Severe Neutropenia is Associated with Better Clinical Outcomes in Patients with Advanced Pancreatic Cancer Who Receive Modified FOLFIRINOX Therapy
Abstract
1. Introduction
2. Results
2.1. Patient Demographics
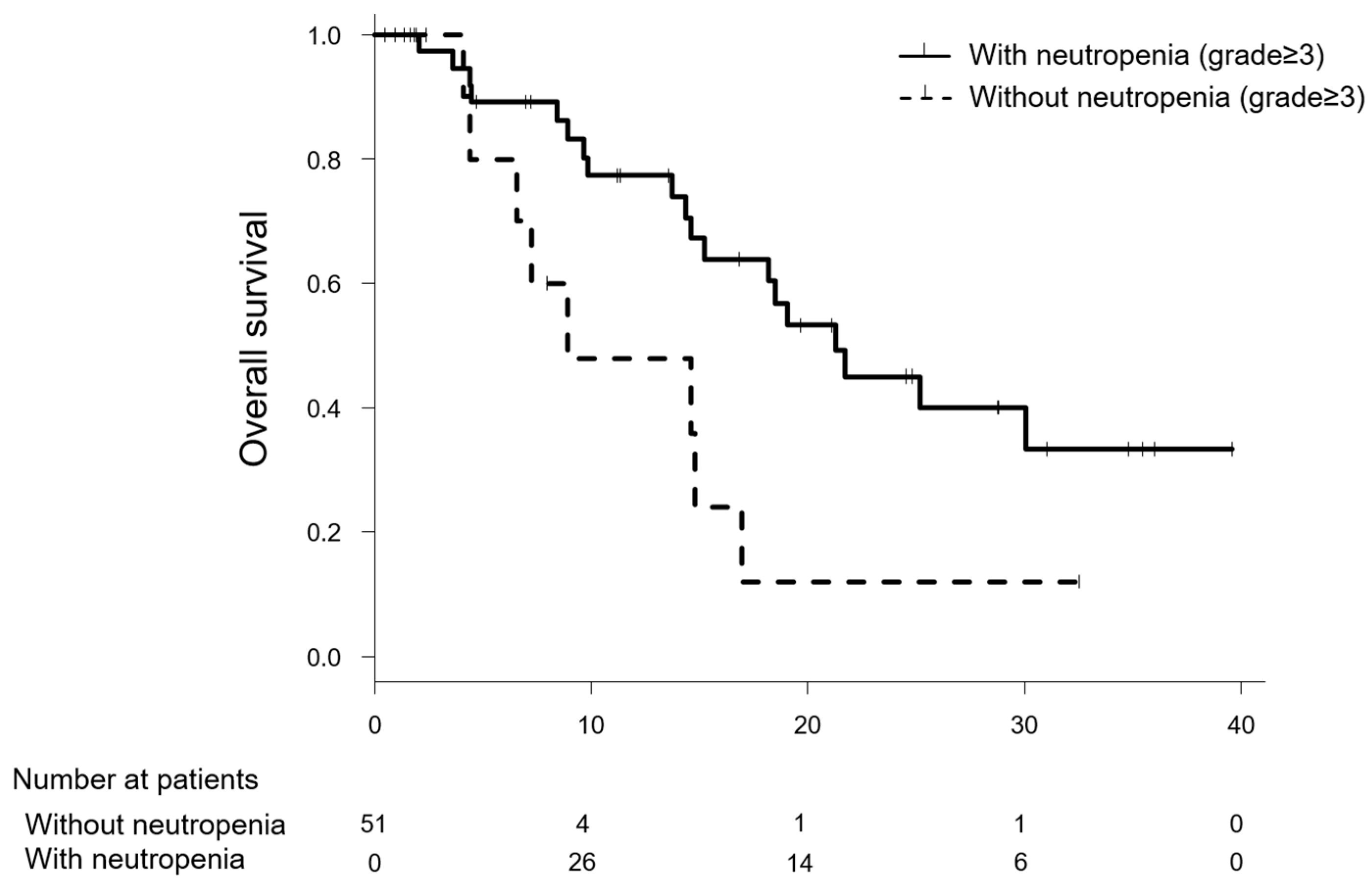
2.2. Comparison of the Efficacy of Modified FOLFIRINOX between Patients with and without Severe Neutropenia
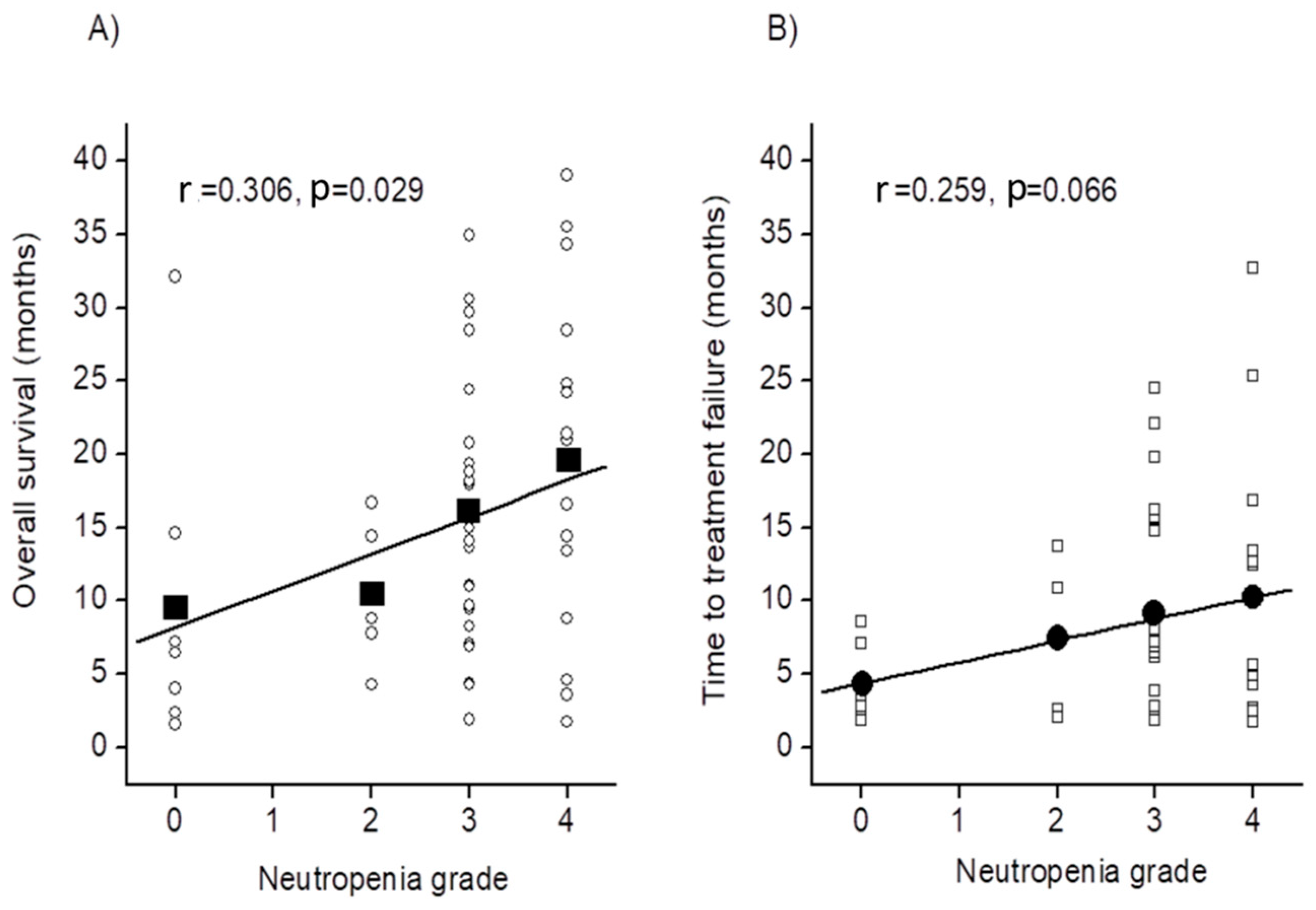
2.3. Relationship between Other Factor and Prolonging Survival
2.4. Risk Factors for Severe Neutropenia
2.5. Comparison of the Incidence of Other Adverse Events between Patients with and without Severe Neutropenia
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Chemotherapy
4.3. Assessment of Adverse Events
4.4. Risk Analysis for Grade ≥3 Neutropenia
4.5. Efficacy of Chemotherapy
4.6. Risk Analysis for Prolonging Survival
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ito, Y.; Miyashiro, I.; Ito, H.; Hosono, S.; Chihara, D.; Nakata-Yamada, K.; Nakayama, M.; Matsuzaka, M.; Hattori, M.; Sugiyama, H.; et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 2014, 105, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Ajiki, W.; Marugame, T.; Ioka, A.; Tsukuma, H.; Sobue, T. Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: A chronological and international comparative study. Jpn. J. Clin. Oncol. 2011, 41, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; De la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Ikeda, M.; Fukutomi, A.; Ioka, T.; Furuse, J.; Ohkawa, S.; Isayama, H.; Boku, N. Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014, 105, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Mahaseth, H.; Brutcher, E.; Kauh, J.; Hawk, N.; Kim, S.; Chen, Z.; Kooby, D.A.; Maithel, S.K.; Landry, J.; El-Rayes, B.F. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013, 42, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Iwashita, T.; Uemura, S.; Maruta, A.; Okuno, M.; Ando, N.; Iwata, K.; Kawaguchi, J.; Mukai, T.; Shimizu, M. A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget 2017, 8, 111346–111355. [Google Scholar] [CrossRef] [PubMed]
- Ozaka, M.; Ishii, H.; Sato, T.; Ueno, M.; Ikeda, M.; Uesugi, K.; Sata, N.; Miyashita, K.; Mizuno, N.; Tsuji, K.; et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2018, 81, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, E.; Wong, H.H.; Hewitt, C.; Calder, J.; Corrie, P.; Basu, B. Safety and efficacy of modified FOLFIRINOX for advanced pancreatic adenocarcinoma: A UK Single-Centre Experience. Oncology 2015, 89, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Blazer, M.; Wu, C.; Goldberg, R.M.; Phillips, G.; Schmidt, C.; Muscarella, P.; Wuthrick, E.; Williams, T.M.; Reardon, J.; Ellison, E.C.; et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann. Surg. Oncol. 