Fucosyl-Agalactosyl IgG1 Induces Cholangiocarcinoma Metastasis and Early Recurrence by Activating Tumor-Associated Macrophage
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Purification of IgG and Generation of Agalactosyl IgG
2.4. Cell Culture and Treatment
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blot Analysis
2.7. Immunohistochemistry
2.8. Preparation of Tryptic Peptides
2.9. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.10. Statistical Analysis
3. Results
3.1. Characteristics of Subjects
3.2. IgG-Fc N-Glycoprofiles in Cholangiocarcinoma
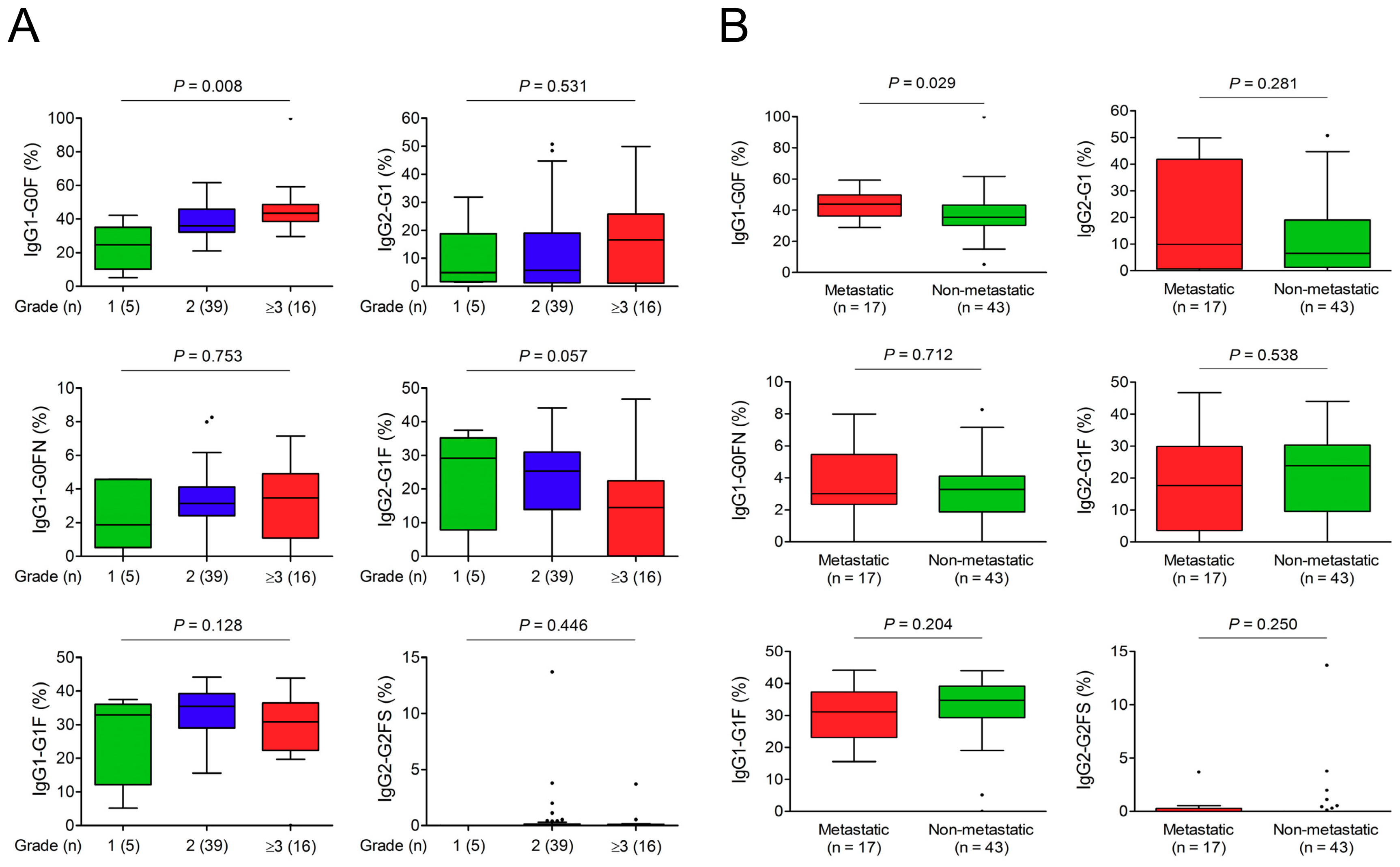
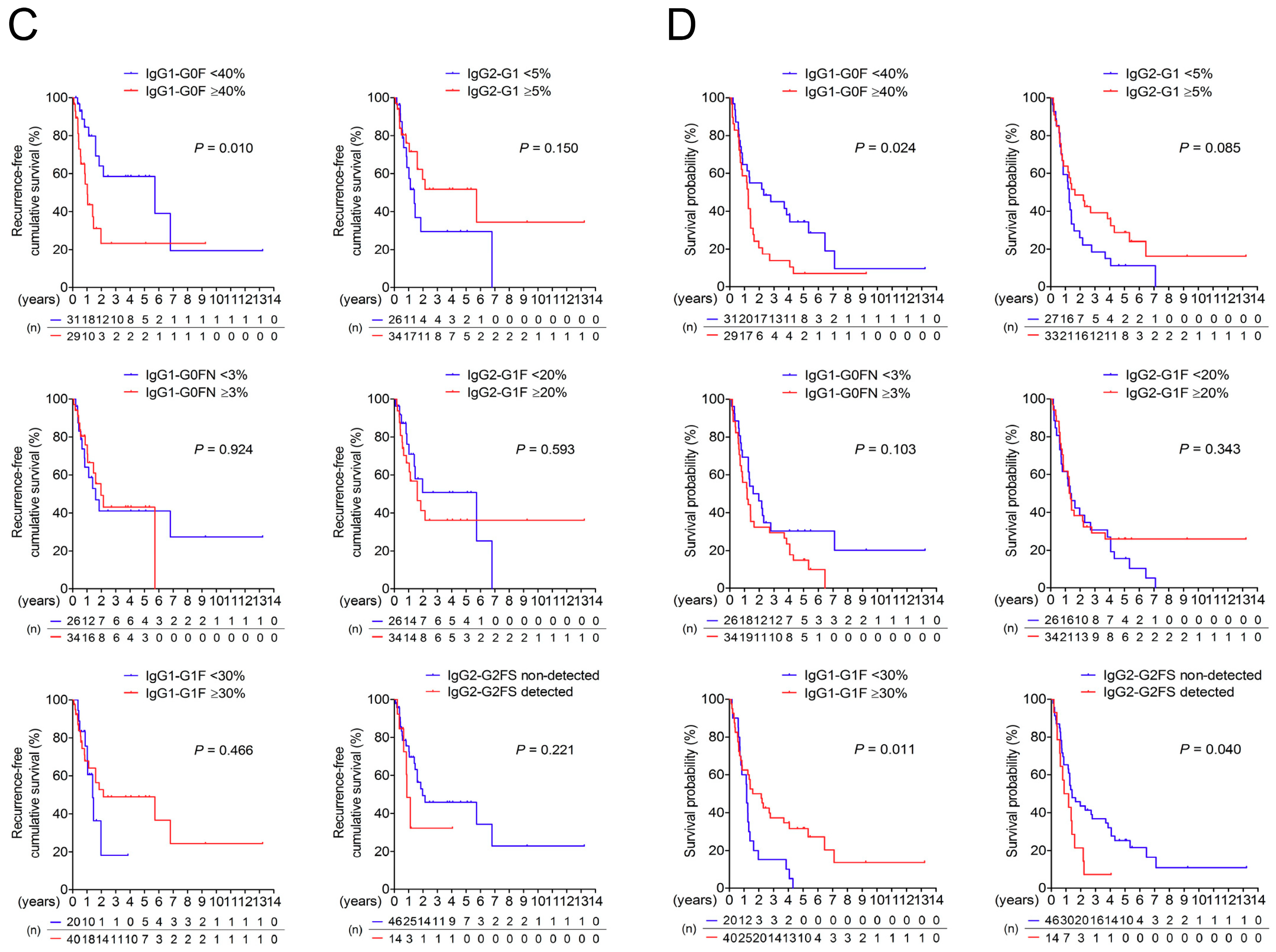
3.3. Association of IgG-Fc Glycans with Cholangiocarcinoma Differentiation, Metastasis, and Recurrence
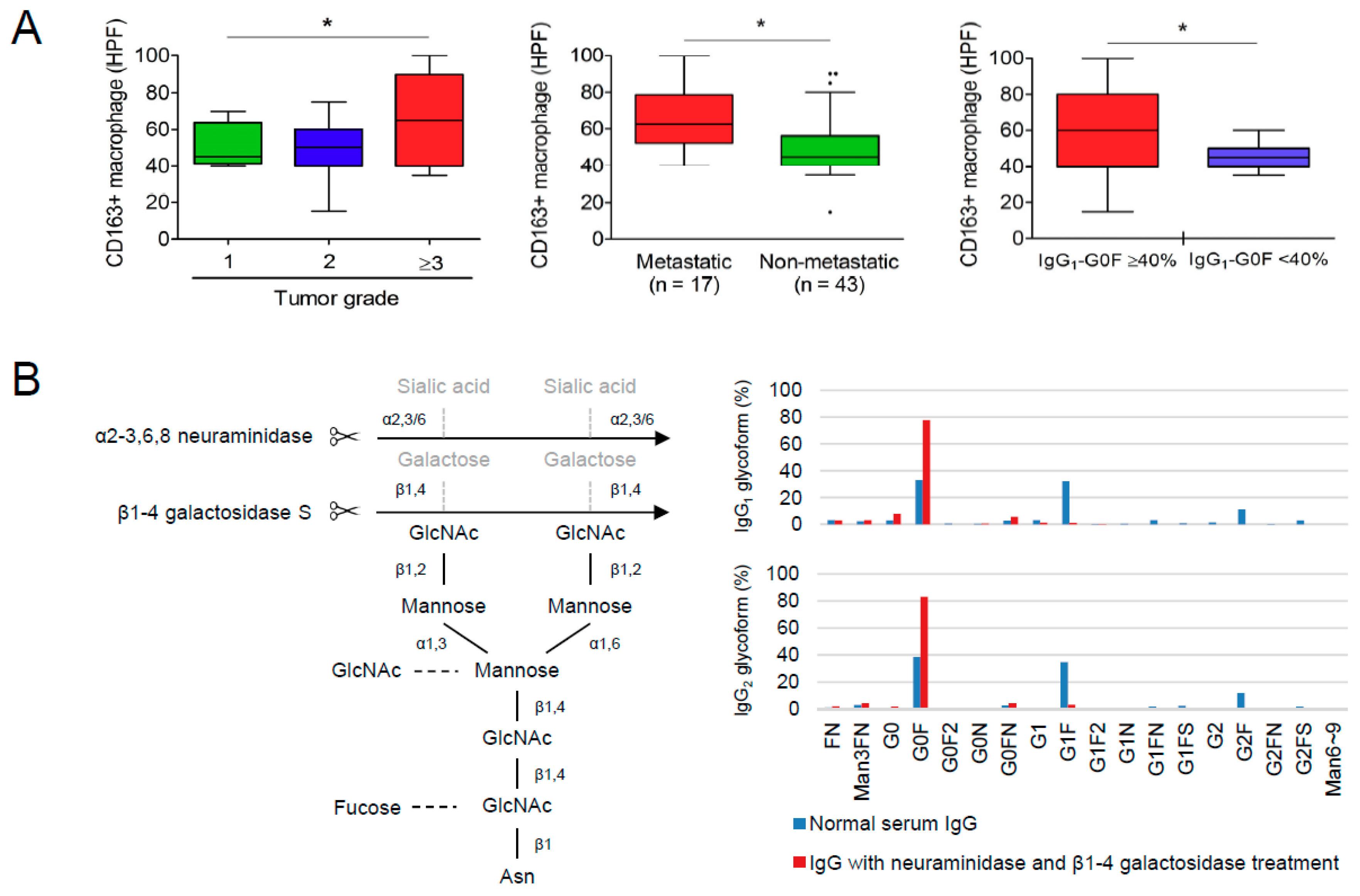
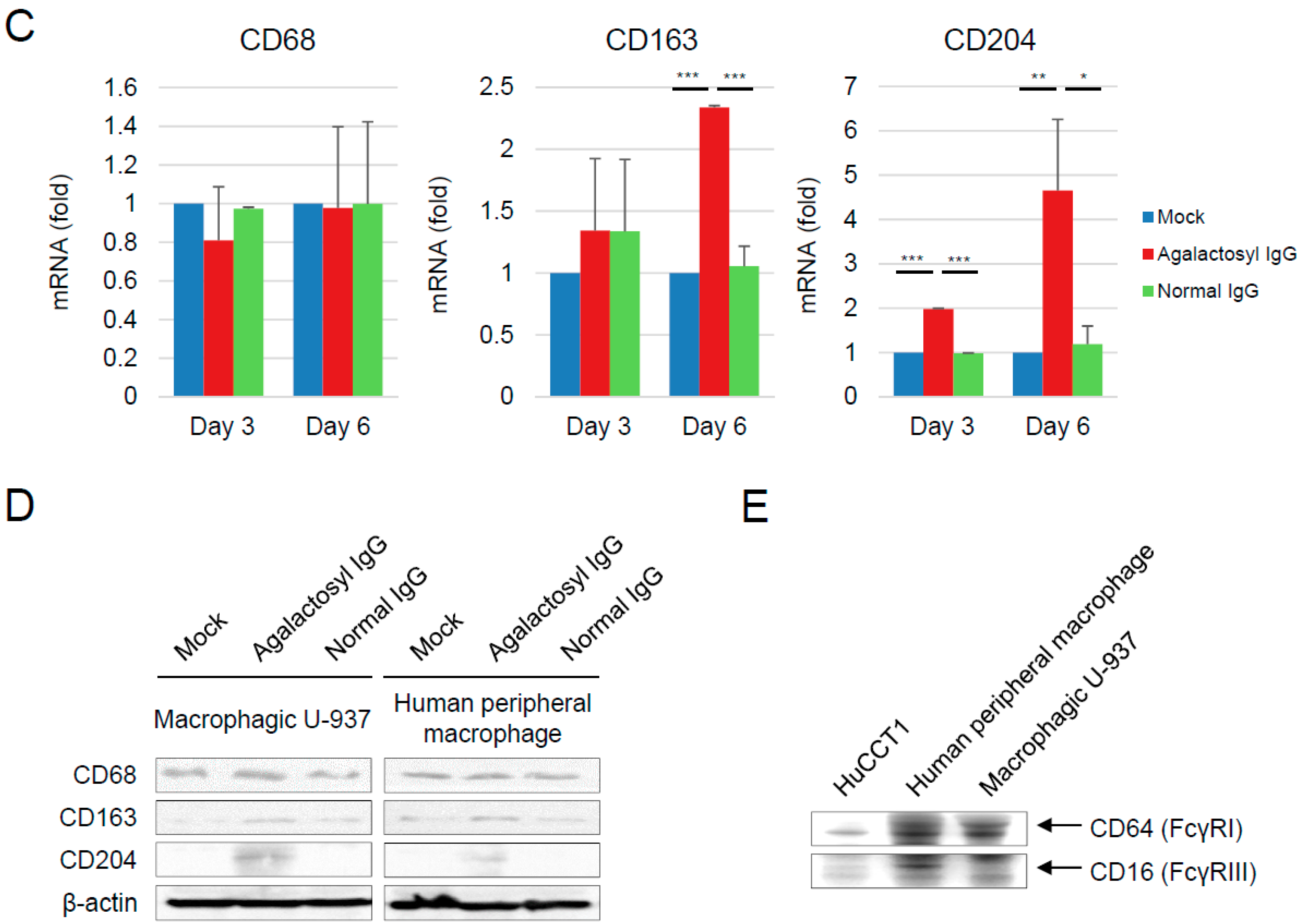
3.4. Reciprocal Regulations of IgG-G0F and Tumor-Associated Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| dHex | deoxyhexose |
| ELISA | enzyme-linked immunosorbent assay |
| F for glycoform | fucosylated |
| Fc | crystallizable fragment |
| FcγR | gamma receptor for crystallizable fragment |
| G0 for glycoform | agalactosylated |
| G1 for glycoform | partially galactosylated |
| G2 for glycoform | fully galactosylated |
| GlcNAc or N for glycoform | N-acetylglucosamine |
| Hex | hexose |
| HexNAc | N-acetylhexoseamine |
| IFN | interferon |
| IgG | immunoglobulin G |
| IL | interleukin |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| Man | mannosylated |
| ROC curve | Receiver operating characteristic curve |
| S for glycoform | sialylated |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
References
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Nakeeb, A.; Pitt, H.A.; Sohn, T.A.; Coleman, J.; Abrams, R.A.; Piantadosi, S.; Hruban, R.H.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996, 224, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Oh, J.K.; Masuyer, E.; Curado, M.P.; Bouvard, V.; Fang, Y.; Wiangnon, S.; Sripa, B.; Hong, S.T. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac. J. Cancer Prev. 2010, 11, 1159–1166. [Google Scholar] [PubMed]
- Ustundag, Y.; Bayraktar, Y. Cholangiocarcinoma: a compact review of the literature. World J. Gastroenterol. 2008, 14, 6458–6466. [Google Scholar] [CrossRef] [PubMed]
- Poomphakwaen, K.; Promthet, S.; Kamsa-Ard, S.; Vatanasapt, P.; Chaveepojnkamjorn, W.; Klaewkla, J.; Sujirarat, D.; Pichainarong, N. Risk factors for cholangiocarcinoma in Khon Kaen, Thailand: a nested case-control study. Asian Pac. J. Cancer Prev. 2009, 10, 251–258. [Google Scholar] [PubMed]
