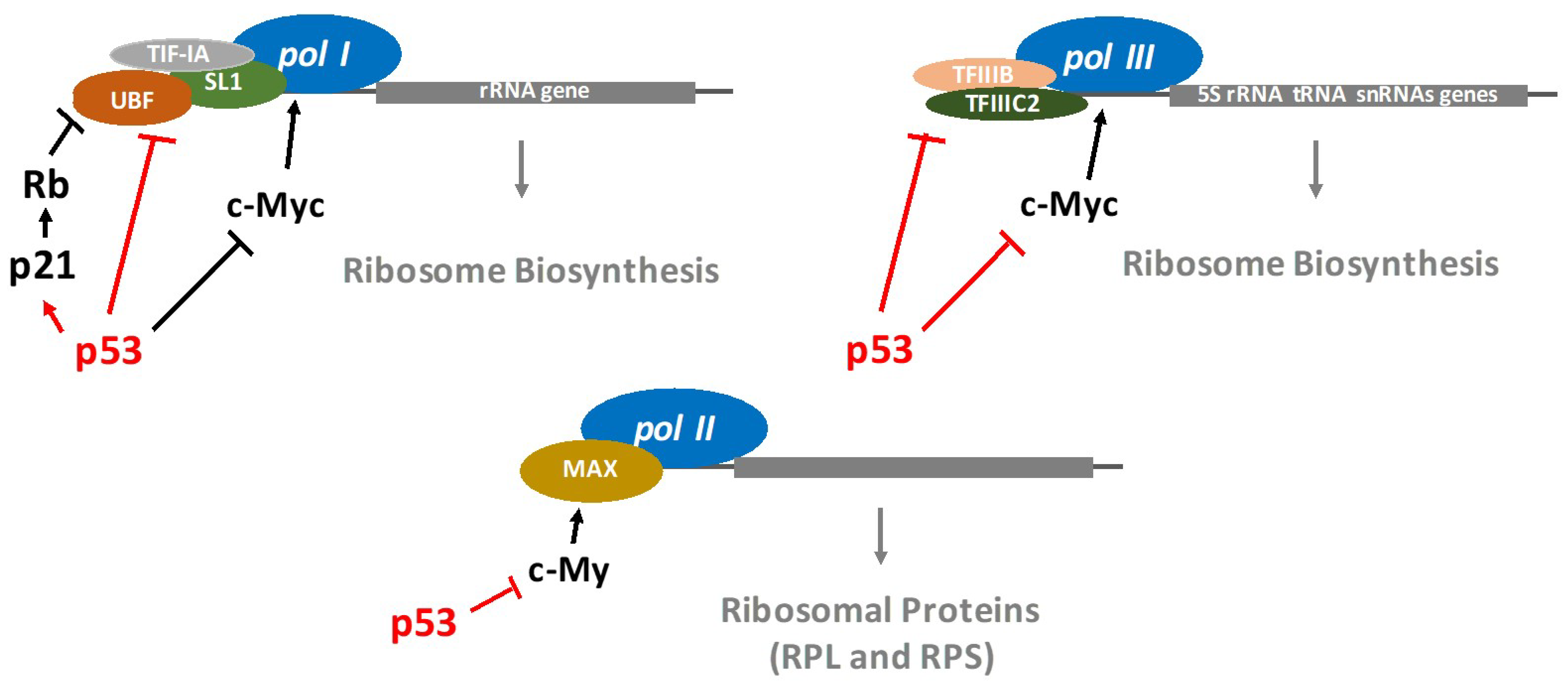
2. p53 Restricts the Ribosome Biogenesis
3. p53 Regulates the Transcription of RP Genes
4. p53 Regulates Ternary Complex and eIF4F Assembly Regulation
5. Genome-Wide Analysis
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chu, J.; Pelletier, J. Therapeutic Opportunities in Eukaryotic Translation. Cold Spring Harb. Perspect. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 2014, 83, 779–812. [Google Scholar] [CrossRef] [PubMed]
- Pain, V.M. Initiation of protein synthesis in eukaryotic cells. Eur. J. Biochem. 1996, 236, 747–771. [Google Scholar] [CrossRef] [PubMed]
- Brostrom, C.O.; Chin, K.V.; Wong, W.L.; Cade, C.; Brostrom, M.A. Inhibition of translational initiation in eukaryotic cells by calcium ionophore. J. Biol. Chem. 1989, 264, 1644–1649. [Google Scholar] [PubMed]
- Aktas, H.; Fluckiger, R.; Acosta, J.A.; Savage, J.M.; Palakurthi, S.S.; Halperin, J.A. Depletion of intracellular Ca2+ stores, phosphorylation of eIF2alpha, and sustained inhibition of translation initiation mediate the anticancer effects of clotrimazole. Proc. Natl. Acad. Sci. USA 1998, 95, 8280–8285. [Google Scholar] [CrossRef] [PubMed]
- Benzaquen, L.R.; Brugnara, C.; Byers, H.R.; Gatton-Celli, S.; Halperin, J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat. Med. 1995, 1, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Voigts-Hoffmann, F.; Klinge, S.; Ban, N. Structural insights into eukaryotic ribosomes and the initiation of translation. Curr. Opin. Struct. Biol. 2012, 22, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Bjornsti, M.A.; Houghton, P.J. Lost in translation: Dysregulation of cap-dependent translation and cancer. Cancer Cell 2004, 5, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 1991, 266, 19867–19870. [Google Scholar] [PubMed]
- Malka-Mahieu, H.; Newman, M.; Desaubry, L.; Robert, C.; Vagner, S. Molecular Pathways: The eIF4F Translation Initiation Complex-New Opportunities for Cancer Treatment. Clin. Cancer Res. 2017, 23, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.C.; Raught, B.; Gygi, S.P.; Niedzwiecka, A.; Miron, M.; Burley, S.K.; Polakiewicz, R.D.; Wyslouch-Cieszynska, A.; Aebersold, R.; Sonenberg, N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001, 15, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Cho, M.H.; Zakowicz, H.; Hegamyer, G.; Sonenberg, N.; Colburn, N.H. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol. Cell. Biol. 2004, 24, 3894–3906. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Topisirovic, I. Signaling pathways involved in the regulation of mRNA translation. Mol. Cell. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.; Melnikov, S.; de Loubresse, N.G.; Ben-Shem, A.; Iskakova, M.; Urzhumtsev, A.; Meskauskas, A.; Dinman, J.; Yusupova, G.; Yusupov, M. Crystal structure of the 80S yeast ribosome. Curr. Opin. Struct. Biol. 2012, 22, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Anger, A.M.; Armache, J.P.; Berninghausen, O.; Habeck, M.; Subklewe, M.; Wilson, D.N.; Beckmann, R. Structures of the human and Drosophila 80S ribosome. Nature 2013, 497, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.G.; Branco, A.T.; Godinho, S.A.; Yu, S.; Lemos, B. Concerted copy number variation balances