2015, 22, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Usón Junior, P.L.S.; Rother, E.T.; Maluf, F.C.; Bugano, D.D.G. Meta-analysis of modified FOLFIRINOX regimens for patients with metastatic pancreatic cancer. Clin. Colorectal Cancer 2018, 17, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Caroli-Bosc, F.X.; Van Laethem, J.L.; Michel, P.; Gay, F.; Hendlisz, A.; Forget, F.; Bleiberg, H. A weekly 24-h infusion of high-dose 5-fluorouracil (5-FU)+leucovorin and bi-weekly cisplatin (CDDP) was active and well tolerated in patients with non-colon digestive carcinomas. Eur. J. Cancer 2001, 37, 1828–1832. [Google Scholar] [CrossRef]
- Khan, S.; Dhadda, A.; Fyfe, D.; Sundar, S. Impact of neutropenia on delivering planned chemotherapy for solid tumours. Eur. J. Cancer Care 2008, 17, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Treister, N.; Sonis, S. Mucositis: biology and management. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Links, M.; Lewis, C. Chemoprotectants: a review of their clinical pharmacology and therapeutic efficacy. Drugs 1999, 57, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Tucci, A.; Rinaldi, A.; Colarusso, D.; Pizza, C.; Reggiardo, G.; Manzione, L. A phase II study of irinotecan alternated with a weekly schedule of oxaliplatin, high-dose leucovorin and 48-hour infusion 5-fluorouracil in patients with advanced colorectal cancer. Oncology 2004, 66, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Lim, H.S.; Shin, E.S.; Yoo, Y.K.; Park, Y.H.; Lee, J.E.; Jang, I.J.; Lee, D.H.; Lee, J.S. Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J. Clin. Oncol. 2006, 24, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, E.; Altés, A.; Menoyo, A.; Del Rio, E.; Gómez-Pardo, M.; Baiget, M. UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br. J. Cancer 2004, 91, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Matsuo, K.; Takahari, D.; Yokota, T.; Inaba, Y.; Yamaura, H.; Sato, Y.; Najima, M.; Ura, T.; Muro, K. Neutropaenia as a prognostic factor in metastatic colorectal cancer patients undergoing chemotherapy with first-line FOLFOX. Eur. J. Cancer 2009, 45, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, Y.; Yan, H.; Wang, Y.R.; Dai, G.H. Timing of chemotherapy-induced neutropenia: the prognostic factor in advanced pancreatic cancer patients treated with gemcitabine/gemcitabine-based chemotherapy. Oncotarget 2017, 8, 66593–66600. [Google Scholar] [CrossRef] [PubMed]
