Establishment and Characterization of a Reliable Xenograft Model of Hodgkin Lymphoma Suitable for the Study of Tumor Origin and the Design of New Therapies
Abstract
1. Introduction
2. Results
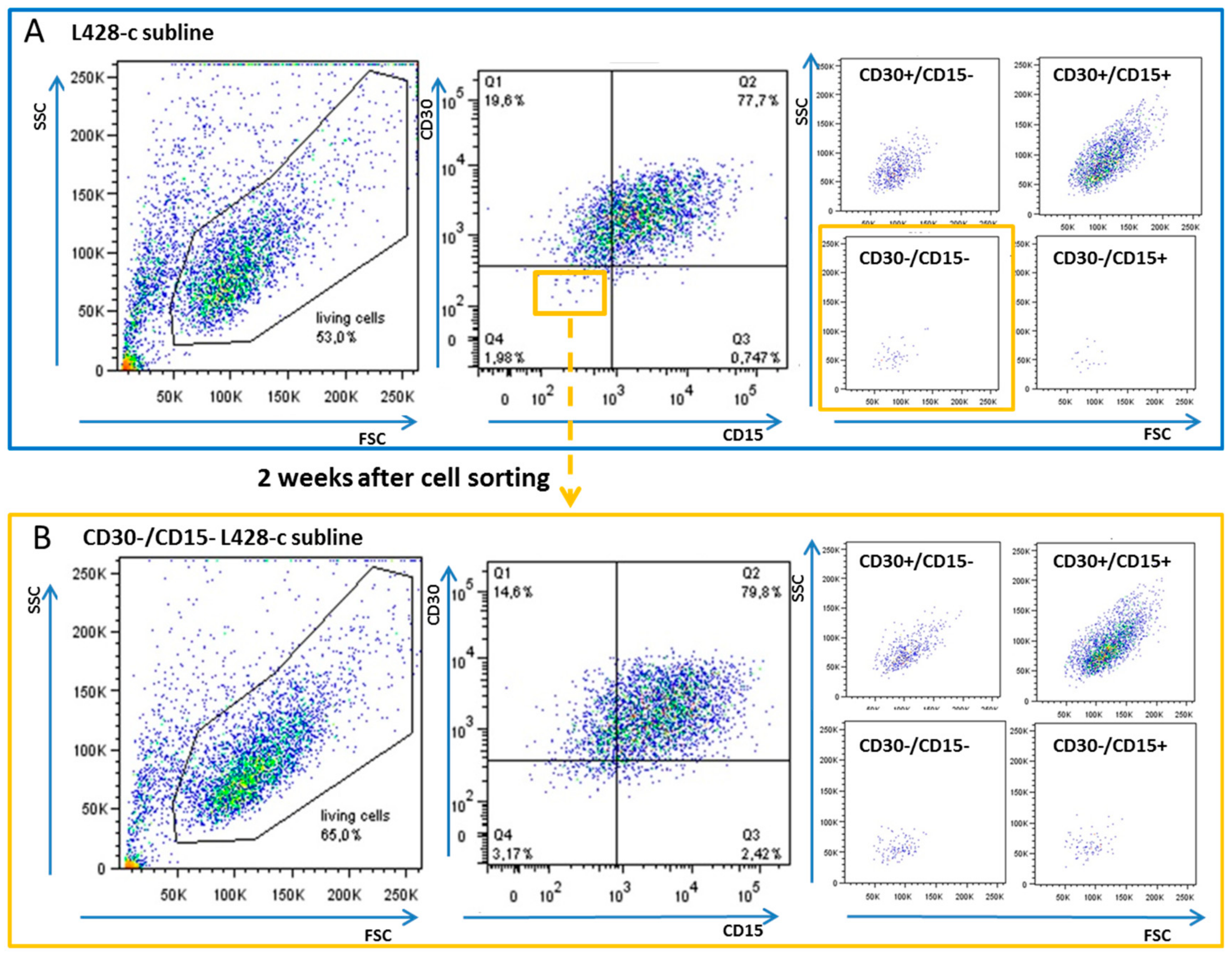
2.1. CD30−/CD15− HL Cells Can Acquire a Phenotype Similar to That of Their Parental HL Cell Lines
2.2. Ability of L428-c Subline to Engraft in NSG Mice
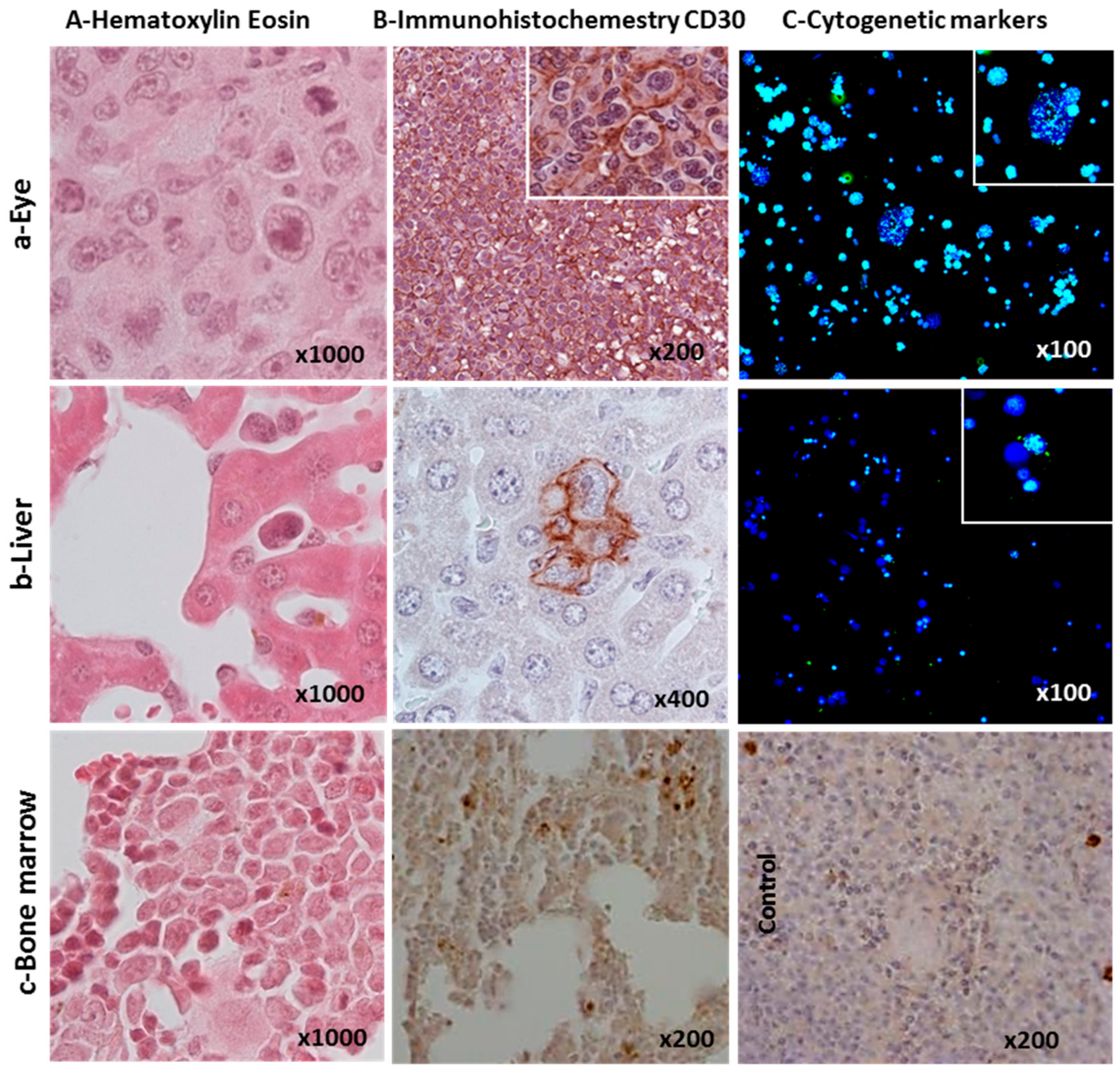
2.3. Cytogenetic Characterization of HL Cells Grown In Vitro and In Vivo
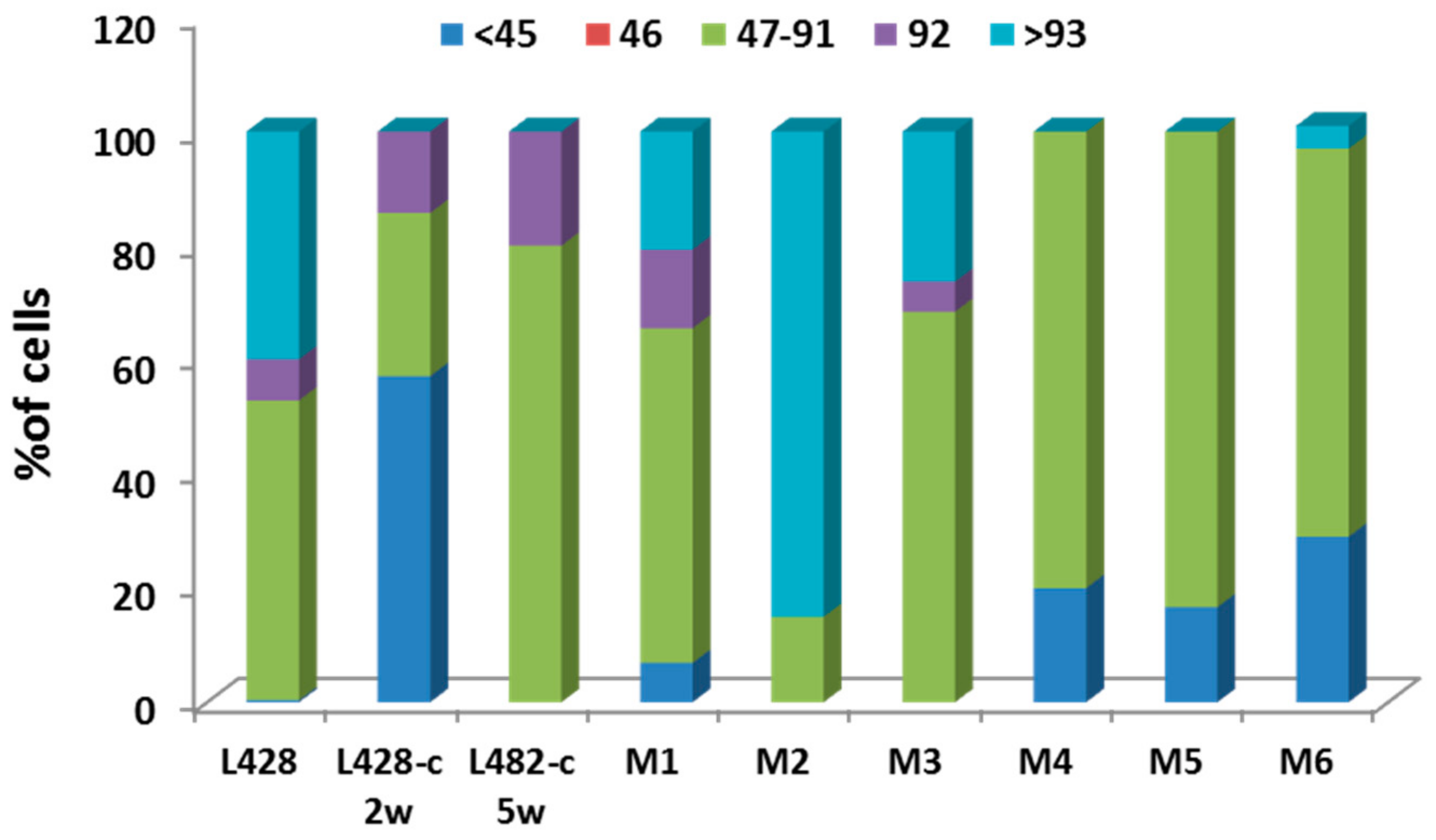
2.3.1. Ploidy Deviations of In Vitro and In Vivo Growing HL Cells
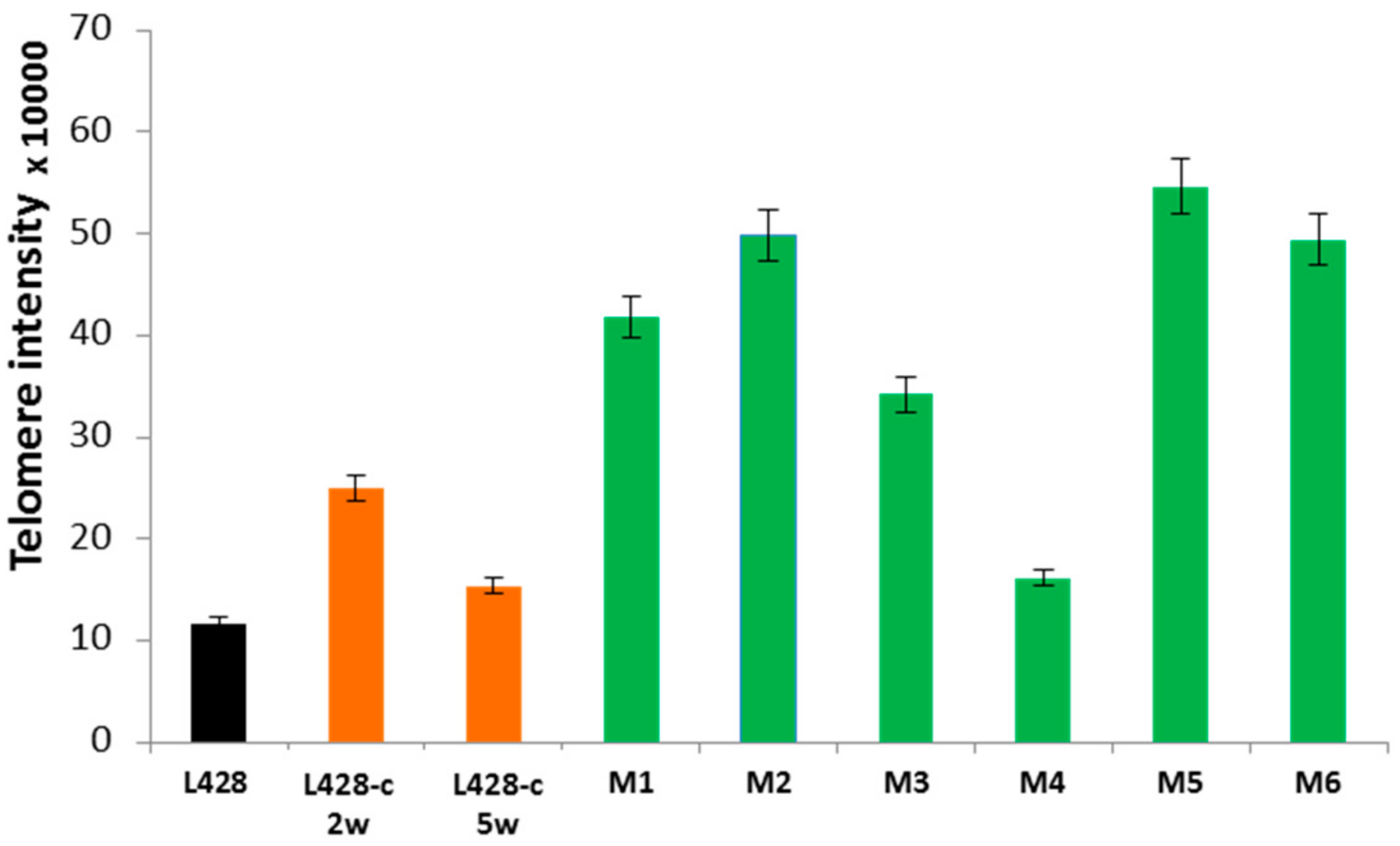
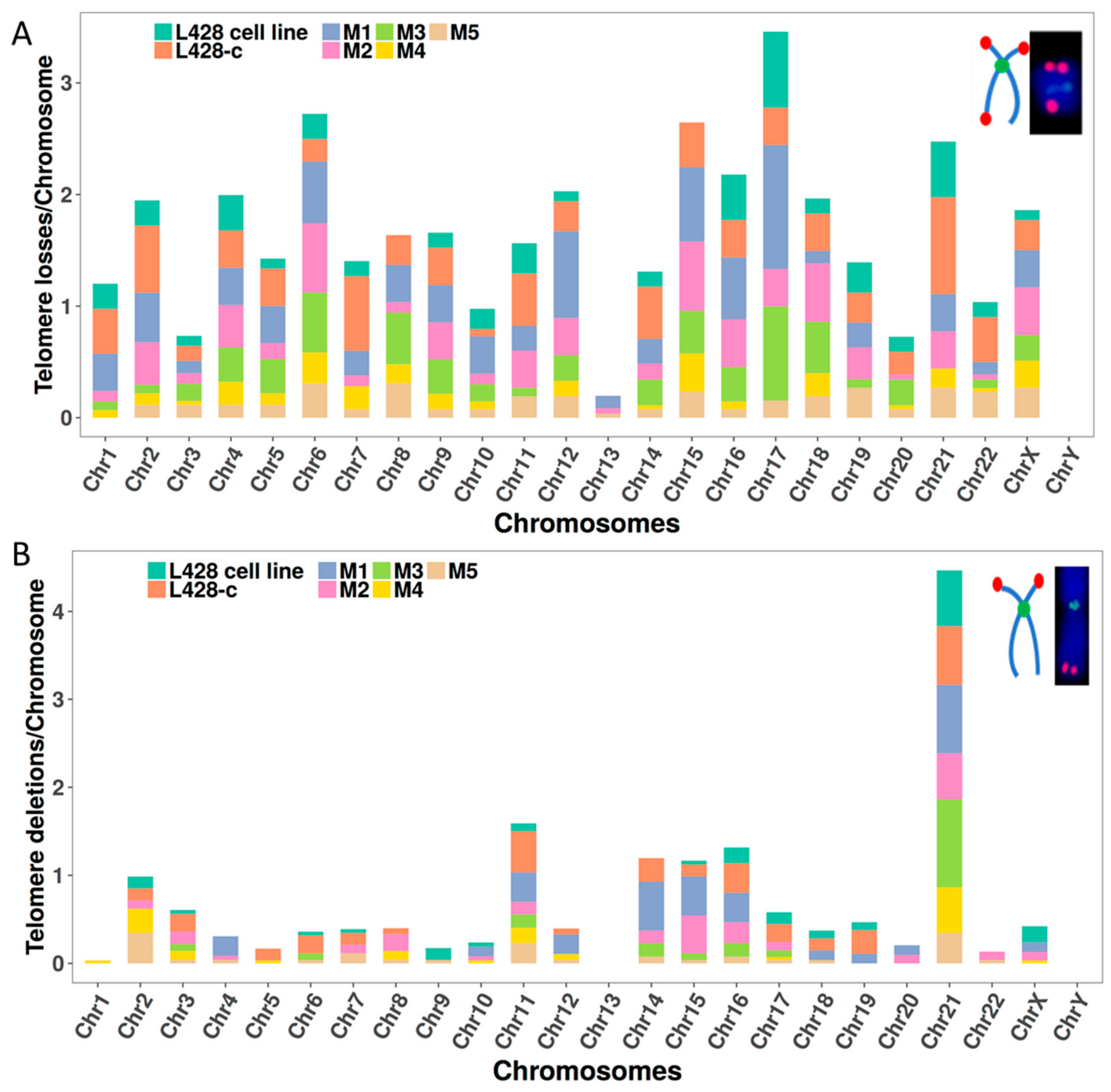
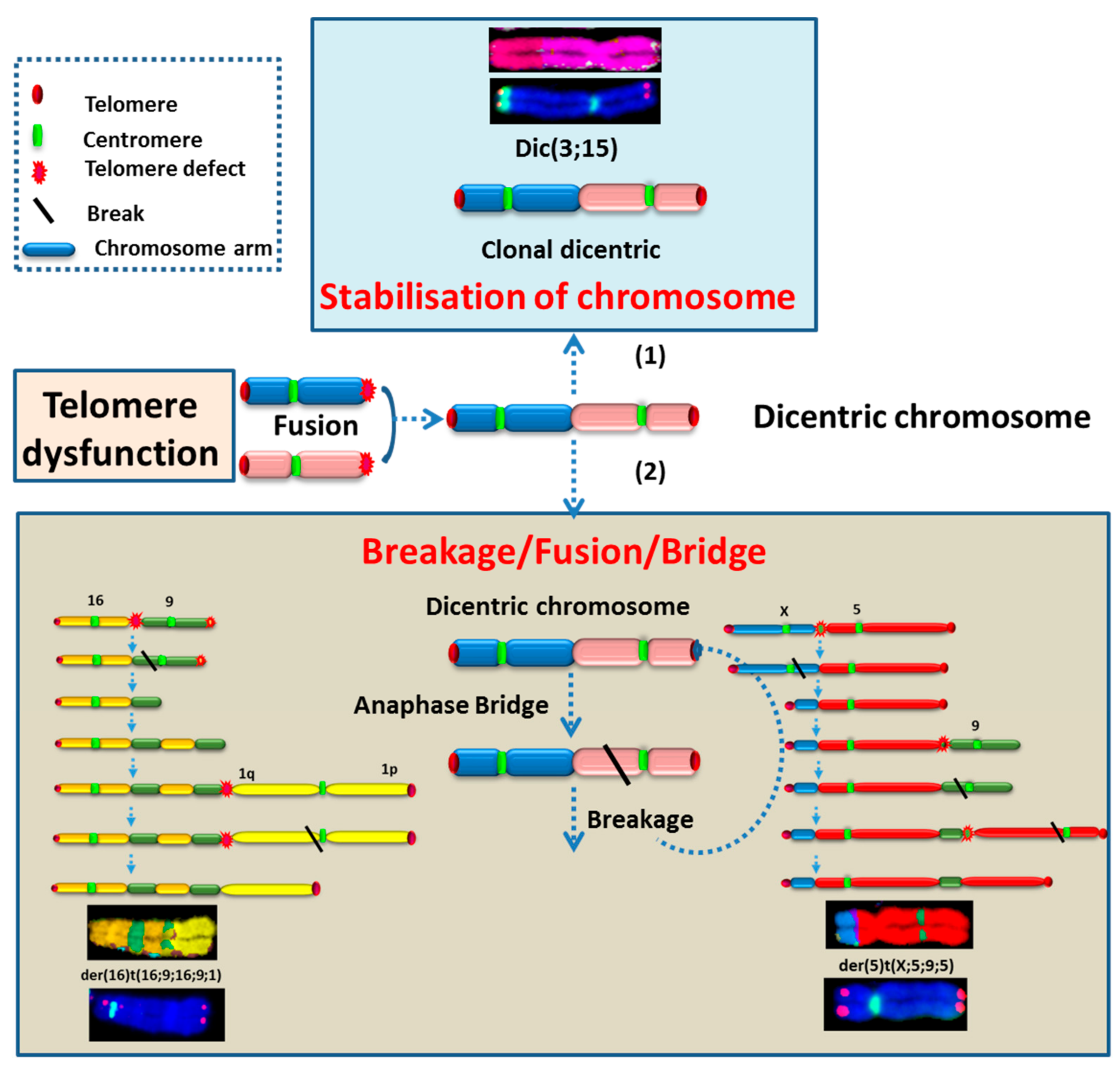
2.3.2. Telomere Dysfunction
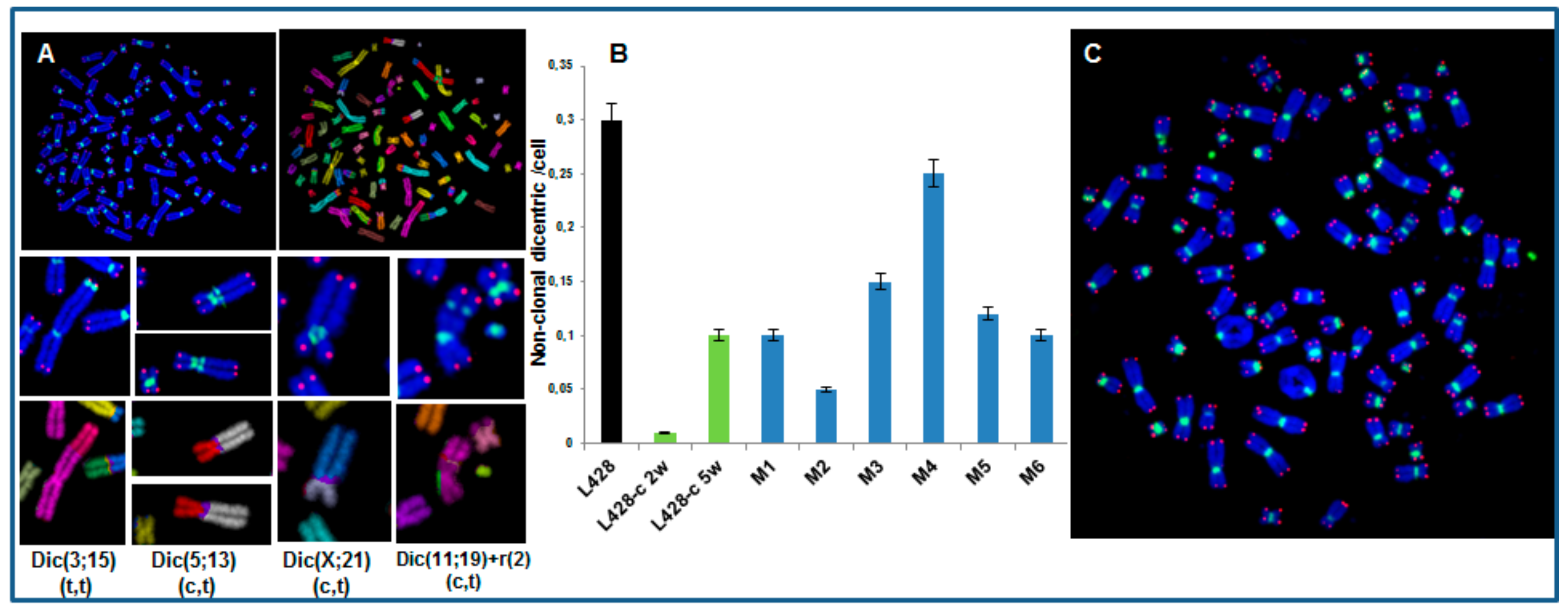
2.3.3. High Incidence of Dicentric Chromosome and Centric Ring Formation in HL Cells
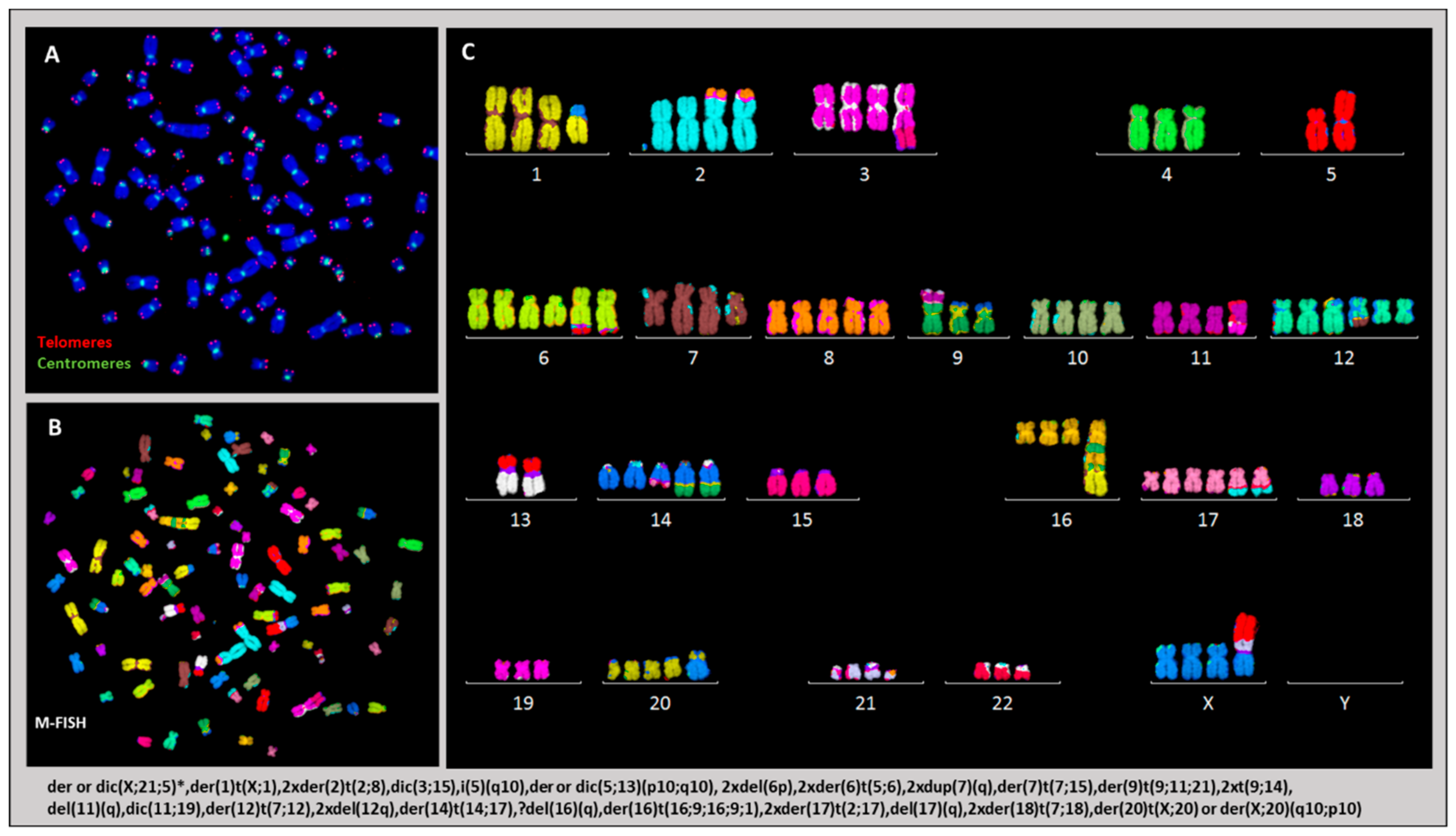
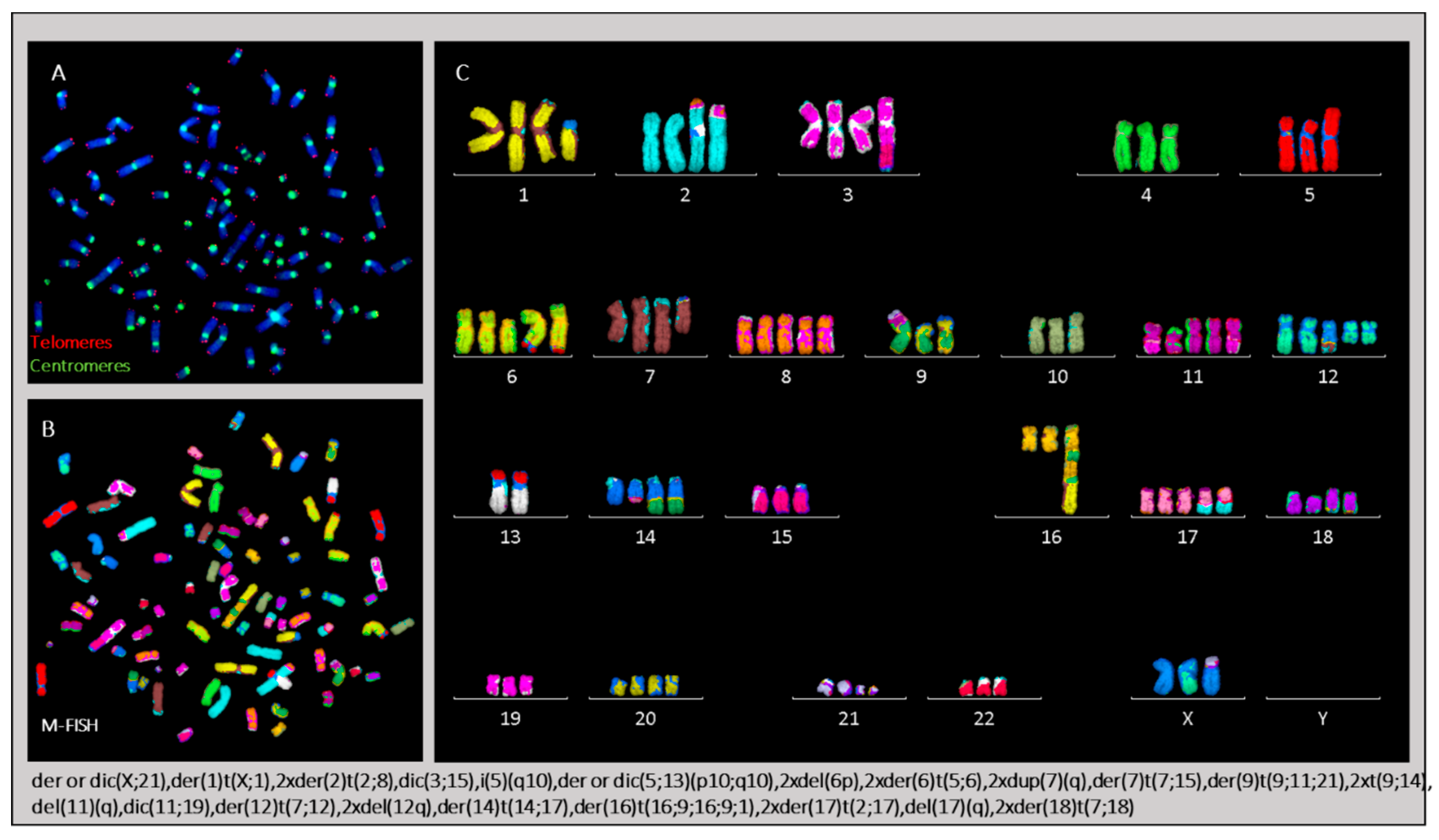
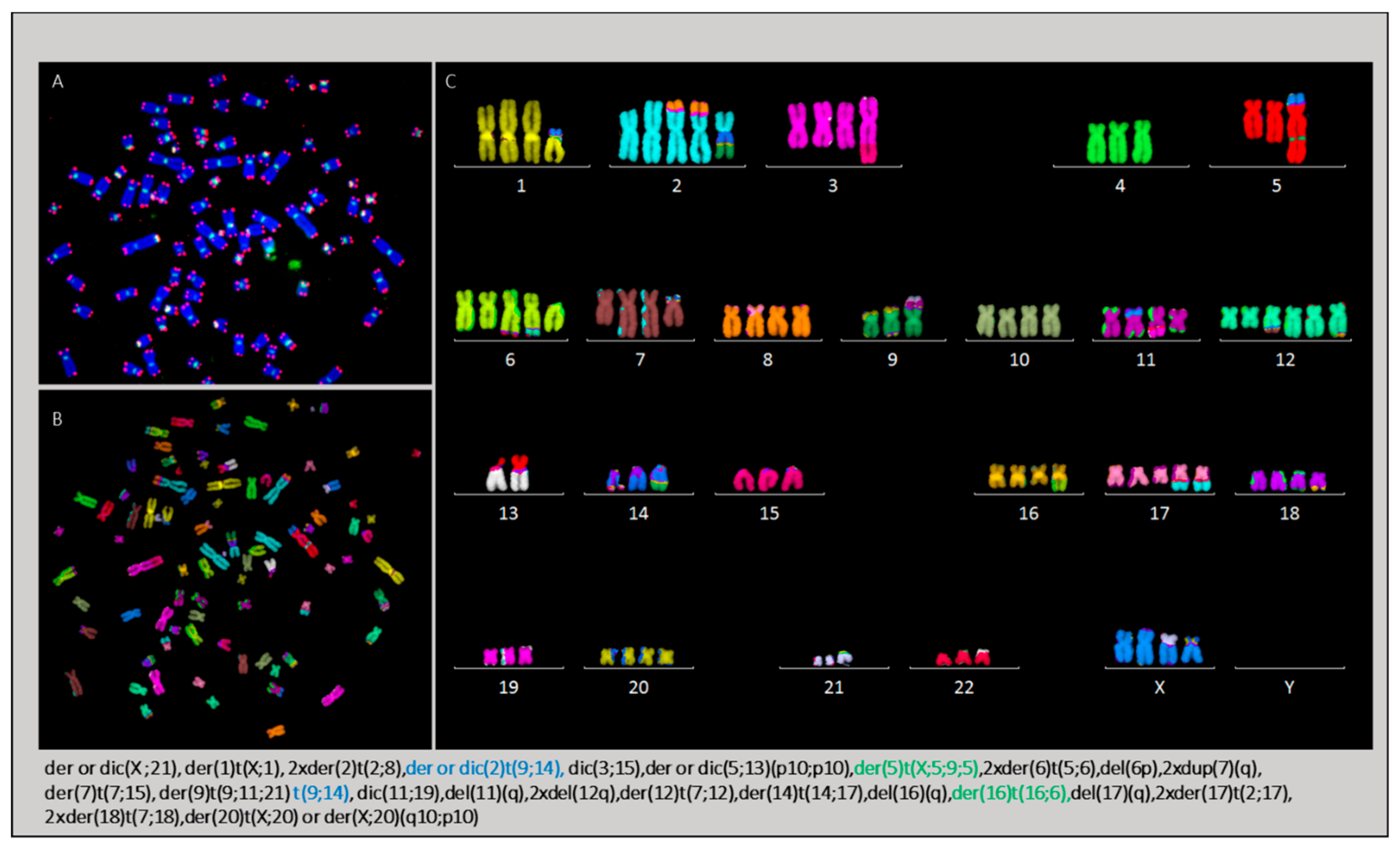
2.3.4. Karyotype of Cells Grown In Vitro and In Vivo
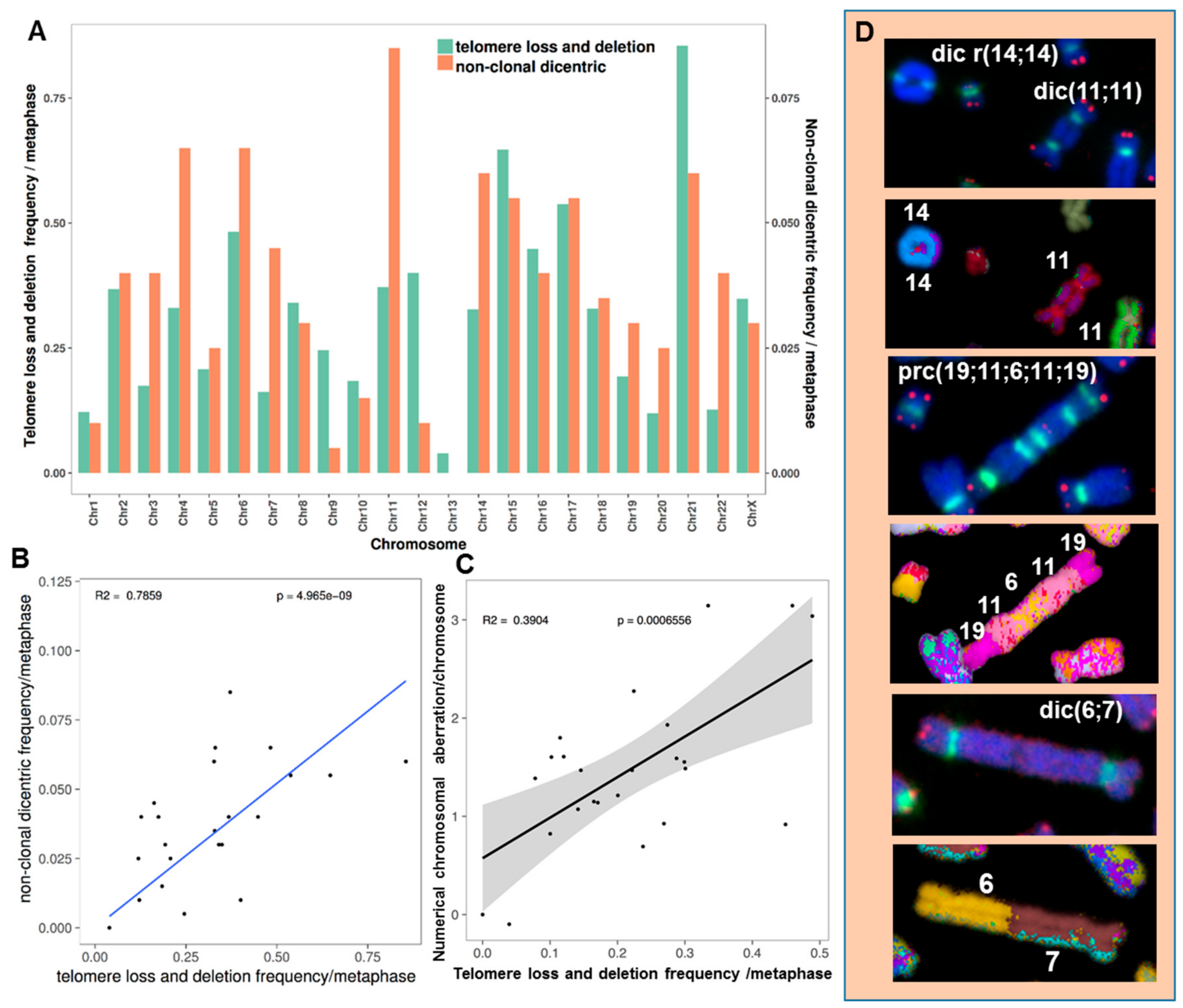
2.3.5. The Occurrence of Chromosomes Involved in Chromosomal Aberrations Correlates with Their Telomere Dysfunction Profile
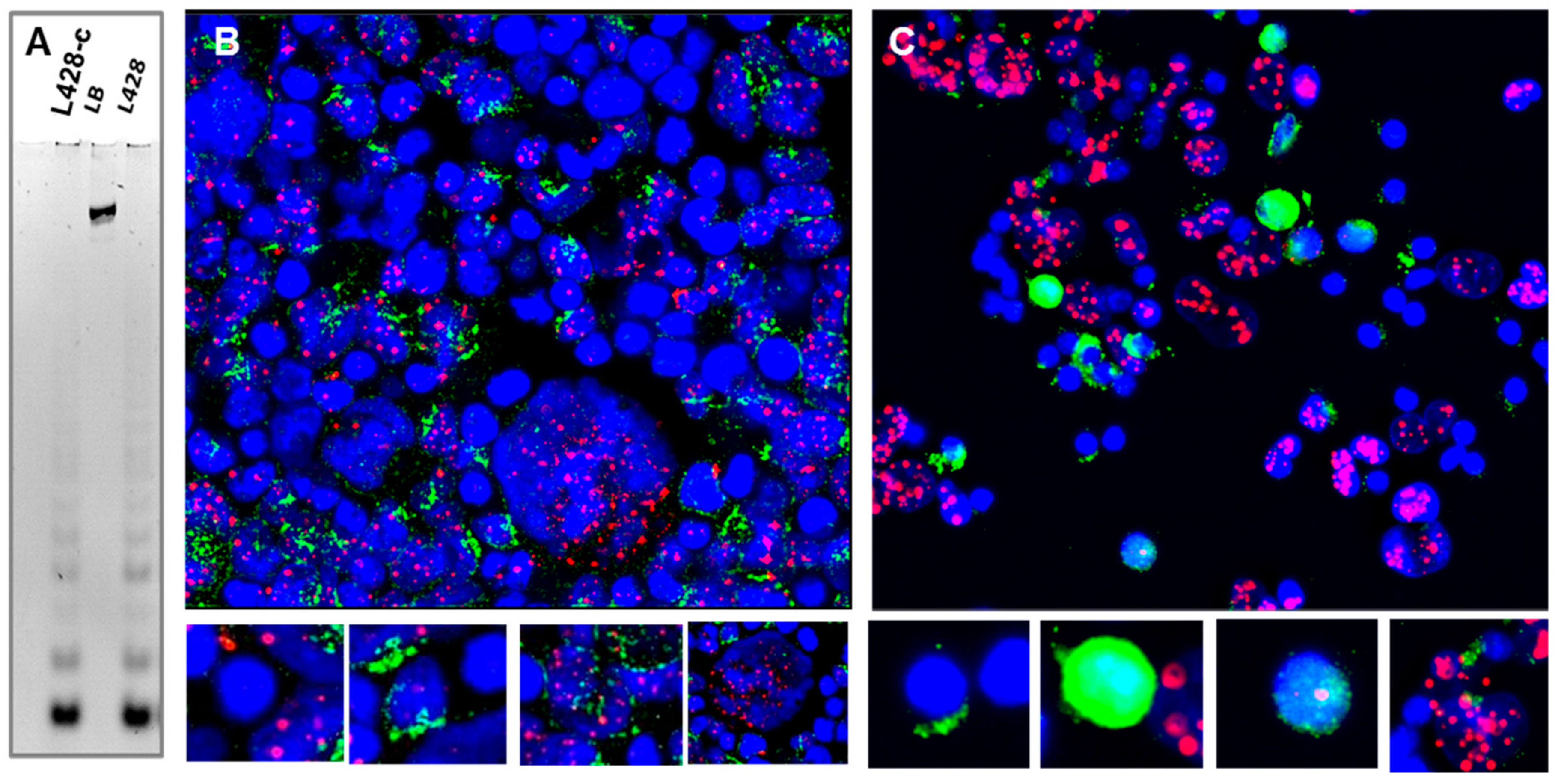
2.3.6. Telomere Maintenance of HL Cells Grown In Vitro and In Vivo
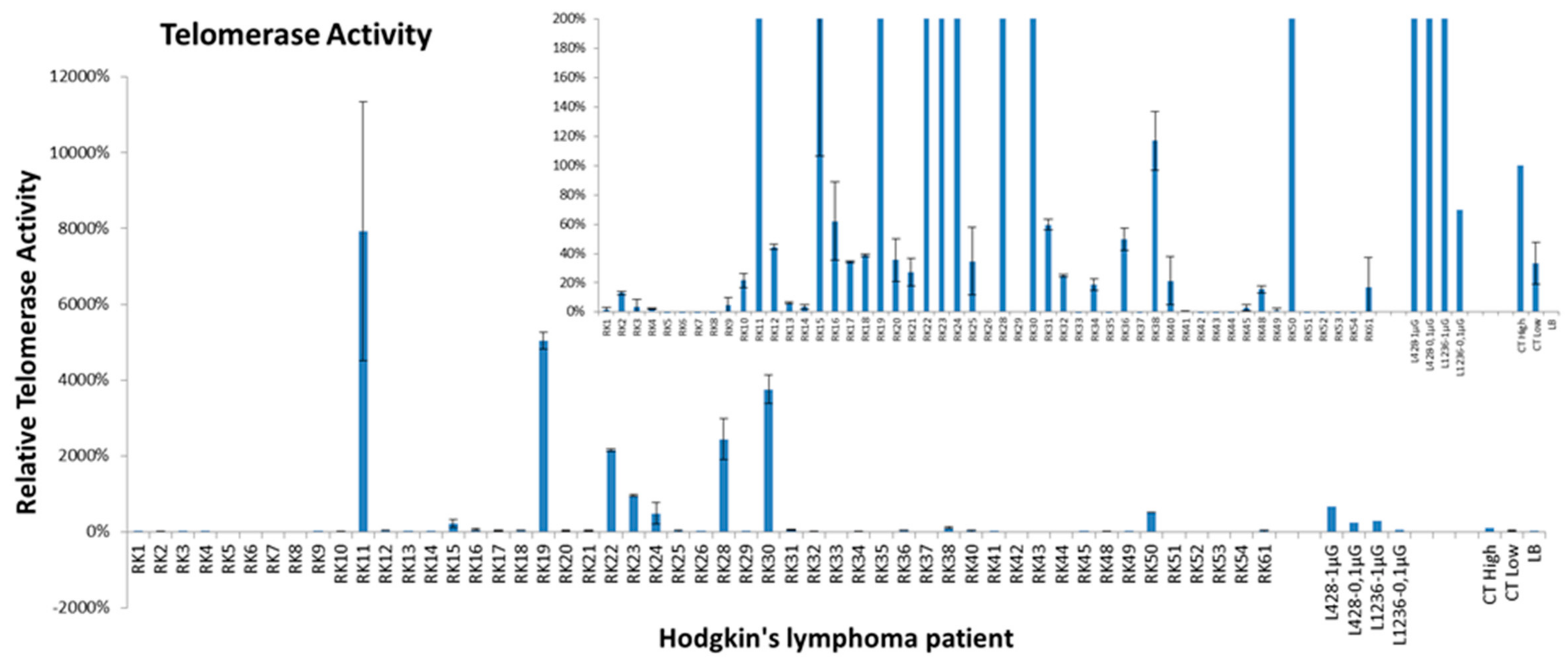
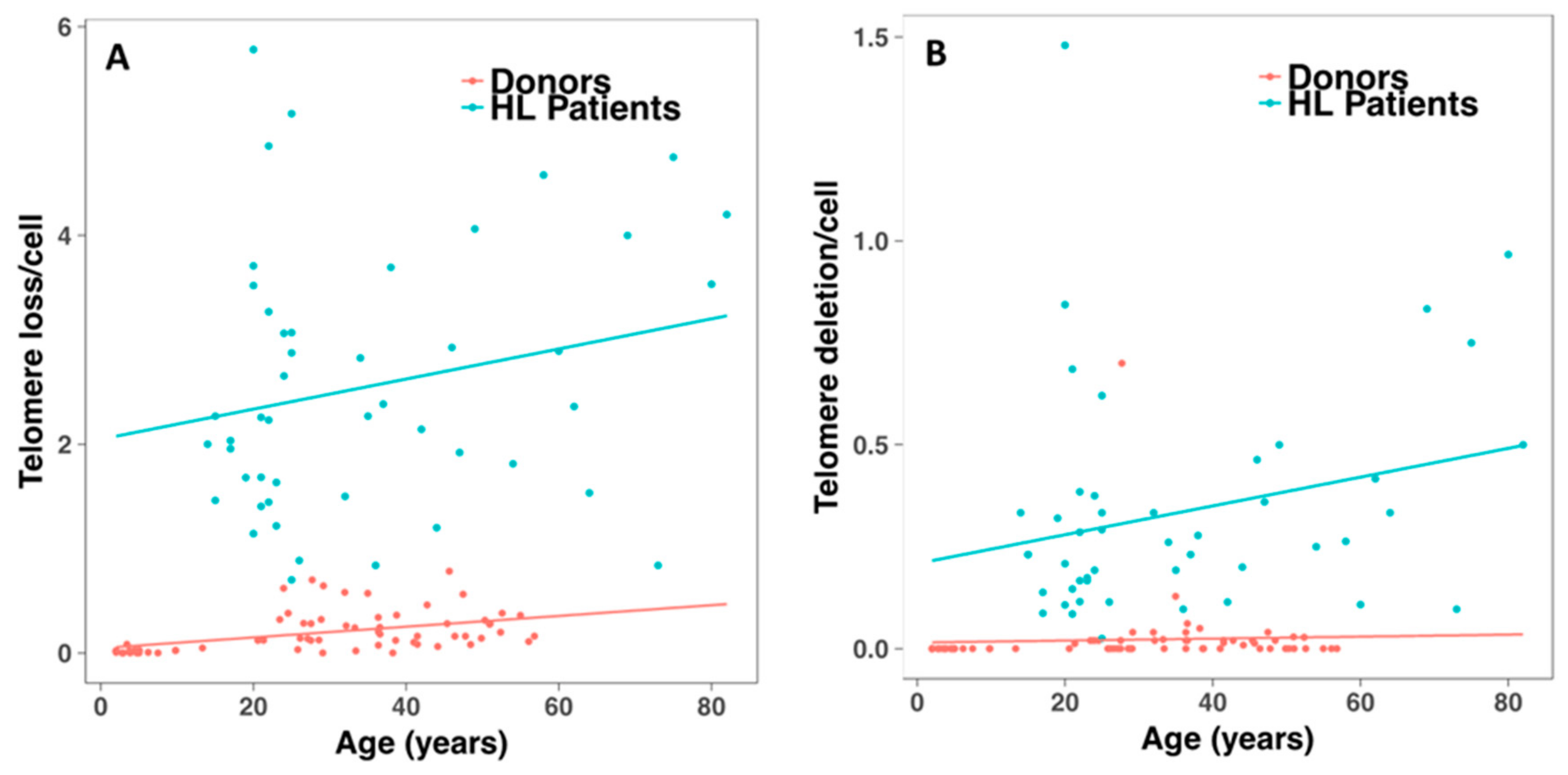
2.4. Telomerase Activity and Telomere Dysfunction in Circulating Lymphocytes of HL Patients