- Nehls, O.; Gregor, M.; Klump, B. Serum and bile markers for cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 139–154. [Google Scholar] [PubMed]
- Oh, S.W.; Yoon, Y.S.; Shin, S.A. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J. Clin. Oncol. 2005, 23, 4742–4754. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Kuno, A.; Matsuzaki, H.; Kawamoto, T.; Shikanai, T.; Nakanuma, Y.; Yamamoto, M.; Ohkohchi, N.; Ikehara, Y.; Shoda, J.; et al. Glycoproteomics-based cancer marker discovery adopting dual enrichment with Wisteria floribunda agglutinin for high specific glyco-diagnosis of cholangiocarcinoma. J. Proteomics 2013, 85, 1–11. [Google Scholar] [PubMed]
- Indramanee, S.; Silsirivanit, A.; Pairojkul, C.; Wongkham, C.; Wongkham, S. Aberrant glycosylation in cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac. J. Cancer Prev. 2012, 13 Suppl, 119–124. [Google Scholar]
- Malhotra, R.; Wormald, M.R.; Rudd, P.M.; Fischer, P.B.; Dwek, R.A.; Sim, R.B. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1995, 1, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Anthony, R.M.; Ravetch, J.V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. USA 2007, 104, 8433–8437. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Chien, R.N.; Cheng, P.N.; Liu, C.K.; Su, C.S.; Wu, I.C.; Liu, W.C.; Chen, S.H.; Chang, T.T. Association of serum IgG N-glycome and transforming growth factor-beta1 with hepatitis B virus e antigen seroconversion during entecavir therapy. Antiviral Res. 2014, 111, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Chien, R.N.; Cheng, P.N.; Liu, J.H.; Liu, C.K.; Su, C.S.; Wu, I.C.; Li, I.C.; Tsai, H.W.; Wu, S.L.; et al. Chang. Aberrant serum immunoglobulin G glycosylation in chronic hepatitis B is associated with histological liver damage and reversible by antiviral therapy. J. Infect. Dis. 2015, 211, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Tsai, H.W.; Lee, C.Y.; Huang, L.J.; Chien, R.N.; Wu, I.C.; Chiu, Y.C.; Liu, W.C.; Cheng, P.N.; Chang, T.T.; et al. Favorable Response to Long-term Nucleos(t)ide Analogue Therapy in HBeAg-positive Patients with High Serum Fucosyl-Agalactosyl IgG. Sci. Rep. 2017, 7, 1957. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Lin, J.; Lin, Y.S.; Wang, S.; Yeh, T.M. Dengue Virus Nonstructural Protein 1-Induced Antibodies Cross-React with Human Plasminogen and Enhance Its Activation. J. Immunol. 2016, 196, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Kulczycki, A., Jr. Bovine IgG can aggregate at conditions simulating pasteurization and binds to some human Fc gamma receptors. Mol. Immunol. 1987, 24, 259–266. [Google Scholar] [CrossRef]