- Otake, A.; Tsuji, D.; Taku, K.; Kawasaki, Y.; Yokoi, M.; Nakamori, H.; Osada, M.; Matsumoto, M.; Inoue, K.; Hirai, K.; et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with metastatic pancreatic cancer treated with gemcitabine. Eur. J. Clin. Pharmacol. 2017, 73, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Q.; Fan, J.; Cheng, S.; Ding, W.; Hua, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin. Chim. Acta. 2018, 479, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, N.G.; Griffith, D.; Hammoda, M.; Shingler, G.; Kambal, A.; Al-Sarireh, B. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB (Oxford). 2018, 20, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, W.; Cho, S.K.; Park, S.G. Characterization of multiple cytokine combinations and TGF-β on differentiation and functions of myeloid-derived suppressor cells. Int. J. Mol. Sci. 2018, 19, pii: E869. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, P.; Mitchem, J.B.; Porembka, M.R.; Tan, M.C.; Belt, B.A.; Wang-Gillam, A.; Gillanders, W.E.; Hawkins, W.G.; Linehan, D.C. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr. Cancer Drug Targets 2011, 11, 734–751. [Google Scholar] [CrossRef] [PubMed]
- Porembka, M.R.; Mitchem, J.B.; Belt, B.A.; Hsieh, C.S.; Lee, H.M.; Herndon, J.; Gillanders, W.E.; Linehan, D.C.; Goedegebuure, P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol. Immunother. 2012, 61, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Khaled, Y.S.; Ammori, B.J.; Elkord, E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J. Immunol. Res. 2014, 2014, 879897. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.; Brooks, T.R.; Duggan, M.C.; Paul, B.K.; Pan, X.; Wei, L.; Abrams, Z.; Luedke, E.; Lesinski, G.B.; Mundy-Bosse, B.; et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol. Immunother. 2015, 64, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Hu, J.; Wang, M.; Peng, F.; Tian, R.; Guo, X.J.; Xie, Y.; Qin, R.Y. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 99–105. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rebe, C.; Ghiringhelli, F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Zhang, Y.; Shang, Y.; Gao, Q. MDSC-decreasing chemotherapy increases the efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma and pancreatic cancer. Oncotarget 2016, 7, 4760–4769. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Kroetz, D.L.; Schuetz, E.; Dolan, M.E.; Ramírez, J.; Relling, M.; Chen, P.; Das, S.; Rosner, G.L. Ratain M.J. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J. Clin. Oncol. 2009, 27, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.; Doherty, M.M. Tumoral drug metabolism: overview and its implications for cancer therapy. J. Clin. Oncol. 2005, 23, 205–229. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. Available online: https://www.eortc.be/services/doc/ctc/ (accessed on 01 September 2018).