2.5. Preclinical Effect of a First In-Class Alkylating HDAC Inhibitor Fusion Molecule (EDO-S101) in the HL Animal Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Cell Lines, Patient Samples and Cell Cultures
4.1.2. Xenograft Models
4.1.3. In Vivo EDO-S101 Activity
4.2. Methods
4.2.1. Fluorescence-Activated Cell Sorting and Flow Cytometry Analyses
4.2.2. Clonogenic Assays
4.2.3. Tissue and Slide Preparation
4.2.4. Immunohistochemistry Analysis
4.2.5. Immunofluorescence to Assess CD30 Expression
4.2.6. Cytogenetic Analysis
4.2.7. Telomerase Activity and Telomere Maintenance Mechanisms in Clonogenic Cells
4.2.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuppers, R.; Rajewsky, K.; Zhao, M.; Simons, G.; Laumann, R.; Fischer, R.; Hansmann, M.L. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc. Natl. Acad. Sci. USA 1994, 91, 10962–10966. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.; Meyer, C.; Quentmeier, H.; Kaufmann, M.; Drexler, H.G.; MacLeod, R.A. Chromothripsis in Hodgkin lymphoma. Genes Chromosomes Cancer 2013, 52, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.Z.; Watanabe, M.; Ahmed, S.; Terashima, K.; Horiuchi, S.; Sata, T.; Honda, M.; Ito, M.; Watanabe, T.; Horie, R.; et al. Hodgkin’s lymphoma cells are efficiently engrafted and tumor marker CD30 is expressed with constitutive nuclear factor-κ activity in unconditioned NOD/SCID/gammac(null) mice. Cancer Sci. 2005, 96, 466–473. [Google Scholar] [CrossRef] [PubMed]