- Miura, T.; Yoshizawa, T.; Hirai, H.; Seino, H.; Morohashi, S.; Wu, Y.; Wakiya, T.; Kimura, N.; Kudo, D.; Ishido, K.; et al. Prognostic Impact of CD163+ Macrophages in Tumor Stroma and CD8+ T-Cells in Cancer Cell Nests in Invasive Extrahepatic Bile Duct Cancer. Anticancer Res 2017, 37, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Thanee, M.; Loilome, W.; Techasen, A.; Namwat, N.; Boonmars, T.; Pairojkul, C.; Yongvanit, P. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac. J. Cancer Prev. 2015, 16, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Hasita, H.; Komohara, Y.; Okabe, H.; Masuda, T.; Ohnishi, K.; Lei, X.F.; Beppu, T.; Baba, H.; Takeya, M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010, 101, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, G.; Hau, H.M.; Dietel, C.; Benzing, C.; Krenzien, F.; Brandl, A.; Wiltberger, G.; Matia, I.; Prager, I.; Schierle, K.; et al. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer 2015, 15, 790. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, N.; van Vlierberghe, H.; van Hecke, A.; Laroy, W.; Delanghe, J.; Contreras, R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat. Med. 2004, 10, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Morelle, W.; Flahaut, C.; Michalski, J.C.; Louvet, A.; Mathurin, P.; Klein, A. Mass spectrometric approach for screening modifications of total serum N-glycome in human diseases: Application to cirrhosis. Glycobiology 2006, 16, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, H.; Xue, R.; Liu, T.; Dong, L.; Yao, J.; Zhang, Y.; Shen, X. Identification of N-glycosylation in hepatocellular carcinoma patients’ serum with a comparative proteomic approach. PLoS ONE 2013, 8, e77161. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Cui, T.; Wang, Y.; Sun, S.; Ma, T.; Wang, T.; Chen, Q.; Li, Z. Selective isolation and analysis of glycoprotein fractions and their glycomes from hepatocellular carcinoma sera. Proteomics 2010, 13, 1481–1498. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Ocho, M.; Togayachi, A.; Kuno, A.; Sogabe, M.; Ohkura, T.; Nozaki, H.; Angata, T.; Chiba, Y.; Ozaki, H.; et al. Glycoproteomic discovery of serological biomarker candidates for HCV/HBV infection-associated liver fibrosis and hepatocellular carcinoma. J. Proteome Res. 2013, 12, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Carre, Y.; Louvet, A.; Michalski, J.C.; Morelle, W. Immunoglobulins are the major glycoproteins involved in the modifications of total serum N-glycome in cirrhotic patients. Proteomics Clin. Appl. 2010, 4, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, T.W.; Williams, P.; Dwek, R.A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 6123–6127. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Irani, V.; Guy, A.J.; Andrew, D.; Beeson, J.G.; Ramsland, P.A.; Richards, J.S. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 2015, 67, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Wuhrer, M.; Stam, J.C.; van de Geijn, F.E.; Koeleman, C.A.; Verrips, C.T.; Dolhain, R.J.; Hokke, C.H.; Deelder, A.M. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 2007, 7, 4070–4081. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. Inflammation: a driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S.E.; Selman, M.H.; Adegnika, A.A.; Amoah, A.S.; van Riet, E.; Kruize, Y.C.; Raynes, J.G.; Rodriguez, A.; Boakye, D.; von Mutius, E.; et al. IgG1 Fc N-glycan galactosylation as a biomarker for immune activation. Sci. Rep. 2016, 6, 28207. [Google Scholar] [PubMed]
- Dekkers, G.; Rispens, T.; Vidarsson, G. Novel Concepts of Altered Immunoglobulin G Galactosylation in Autoimmune Diseases. Front. Immunol. 2018, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Tsai, M.H.; Li, S.T.; Tsai, T.I.; Chu, K.C.; Liu, Y.C.; Lai, M.Y.; Wu, C.Y.; Tseng, Y.C.; Shivatare, S.S.; et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc. Natl. Acad. Sci. USA 2015, 112, 10611–10616. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Jayapal, M.; Tay, H.K.; Reghunathan, R.; Lin, G.; Too, C.T.; Lim, Y.T.; Chan, S.H.; Kemeny, D.M.; Floto, R.A.; et al. Differential signal transduction, membrane trafficking, and immune effector functions mediated by FcgammaRI versus FcgammaRIIa. Blood 2009, 114, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, X.H. Study of the interaction of the C-reactive protein monomer with the U937 monocyte. Cell. Mol. Biol. Lett. 2010, 15, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kecse-Nagy, C.; Szittner, Z.; Papp, K.; Hegyi, Z.; Rovero, P.; Migliorini, P.; Lorand, V.; Homolya, L.; Prechl, J. Characterization of NF-kappaB Reporter U937 Cells and Their Application for the Detection of Inflammatory Immune-Complexes. PLoS ONE 2016, 11, e0156328. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahrani, R.; Abuetabh, Y.; Zeitouni, N.; Sergi, C. Cholangiocarcinoma: risk factors, environmental influences and oncogenesis. Ann. Clin. Lab. Sci. 2013, 43, 195–210. [Google Scholar] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]