- Ichikawa, W.; Uehara, K.; Minamimura, K.; Tanaka, C.; Takii, Y.; Miyauchi, H.; Sadahiro, S.; Fujita, K.; Moriwaki, T.; Nakamura, M.; et al. An internally and externally validated nomogram for predicting the risk of irinotecan-induced severe neutropenia in advanced colorectal cancer patients. Br. J. Cancer 2015, 112, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Lee, J.; Park, S.H.; Lee, K.T.; Lee, J.K.; Lee, K.H.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Lim, D.H.; et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology 2011, 80, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikeda, M.; Okusaka, T.; Ueno, H.; Morizane, C.; Hagihara, A.; Iwasa, S.; Kojima, Y. Prognostic factors in japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn. J. Clin. Oncol. 2008, 38, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Stotz, M.; Absenger, G.; Stojakovic, T.; Samonigg, H.; Kornprat, P.; Schaberl-Moser, R.; Alzoughbi, W.; Lackner, C.; Ress, A.L.; et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br. J. Cancer. 2014, 110, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Kim, T.Y.; Lee, K.H.; Han, S.W.; Oh, D.Y.; Im, S.A.; Kim, T.Y.; Bang, Y.J. Efficacy of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) in the treatment of patients with gemcitabine-pretreated pancreatic cancer and analysis of prognostic factors in a salvage setting. Cancer Chemother. Pharmacol. 2011, 68, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.K.; Wei, L.; Trolli, E.; Bekaii-Saab, T. Elevated baseline CA19-9 levels correlate with adverse prognosis in patients with early- or advanced-stage pancreas cancer. Med. Oncol. 2012, 29, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Grimes, N.; Farid, S.; Morris-Stiff, G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: A systematic review. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 474–481. [Google Scholar] [CrossRef]
- Ben, Q.; An, W.; Wang, L.; Wang, W.; Yu, L.; Yuan, Y. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 2015, 44, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Long, F.; Jaiswar, M.; Yang, L.; Wang, C.; Zhou, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis. Sci. Rep. 2015, 5, 11026. [Google Scholar] [CrossRef] [PubMed]
- Stotz, M.; Gerger, A.; Eisner, F.; Szkandera, J.; Loibner, H.; Ress, A.L.; Kornprat, P.; AlZoughbi, W.; Seggewies, F.S.; Lackner, C.; et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer. 2013, 109, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Stotz, M.; Eisner, F.; Absenger, G.; Stojakovic, T.; Samonigg, H.; Kornprat, P.; Schaberl-Moser, R.; Alzoughbi, W.; Ress, A.L.; et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS ONE 2013, 8, e78225. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Kanai, M.; Mori, Y.; Nishimura, T.; Uza, N.; Kodama, Y.; Kawaguchi, Y.; Takaori, K.; Matsumoto, S.; Uemoto, S.; et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014, 3, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, H.; Mizuno, N.; Hara, K.; Hijioka, S.; Tajika, M.; Tanaka, T.; Ishihara, M.; Yogi, T.; Tsutsumi, H.; Fujiyoshi, T.; et al. Evaluation of Modified Glasgow Prognostic Score for Pancreatic Cancer: A Retrospective Cohort Study. Pancreas 2016, 45, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Makuch, R.W. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus nonresponder bias. Stat. Med. 1984, 3, 35–44. [Google Scholar] [CrossRef] [PubMed]
| Number of patients (male/female) | 51 | (30/21) |
| Age, median (mini–max) | 64 | (49–74) |
| Heterozygous for UGT1A1*6 or *28 (with/without) | 37.3 | (19/32) |
| Height (cm) | 162 | (156.0–166.5) |
| Body weight (kg) | 57 | (49.0–61.5) |
| Body surface area (m2) | 1.58 | (1.51–1.69) |
| Body mass index | 21.3 | (19.1–23.7) |
| Aspartate aminotransferase (IU/L) | 20 | (16–31) |
| Alanine aminotransferase (IU/L) | 19 | (13–34) |
| Serum creatinine (mg/dL) | 0.69 | (0.53–0.82) |
| Total bilirubin (mg/dL) | 0.7 | (0.5–0.8) |
| Neutrophil (/μL) | 3560 | (2955–4455) |
| White blood cells (/μL) | 5460 | (4835–6345) |
| Hemoglobin (g/dL) | 12.2 | (11.5–13.1) |
| Platelet (104/μL) | 19.7 | (16.1–24.6) |
| HbA1c (%) | 6.2 | (5.7–6.8) |
| C-reactive protein (CRP, mg/dL) | 0.29 | (0.05–1.42) |
| Carbohydrate antigen 19-9(CA19-9, U/mL) | 578.9 | (141.5–3983.7) |
| Biliary stent or drainage (%) (with/without) | 29.4 | (15/36) |
| Distant metastasis (%) (presence/absence) | 54.9 | (28/23) |
| Neutrophil-lymphocyte ratio (NLR) | 2.89 | (2.22–3.85) |
| Modified Glasgow prognostic score (mGPS, 0/1/2) | (33/10/7) | |
| Initial dose of chemotherapy drug | ||
| Irinotecan (mg/m2) | 150 | |
| Oxaliplatin (mg/m2) | 85 | |
| 5-Fluorouracil (mg/m2) | 2400 | |
| Factors | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|
| Neutropenia | 0.40 | (0.17–0.95) | 0.039 |
| Age (IQR:59–68) | 0.90 | (0.48–1.69) | 0.738 |
| NLR (IQR:2.2–3.8) | 1.25 | (1.02–1.52) | 0.029 |
| Effect | Without Neutropenia (n = 12) | With Neutropenia (n = 39) | p-value | ||
|---|---|---|---|---|---|
| Median time to treatment failure (months, 95% CI) | 3.7 | (2.0-12.1) | 7.0 | (1.9-24.5) | 0.079 |
| Tumor response rate (%) | |||||
| Response rate (CR+PR) | 16.7 | (2/12) | 35.9 | (14/39) | 0.296 |
| Disease control rate (CR+PR+SD) | 66.7 | (8/12) | 76.9 | (30/39) | 0.474 |
| One-year survival (%) | 41.7 | (5/12) | 71.8 | (28/39) | 0.085 |
| Factors | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| With distant metastasis | 2.07 | (0.83–5.16) | 0.119 | 2.11 | (0.84–5.30) | 0.113 |