- von Kalle, C.; Wolf, J.; Becker, A.; Sckaer, A.; Munck, M.; Engert, A.; Kapp, U.; Fonatsch, C.; Komitowski, D.; Feaux de Lacroix, W.; et al. Growth of hodgkin cell lines in severely combined immunodeficient mice. Int. J. Cancer 1992, 52, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, S.L.; Giacomini, A.; Guidetti, A.; Cleris, L.; Mortarini, R.; Anichini, A.; Gianni, A.M.; Carlo-Stella, C. Perifosine and Sorafenib combination induces mitochondrial cell death and antitumor effects in NOD/SCID mice with hodgkin lymphoma cell line xenografts. Leukemia 2013, 27, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.; Hajdu, M.; Varadi, Z.; Sticz, T.B.; Nagy, N.; Csomor, J.; Berczi, L.; Varga, V.; Csoka, M.; Kopper, L.; et al. Characteristic mtor activity in Hodgkin-lymphomas offers a potential therapeutic target in high risk disease—A combined tissue microarray, in vitro and in vivo study. BMC Cancer 2013, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Zhang, M.; Wilson, K.M.; Petrus, M.N.; Bamford, R.N.; Zhang, X.; Guha, R.; Ferrer, M.; Thomas, C.J.; Waldmann, T.A. Augmented efficacy of Brentuximab Vedotin combined with Ruxolitinib and/or Navitoclax in a murine model of human Hodgkin’s lymphoma. Proc. Natl. Acad. Sci. USA 2016, 113, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Kapp, U.; Wolf, J.; Hummel, M.; Pawlita, M.; von Kalle, C.; Dallenbach, F.; Schwonzen, M.; Krueger, G.R.; Muller-Lantzsch, N.; Fonatsch, C.; et al. Hodgkin’s lymphoma-derived tissue serially transplanted into severe combined immunodeficient mice. Blood 1993, 82, 1247–1256. [Google Scholar] [PubMed]