ribosomal DNA dosage in human and mouse genomes. Proc. Natl. Acad. Sci. USA 2015, 112, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Stults, D.M.; Killen, M.W.; Pierce, H.H.; Pierce, A.J. Genomic architecture and inheritance of human ribosomal RNA gene clusters. Genome Res. 2008, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gentilella, A.; Kozma, S.C.; Thomas, G. A liaison between mTOR signaling, ribosome biogenesis and cancer. Biochim. Biophys. Acta. 2015, 1849, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.S.; Park, J.; Qin, Y.; Li, X.; Parsawar, K.; Larson, M.H.; Cox, J.; Cheng, Y.; Lambowitz, A.M.; Weissman, J.S.; et al. Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 2015, 347, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, N.; Sonenberg, N. Translational control and the cancer cell response to stress. Curr. Opin. Cell Biol. 2017, 45, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Quin, J.E.; Devlin, J.R.; Cameron, D.; Hannan, K.M.; Pearson, R.B.; Hannan, R.D. Targeting the nucleolus for cancer intervention. Biochim. Biophys. Acta 2014, 1842, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Sulima, S.O.; Hofman, I.J.F.; De Keersmaecker, K.; Dinman, J.D. How Ribosomes Translate Cancer. Cancer Discov. 2017, 7, 1069–1087. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P.; Armache, K.J.; Baumli, S.; Benkert, S.; Brueckner, F.; Buchen, C.; Damsma, G.E.; Dengl, S.; Geiger, S.R.; Jasiak, A.J.; et al. Structure of eukaryotic RNA polymerases. Annu. Rev. Biophys. 2008, 37, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Budde, A.; Grummt, I. p53 represses ribosomal gene transcription. Oncogene 1999, 18, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Comai, L. Repression of RNA polymerase I transcription by the tumor suppressor p53. Mol. Cell. Biol. 2000, 20, 5930–5938. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.; Ma, W.; Mao, D.Y.; Benchimol, S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol. Cell. Biol. 2005, 25, 7423–7431. [Google Scholar] [CrossRef] [PubMed]
- Bywater, M.J.; Poortinga, G.; Sanij, E.; Hein, N.; Peck, A.; Cullinane, C.; Wall, M.; Cluse, L.; Drygin, D.; Anderes, K.; et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012, 22, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Cairns, C.A.; White, R.J. p53 is a general repressor of RNA polymerase III transcription. EMBO J. 1998, 17, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Chesnokov, I.; Chu, W.M.; Botchan, M.R.; Schmid, C.W. p53 inhibits RNA polymerase III-directed transcription in a promoter-dependent manner. Mol. Cell. Biol. 1996, 16, 7084–7088. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.; Crighton, D.; Boyle, J.M.; Varley, J.M.; White, R.J. RNA polymerase III transcription can be derepressed by oncogenes or mutations that compromise p53 function in tumours and Li-Fraumeni syndrome. Oncogene 2002, 21, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Wang, H.; Wang, M.H.; Xu, W.; Zhang, R. Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop. Oncogene 2013, 32, 2782–2791. [Google Scholar] [CrossRef] [PubMed]
- Loging, W.T.; Reisman, D. Elevated expression of ribosomal protein genes L37, RPP-1, and S2 in the presence of mutant p53. Cancer Epidemiol. Biomark. Prev. 1999, 8, 1011–1016. [Google Scholar]