| CRP | 1.01 | (0.84–1.22) | 0.908 | 0.99 | (0.82–1.21) | 0.953 |
| CA19-9 | 1.00 | (0.99–1.00) | 0.122 | 1.00 | (0.99–1.00) | 0.181 |
| NLR | 1.18 | (1.02–1.36) | 0.030 | 1.15 | (1.00–1.34) | 0.048 |
| mGPS | 0.90 | (0.55–1.47) | 0.676 | 0.92 | (0.56–1.53) | 0.755 |
| Factors | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Heterozygous for UGT1A1*6 or *28 | 1.41 (0.74–2.70) | 0.300 | 1.35 (0.69–2.65) | 0.373 |
| Neutrophil | 1.00 (0.99–1.00) | 0.062 | 1.00 (0.99–1.00) | 0.074 |
| Total bilirubin | 2.79 (1.30–6.10) | 0.010 | 2.65 (1.13–6.19) | 0.024 |
| Adverse Effect | Without Neutropenia (n = 12) | With Neutropenia (n = 39) | p-value | ||
|---|---|---|---|---|---|
| % | (presence/absence) | % | (presence/absence) | ||
| Nausea | 58.3 | (7/5) | 53.8 | (21/18) | 1.000 |
| Vomiting | 8.3 | (1/11) | 10.3 | (4/35) | 1.000 |
| Oral mucositis | 16.7 | (2/10) | 20.5 | (8/31) | 1.000 |
| Dysgeusia | 8.3 | (1/11) | 25.6 | (10/29) | 0.422 |
| Peripheral neuropathy | 41.7 | (5/7) | 28.2 | (11/28) | 0.481 |
| Diarrhea | 33.3 | (4/8) | 25.6 | (10/29) | 0.715 |
| Leukopenia | 33.3 | (4/8) | 100 | (39/0) | <0.001 |
| Thrombocytopenia | 16.7 | (2/10) | 25.6 | (10 / 29) | 0.706 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Fujii, H.; Watanabe, D.; Kato-Hayashi, H.; Ohata, K.; Kobayashi, R.; Ishihara, T.; Uemura, S.; Iwashita, T.; Shimizu, M.; et al. Severe Neutropenia is Associated with Better Clinical Outcomes in Patients with Advanced Pancreatic Cancer Who Receive Modified FOLFIRINOX Therapy. Cancers 2018, 10, 454. https://doi.org/10.3390/cancers10110454
Yamada Y, Fujii H, Watanabe D, Kato-Hayashi H, Ohata K, Kobayashi R, Ishihara T, Uemura S, Iwashita T, Shimizu M, et al. Severe Neutropenia is Associated with Better Clinical Outcomes in Patients with Advanced Pancreatic Cancer Who Receive Modified FOLFIRINOX Therapy. Cancers. 2018; 10(11):454. https://doi.org/10.3390/cancers10110454
Chicago/Turabian StyleYamada, Yunami, Hironori Fujii, Daichi Watanabe, Hiroko Kato-Hayashi, Koichi Ohata, Ryo Kobayashi, Takuma Ishihara, Shinya Uemura, Takuji Iwashita, Masahito Shimizu, and et al. 2018. "Severe Neutropenia is Associated with Better Clinical Outcomes in Patients with Advanced Pancreatic Cancer Who Receive Modified FOLFIRINOX Therapy" Cancers 10, no. 11: 454. https://doi.org/10.3390/cancers10110454
APA StyleYamada, Y., Fujii, H., Watanabe, D., Kato-Hayashi, H., Ohata, K., Kobayashi, R., Ishihara, T., Uemura, S., Iwashita, T., Shimizu, M., & Suzuki, A. (2018). Severe Neutropenia is Associated with Better Clinical Outcomes in Patients with Advanced Pancreatic Cancer Who Receive Modified FOLFIRINOX Therapy. Cancers, 10(11), 454. https://doi.org/10.3390/cancers10110454