- Borchmann, P.; Treml, J.F.; Hansen, H.; Gottstein, C.; Schnell, R.; Staak, O.; Zhang, H.F.; Davis, T.; Keler, T.; Diehl, V.; et al. The human anti-CD30 antibody 5F11 shows in vitro and in vivo activity against malignant lymphoma. Blood 2003, 102, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.; Bhalla, S.; Gartenhaus, R.B.; Crombie, J.; Kandela, I.; Sharma, J.; Mazar, A.; Evens, A.M. The novel organic arsenical darinaparsin induces MAPK-mediated and SHP1-dependent cell death in T-cell lymphoma and Hodgkin lymphoma cells and human xenograft models. Clin. Cancer Res. 2014, 20, 6023–6033. [Google Scholar] [CrossRef] [PubMed]
- Reiners, K.S.; Gossmann, A.; von Strandmann, E.P.; Boll, B.; Engert, A.; Borchmann, P. Effects of the anti-VEGF monoclonal antibody Bevacizumab in a preclinical model and in patients with refractory and multiple relapsed Hodgkin Lymphoma. J. Immunother. 2009, 32, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Newcom, S.R.; Kadin, M.E.; Phillips, C. L-428 Reed-Sternberg cells and mononuclear Hodgkin’s cells arise from a single cloned mononuclear cell. Int. J. Cell Cloning 1988, 6, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Gocke, C.D.; Kasamon, Y.L.; Miller, C.B.; Perkins, B.; Barber, J.P.; Vala, M.S.; Gerber, J.M.; Gellert, L.L.; Siedner, M.; et al. Circulating clonotypic B cells in classic Hodgkin Lymphoma. Blood 2009, 113, 5920–5926. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.P.; Hopman, A.H.; Haesevoets, A.M.; Gennotte, I.A.; Bot, F.J.; Arends, J.W.; Ramaekers, F.C.; Schouten, H.C. Chromosomal abnormalities in Hodgkin’s disease are not restricted to Hodgkin/Reed-sternberg cells. J. Pathol. 1998, 185, 145–152. [Google Scholar] [CrossRef]
- Jansen, M.P.; Hopman, A.H.; Bot, F.J.; Haesevoets, A.; Stevens-Kroef, M.J.; Arends, J.W.; Jox, A.; Wolf, J.; Ramaekers, F.C.; Schouten, H.C. Morphologically normal, CD30-negative B-lymphocytes with chromosome aberrations in classical Hodgkin’s disease: The progenitor cell of the malignant clone? J. Pathol. 1999, 189, 527–532. [Google Scholar] [CrossRef]
- Sanchez-Aguilera, A.; Montalban, C.; de la Cueva, P.; Sanchez-Verde, L.; Morente, M.M.; Garcia-Cosio, M.; Garcia-Larana, J.; Bellas, C.; Provencio, M.; Romagosa, V.; et al. Tumor microenvironment and mitotic checkpoint are key factors in the outcome of classic Hodgkin Lymphoma. Blood 2006, 108, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Kuppers, R. Clonotypic B cells in classic Hodgkin Lymphoma. Blood 2009, 114, 3970–3971; author reply 3971–3972. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, R.D. Stem cells in Hodgkin lymphoma? Blood 2009, 113, 5694. [Google Scholar] [CrossRef] [PubMed]