- Takagi, M.; Absalon, M.J.; McLure, K.G.; Kastan, M.B. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 2005, 123, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, K.; Kastan, M.B. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J. Biol. Chem. 2012, 287, 16467–16476. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, J.; Zhuang, L.; Banerjee, B.; Yang, X.; Chau, J.F.L.; Lee, P.L.; Hande, M.P.; Li, B.; Yu, Q. Ribosomal protein S27-like, a p53-inducible modulator of cell fate in response to genotoxic stress. Cancer Res. 2007, 67, 11317–11326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, M.; Liu, X.; Xiong, X.; Sun, Y. Inactivation of ribosomal protein S27-like confers radiosensitivity via the Mdm2-p53 and Mdm2-MRN-ATM axes. Cell Death Dis. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Shishova, K.V.; Khodarovich, Y.M.; Lavrentyeva, E.A.; Zatsepina, O.V. High-resolution microscopy of active ribosomal genes and key members of the rRNA processing machinery inside nucleolus-like bodies of fully-grown mouse oocytes. Exp. Cell Res. 2015, 337, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Marcel, V.; Ghayad, S.E.; Belin, S.; Therizols, G.; Morel, A.P.; Solano-Gonzàlez, E.; Vendrell, J.A.; Hacot, S.; Mertani, H.C.; Albaret, M.A.; et al. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell 2013, 24, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Masvidal, L.; Cargnello, M.; Gyenis, L.; McLaughlan, S.; Cai, Y.; Tenkerian, C.; Morita, M.; Balanathan, P.; Jean-Jean, O. mTORC1 and CK2 coordinate ternary and eIF4F complex assembly. Nat. Commun. 2016, 7, 11127. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ruzzene, M.; Pinna, L.A. Addiction to protein kinase CK2: A common denominator of diverse cancer cells? Biochim. Biophys. Acta 2010, 1804, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Horton, L.E.; Bushell, M.; Barth-Baus, D.; Tilleray, V.J.; Clemens, M.J.; Hensold, J.O. p53 activation results in rapid dephosphorylation of the eIF4E-binding protein 4E-BP1, inhibition of ribosomal protein S6 kinase and inhibition of translation initiation. Oncogene 2002, 21, 5325–5334. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Ageberg, M.; Sanden, C.; Olofsson, T.; Gullberg, U.; Drott, K. The p53 target gene TRIM22 directly or indirectly interacts with the translation initiation factor eIF4E and inhibits the binding of eIF4E to eIF4G. Biol. Cell 2012, 104, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Clemens, M.J. Regulation of the phosphorylation and integrity of protein synthesis initiation factor eIF4GI and the translational repressor 4E-BP1 by p53. Oncogene 2005, 24, 4839–4850. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Clemens, M.J. Regulation of translation factors eIF4GI and 4E-BP1 during recovery of protein synthesis from inhibition by p53. Cell Death Differ. 2007, 14, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Petroulakis, E.; Parsyan, A.; Dowling, R.J.; LeBacquer, O.; Martineau, Y.; Bidinosti, M.; Larsson, O.; Alain, T.; Rong, L.; Mamane, Y.; et al. p53-dependent translational control of senescence and transformation via 4E-BPs. Cancer Cell 2009, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.O.; Sanders, K.; Abreo, F.W.; Nassar, R.; Glass, J. Correlation of p53 and the proto-oncogene eIF4E in larynx cancers: Prognostic implications. Cancer Res. 2000, 60, 3599–3604. [Google Scholar] [PubMed]