| Variable | Healthy Control (n = 55) | Intrahepatic CC (n = 50) | Perihilar CC (n = 10) | p-Value 1 | p-Value 2 | p-Value 3 |
|---|---|---|---|---|---|---|
| Demographic and cancer data | ||||||
| Male, n (%) | 35 (63.6) | 25 (50.0) | 6 (60.0) | 0.173 | 1.000 | 0.732 |
| Age (years) | 60 (56–80) | 67 (33–85) | 60 (38–77) | 0.055 | 0.635 | 0.258 |
| Tumor staging, n (%) | ||||||
| I | 10 (20) | 1 (10) | ||||
| II | 17 (34) | 5 (50) | ||||
| III | 4 (8) | 2 (20) | ||||
| IVA | 16 (32) | 2 (20) | ||||
| IVB | 3 (6) | 0 (0) | ||||
| Follow-up period (years) | 1.3 (0.2–13.2) | 1.7 (0.1–5.5) | 0.648 | |||
| 5-year recurrence, n (%) | 20 (40) | 5 (50) | 0.728 | |||
| 5-year survivals, n (%) | 8 (16) | 2 (20) | 0.668 | |||
| Biochemical tests | ||||||
| Alanine transaminase (U/L) | 21 (9–45) | 34 (10–199) | 86 (13–140) | <0.001 | <0.001 | 0.006 |
| Aspartate aminotransferase (U/L) | 23 (15–33) | 42 (17–231) | 62 (28–101) | <0.001 | <0.001 | 0.031 |
| Alkaline phosphatase (U/L) | 64 (9–106) | 118 (25–591) | 246 (168–786) | <0.001 | <0.001 | 0.001 |
| Albumin (g/dL) | 4.6 (4.2–5.2) | 4.3 (2.9–4.9) | 4.1 (3.0–5.2) | <0.001 | <0.001 | 0.200 |
| Immunoglobulin G (g/dL) | 10.5 (4.3–23.5) | 11.4 (8.3–15.8) | 11.1 (8.7–13.0) | 0.069 | 0.363 | 0.913 |
| Total bilirubin (mg/dL) | 0.9 (0.2–2.5) | 0.5 (0.2–11.8) | 5.0 (0.8–11.6) | 0.002 | <0.001 | <0.001 |
| Glucose ante cibum | 87 (71–175) | 103 (80–451) | 109 (91–240) | <0.001 | 0.001 | 0.471 |
| Creatinine (mg/dL) | 0.93 (0.50–1.24) | 0.80 (0.41–7.90) | 0.80 (0.60–1.30) | <0.001 | 0.230 | 0.436 |
| α-fetoprotein (ng/mL) | 3.68 (1.82–16.83) | 2.83 (1.31–474.20) | 4.29 (1.76–5.79) | 0.180 | 0.843 | 0.965 |
| Carcinoembryonic antigen (ng/mL) | 1.43 (0.44–4.09) | 2.71 (0.30–60.42) | 4.37 (1.53–13.18) | <0.001 | <0.001 | 0.662 |
| Carbohydrate antigen 125 (U/mL) | 30.80 (7.95–772.10) | 20.29 (9.15–201.80) | 0.503 | |||
| Carbohydrate antigen 19-9 (U/mL) | 172.1 (0.0–36622.0) | 330.9 (73.9–5791.0) | 0.043 | |||
| Hematological tests | ||||||
| Red blood cell (106/μL) | 4.48 (3.56–5.90) | 4.12 (3.17–5.47) | 4.30 (2.56–5.57) | <0.001 | 0.068 | 0.796 |