- Oki, Y.; Neelapu, S.S.; Fanale, M.; Kwak, L.W.; Fayad, L.; Rodriguez, M.A.; Wallace, M.; Klinger, M.; Carlton, V.; Kong, K.; et al. Detection of classical Hodgkin lymphoma specific sequence in peripheral blood using a next-generation sequencing approach. Br. J. Haematol. 2015, 169, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Kuppers, R.; Brauninger, A.; Muschen, M.; Distler, V.; Hansmann, M.L.; Rajewsky, K. Evidence that hodgkin and Reed-Sternberg cells in Hodgkin disease do not represent cell fusions. Blood 2001, 97, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Guffei, A.; Sarkar, R.; Klewes, L.; Righolt, C.; Knecht, H.; Mai, S. Dynamic chromosomal rearrangements in Hodgkin’s Lymphoma are due to ongoing three-dimensional nuclear remodeling and breakage-bridge-fusion cycles. Haematologica 2010, 95, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Bennaceur-Griscelli, A.; Girinsky, T.; Koscielny, S.; Delhommeau, F.; Dossou, J.; Violot, D.; Leclercq, E.; Courtier, M.H.; Beron-Gaillard, N.; et al. Telomere shortening and associated chromosomal instability in peripheral blood lymphocytes of patients with Hodgkin’s Lymphoma prior to any treatment are predictive of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, C.; Mancini, A.; Colapietro, A.; Gravina, G.L.; Vitale, F.; Marampon, F.; Delle Monache, S.; Pompili, S.; Cristiano, L.; Vetuschi, A.; et al. Correction to: The first-in-class alkylating deacetylase inhibitor molecule Tinostamustine shows antitumor effects and is synergistic with radiotherapy in preclinical models of glioblastoma. J. Hematol. Oncol. 