- Rahman, M.; Lem, C.; Muaddi, H.; Koromilas, A.E. PKR is not a universal target of tumor suppressor p53 in response to genotoxic stress. Cell Cycle 2009, 8, 3606–3607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Budanov, A.V.; Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Dinman, J.D.; Green, R. Translation Elongation and Recoding in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Preukschas, M.; Hagel, C.; Schulte, A.; Weber, K.; Lamszus, K.; Sievert, H.; Pällmann, N.; Bokemeyer, C.; Hauber, J.; Braig, M.; et al. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: Implications for new targeted therapies. PLoS ONE 2012, 7, e43468. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.P.; Kraiss, S.; Montenarh, M. Association of casein kinase II with immunopurified p53. Oncogene 1991, 6, 877–884. [Google Scholar] [PubMed]
- Meek, D.W.; Simon, S.; Kikkawa, U.; Eckhart, W. The p53 tumour suppressor protein is phosphorylated at serine 389 by casein kinase, II. EMBO J. 1990, 9, 3253–3260. [Google Scholar] [PubMed]
- Prowald, A.; Schuster, N.; Montenarh, M. Regulation of the DNA binding of p53 by its interaction with protein kinase CK2. FEBS Lett. 1997, 408, 99–104. [Google Scholar] [CrossRef]
- Schuster, N.; Prowald, A.; Schneider, E.; Scheidtmann, K.H.; Montenarh, M. Regulation of p53 mediated transactivation by the beta-subunit of protein kinase CK2. FEBS Lett. 1999, 447, 160–166. [Google Scholar] [CrossRef]
- Schuster, N.; GoÈtz, C.; Faust, M.; Schneider, E.; Prowald, A.; Jungbluth, A.; Montenarh, M. Wild-type p53 inhibits protein kinase CK2 activity. J. Cell. Biochem. 2001, 81, 172–183. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Ghaemmaghami, S.; Newman, J.R.; Weissman, J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009, 324, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zaccara, S.; Tebaldi, T.; Pederiva, C.; Ciribilli, Y.; Bisio, A.; Inga, A. p53-directed translational control can shape and expand the universe of p53 target genes. Cell Death Differ. 2014, 21, 1522–1534. [Google Scholar] [CrossRef] [PubMed]
- Loayza-Puch, F.; Drost, J.; Rooijers, K.; Lopes, R.; Elkon, R.; Agami, R. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol. 2013, 14, R32. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasteri, J.; Das, D.; Zhong, X.; Persaud, L.; Francis, A.; Muharam, H.; Sauane, M. Translation Control by p53. Cancers 2018, 10, 133. https://doi.org/10.3390/cancers10050133
Kasteri J, Das D, Zhong X, Persaud L, Francis A, Muharam H, Sauane M. Translation Control by p53. Cancers. 2018; 10(5):133. https://doi.org/10.3390/cancers10050133
Chicago/Turabian StyleKasteri, Justina, Dibash Das, Xuelin Zhong, Leah Persaud, Ashleigh Francis, Hilal Muharam, and Moira Sauane. 2018. "Translation Control by p53" Cancers 10, no. 5: 133. https://doi.org/10.3390/cancers10050133
APA StyleKasteri, J., Das, D., Zhong, X., Persaud, L., Francis, A., Muharam, H., & Sauane, M. (2018). Translation Control by p53. Cancers, 10(5), 133. https://doi.org/10.3390/cancers10050133