| Hemoglobin (g/dL) | 14.1 (9.7–17.2) | 12.8 (8.8–15.5) | 12.4 (10.5–14.8) | <0.001 | 0.004 | 0.781 |
| Hematocrit (%) | 40.7 (29.1–49.2) | 37.5 (25.6–44.7) | 36.5 (31.0–42.7) | <0.001 | 0.004 | 0.677 |
| Mean corpuscular hemoglobin (pg/cell) | 31.5 (20.1–35.8) | 31.1 (19.7–34.5) | 31.8 (21.5–34.8) | 0.045 | 0.806 | 0.425 |
| Mean corpuscular hemoglobin concentration (g/dL) | 34.4 (31.8–35.4) | 33.9 (31.8–35.2) | 34.2 (32.0–35.5) | <0.001 | 0.506 | 0.069 |
| Mean corpuscular volume (fL/cell) | 91.2 (63.1–101.5) | 90.7 (61.9–101.0) | 91.2 (67.1–102.5) | 0.720 | 0.964 | 0.872 |
| Red cell distribution width (%) | 13.1 (11.8–21.8) | 13.5 (12.0–17.7) | 15.7 (11.9–25.9) | 0.030 | <0.001 | 0.004 |
| White blood cell (103/μL) | 4.8 (3.1–7.8) | 6.5 (3.6–16.9) | 7.5 (4.2–12.1) | <0.001 | <0.001 | 0.367 |
| Platelet (103/μL) | 212 (70–317) | 204 (84–412) | 306 (149–394) | 0.441 | 0.004 | 0.007 |
| IgG1 | IgG2 | |||||
|---|---|---|---|---|---|---|
| Glycoform | Control | Cholangiocarcinoma | p-Value | Control | Cholangiocarcinoma | p-Value |
| FN | 0.00 (0.00–25.89) | 0.16 (0.00–17.33) | <0.001 | 0.00 (0.00–13.17) | 0.00 (0.00–44.26) | 0.044 |
| Man3FN | 0.00 (0.00–0.31) | 0.21 (0.00–6.42) | <0.001 | 0.00 (0.00–1.73) | 0.00 (0.00–3.67) | <0.001 |
| G0 | 0.00 (0.00–4.29) | 1.97 (0.00–12.33) | <0.001 | 0.00 (0.00–3.15) | 0.00 (0.00–4.05) | 0.010 |
| G0F | 26.15 (0.00–52.62) | 38.08 (5.20–100.00) | <0.001 | 32.55 (0.63–54.86) | 39.68 (0.00–89.51) | 0.012 |
| G0F2 | 0.00 (0.00–0.00) | 0.00 (0.00–8.00) | 0.052 | 0.00 (0.00–34.72) | 0.00 (0.00–15.23) | 0.627 |
| G0N | 0.00 (0.00–1.03) | 0.15 (0.00–1.28) | <0.001 | 0.00 (0.00–0.02) | 0.00 (0.00–0.67) | 0.020 |
| G0FN | 0.00 (0.00–7.05) | 3.21 (0.00–8.27) | <0.001 | 1.55 (0.00–11.55) | 2.58 (0.00–29.24) | 0.023 |
| G1 | 0.00 (0.00–7.50) | 2.54 (0.00–9.18) | <0.001 | 0.00 (0.00–34.27) | 6.86 (0.00–50.76) | <0.001 |
| G1F | 44.38 (8.59–100.00) | 34.57 (0.00–44.14) | <0.001 | 37.02 (0.52–63.06) | 22.81 (0.00–83.35) | <0.001 |
| G1F2 | 0.00 (0.00–0.56) | 0.00 (0.00–3.00) | 0.359 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 |
| G1N | 0.00 (0.00–0.50) | 0.12 (0.00–8.37) | <0.001 | 0.00 (0.00–1.40) | 0.00 (0.00–14.53) | 0.002 |
| G1FN | 0.00 (0.00–65.62) | 2.47 (0.00–52.72) | 0.008 | 0.00 (0.00–2.56) | 0.25 (0.00–3.40) | 0.006 |
| G1FS | 0.00 (0.00–0.61) | 0.00 (0.00–25.99) | <0.001 | 1.74 (0.00–91.22) | 0.09 (0.00–50.00) | <0.001 |
| G2 | 0.00 (0.00–8.30) | 0.89 (0.00–7.27) | 0.002 | 0.00 (0.00–39.00) | 1.28 (0.00–32.92) | 0.035 |