2018, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Stathis, A.; Younes, A. The new therapeutical scenario of Hodgkin Lymphoma. Ann Oncol. 2015, 26, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Garrido-Laguna, I.; Naing, A.; Fu, S.; Falchook, G.S.; Piha-Paul, S.A.; Wheler, J.J.; Hong, D.S.; Tsimberidou, A.M.; Subbiah, V.; et al. Phase I dose-escalation study of the mTOR inhibitor sirolimus and the HDAC inhibitor Vorinostat in patients with advanced malignancy. Oncotarget 2016, 7, 67521–67531. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, L.; Ricoul, M.; Pottier, G.; Murnane, J.P. The loss of a single telomere can result in instability of multiple chromosomes in a human tumor cell line. Mol. Cancer Res. MCR 2005, 3, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Murnane, J.P.; Sabatier, L. Chromosome rearrangements resulting from telomere dysfunction and their role in cancer. BioEssays 2004, 26, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Cuceu, C.; Colicchio, B.; Jeandidier, E.; Junker, S.; Plassa, F.; Shim, G.; Mika, J.; Frenzel, M.; Al Jawhari, M.; Hempel, W.; et al. Independent Mechanisms Lead to Genomic Instability in Hodgkin Lymphoma: Microsatellite or Chromosomal Instability. Cancers 2018, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Marks, D.H.; Chin, Y.; Benezra, R. Whole chromosome loss and associated breakage-fusion-bridge cycles transform mouse tetraploid cells. EMBO J. 2018, 37, 201–218. [Google Scholar] [CrossRef] [PubMed]