| G2F | 19.15 (0.00–53.03) | 10.83 (0.00–25.12) | <0.001 | 7.39 (0.00–35.23) | 2.67 (0.00–21.80) | 0.095 |
| G2FN | 0.00 (0.00–3.29) | 0.16 (0.00–1.36) | <0.001 | 0.00 (0.00–0.14) | 0.00 (0.00–0.77) | 0.035 |
| G2FS | 0.00 (0.00–69.25) | 0.00 (0.00–25.06) | 0.010 | 1.39 (0.00–18.81) | 0.00 (0.00–13.72) | <0.001 |
| Man6~9 | 0.00 (0.00–0.00) | 0.00 (0.00–79.28) | 0.052 | 0.00 (0.00–26.48) | 0.00 (0.00–20.41) | 0.844 |
| IgG1 | IgG2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glycoform | AUC | SE | p-Value | Cut-off (%) | SENS, SPEC | AUC | SE | p-Value | Cut-off (%) | SENS, SPEC |
| FN | 0.702 | 0.050 | <0.001 | 0.02 | 58.3%, 85.5% | 0.578 | 0.053 | 0.147 | ||
| Man3FN | 0.813 | 0.041 | <0.001 | 0.00 | 65.0%, 96.4% | 0.653 | 0.051 | 0.005 | ||
| G0 | 0.777 | 0.044 | <0.001 | 0.26 | 81.7%, 67.3% | 0.606 | 0.053 | 0.050 | ||
| G0F | 0.809 | 0.041 | <0.001 | 30.28 | 81.7%, 70.9% | 0.637 | 0.053 | 0.012 | ||
| G0F2 | 0.533 | 0.054 | 0.538 | 0.507 | 0.054 | 0.893 | ||||
| G0N | 0.762 | 0.046 | <0.001 | 0.01 | 61.7%, 90.9% | 0.559 | 0.054 | 0.279 | ||
| G0FN | 0.795 | 0.043 | <0.001 | 2.30 | 75.0%, 80.0% | 0.621 | 0.052 | 0.025 | ||
| G1 | 0.684 | 0.051 | <0.001 | 0.751 | 0.046 | <0.001 | 1.12 | 78.3%, 70.9% | ||
| G1F | 0.798 * | 0.043 | <0.001 | 38.26 | 72.7%, 78.3% | 0.813 * | 0.041 | <0.001 | 30.49 | 80.0%, 76.7% |
| G1F2 | 0.516 | 0.054 | 0.771 | 0.500 | 0.054 | 1.000 | ||||
| G1N | 0.752 | 0.046 | <0.001 | 0.03 | 56.7%, 92.7% | 0.617 | 0.052 | 0.030 | ||
| G1FN | 0.642 | 0.055 | 0.009 | 0.636 | 0.052 | 0.012 | ||||
| G1FS | 0.622 | 0.052 | 0.024 | 0.715 * | 0.049 | <0.001 | 1.24 | 61.8%, 76.7% | ||
| G2 | 0.662 | 0.053 | 0.003 | 0.608 | 0.054 | 0.046 | ||||
| G2F | 0.780 * | 0.045 | <0.001 | 16.36 | 61.8%, 85.0% | 0.590 | 0.054 | 0.097 | ||
| G2FN | 0.785 | 0.044 | <0.001 | 0.11 | 61.7%, 96.4% | 0.550 | 0.054 | 0.353 | ||
| G2FS | 0.612 | 0.053 | 0.039 | 0.817 * | 0.041 | <0.001 | 0.03 | 80.0%, 76.7% | ||
| Man6~9 | 0.533 | 0.054 | 0.538 | 0.510 | 0.054 | 0.856 | ||||
| Within 1 Year | Within 2 Years | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Sex (Male = 1. Female = 0) | 0.520 (0.189–1.432) | 0.206 | 0.668 (0.298–1.495) | 0.326 | ||||
| Age (years) | 1.005 (0.965–1.047) | 0.810 | 1.024 (0.990–1.059) | 0.174 | ||||
| Tumor type (Intrahepatic = 1, Perihilar = 0) | 1.363 (0.309–6.007) | 0.683 | 0.786 (0.292–2.113) | 0.633 | ||||
| Tumor stage | 1.667 (0.829–3.352) | 0.151 | 1.533 (0.886–2.651) | 0.126 | ||||
| Alanine transaminase (U/L) | 1.000 (0.989–1.012) | 0.984 | 1.005 (0.996–1.014) | 0.305 | ||||
| Aspartate aminotransferase (U/L) | 0.999 (0.987–1.012) | 0.922 | 1.001 (0.991–1.012) | 0.836 | ||||
| Alkaline phosphatase (U/L) | 1.000 (0.995–1.005) | 0.942 | 1.000 (0.995–1.004) | 0.859 | ||||
| Total bilirubin (mg/dL) | 0.970 (0.795–1.185) | 0.767 | 1.077 (0.958–1.211) | 0.216 | ||||
| α-fetoprotein (ng/mL) | 0.964 (0.844–1.101) | 0.588 | 0.992 (0.961–1.024) | 0.604 | ||||
| Carcinoembryonic antigen (ng/mL) | 1.020 (0.990–1.050) | 0.194 | 1.019 (0.990–1.050) | 0.200 | ||||
| Carbohydrate antigen 125 (U/mL) | 1.001 (0.998–1.004) | 0.590 | 1.000 (0.997–1.004) | 0.771 | ||||
| Carbohydrate antigen 19-9 (U/mL) | 1.000 (1.000–1.000) | 0.219 | 1.000 (0.997–1.004) | 0.264 | ||||
| Red blood cell (106/μL) | 1.001 (0.446–2.248) | 0.998 | 0.879 (0.474–1.629) | 0.681 | ||||
| White blood cell (103/μL) | 0.998 (0.831–1.199) | 0.986 | 0.998 (0.847–1.175) | 0.980 | ||||
| Platelet (103/μL) | 1.002 (0.995–1.008) | 0.584 | 1.001 (0.996–1.006) | 0.692 | ||||
| IgG1-G0F ≥40% (Yes =1. No = 0) | 4.228 (1.361–13.133) | 0.013 | 2.765 (0.543–14.092) | 0.221 | 3.505 (1.484–8.278) | 0.004 | 2.521 (0.734–8.662) | 0.142 |
| CD68+ macrophage in tumor foci | 1.023 (0.996–1.051) | 0.098 | 1.019 (0.993–1.045) | 0.156 | ||||
| CD163+ macrophages in tumor foci | 1.031 (1.004–1.059) | 0.024 | 1.022 (0.994–1.051) | 0.124 | 1.026 (1.002–1.050) | 0.036 | 1.016 (0.991–1.041) | 0.223 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-T.; Tsai, H.-W.; Ho, C.-H. Fucosyl-Agalactosyl IgG1 Induces Cholangiocarcinoma Metastasis and Early Recurrence by Activating Tumor-Associated Macrophage. Cancers 2018, 10, 460. https://doi.org/10.3390/cancers10110460
Chang T-T, Tsai H-W, Ho C-H. Fucosyl-Agalactosyl IgG1 Induces Cholangiocarcinoma Metastasis and Early Recurrence by Activating Tumor-Associated Macrophage. Cancers. 2018; 10(11):460. https://doi.org/10.3390/cancers10110460
Chicago/Turabian StyleChang, Ting-Tsung, Hung-Wen Tsai, and Cheng-Hsun Ho. 2018. "Fucosyl-Agalactosyl IgG1 Induces Cholangiocarcinoma Metastasis and Early Recurrence by Activating Tumor-Associated Macrophage" Cancers 10, no. 11: 460. https://doi.org/10.3390/cancers10110460
APA StyleChang, T.-T., Tsai, H.-W., & Ho, C.-H. (2018). Fucosyl-Agalactosyl IgG1 Induces Cholangiocarcinoma Metastasis and Early Recurrence by Activating Tumor-Associated Macrophage. Cancers, 10(11), 460. https://doi.org/10.3390/cancers10110460