- M’kacher, R.; Cuceu, C.; Al Jawhari, M.; Morat, L.; Frenzel, M.; Shim, G.; Lenain, A.; Hempel, W.M.; Junker, S.; Girinsky, T.; et al. The Transition between Telomerase and ALT Mechanisms in Hodgkin Lymphoma and Its Predictive Value in Clinical Outcomes. Cancers 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, K.; Yu, E.Y.; Khurts, S.; Ben-Porath, I.; Currier, J.L.; Metz, G.B.; Brooks, M.W.; Kaneko, S.; Murakami, S.; DeCaprio, J.A.; et al. Telomerase maintains telomere structure in normal human cells. Cell 2003, 114, 241–253. [Google Scholar] [CrossRef]
- M’kacher, R.; Girinsky, T.; Koscielny, S.; Dossou, J.; Violot, D.; Béron-Gaillard, N.; Ribrag, V.; Bourhis, J.; Bernheim, A.; Parmentier, C.; et al. Baseline and treatment-induced chromosomal abnormalities in peripheral blood lymphocytes of Hodgkin’s Lymphoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 321–326. [Google Scholar] [CrossRef]
- M’kacher, R.; Andreoletti, L.; Flamant, S.; Milliat, F.; Girinsky, T.; Dossou, J.; Violot, D.; Assaf, E.; Clausse, B.; Koscielny, S.; et al. JC human polyomavirus is associated to chromosomal instability in peripheral blood lymphocytes of Hodgkin’s Lymphoma patients and poor clinical outcome. Ann. Oncol. 2010, 21, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Besson, C.; Amiel, C.; Le-Pendeven, C.; Plancoulaine, S.; Bonnardel, C.; Ranque, B.; Abbed, K.; Brice, P.; Fermé, C.; Carde, P.; et al. Strong correlations of anti-viral capsid antigenantibody levels in first-degree relatives from families with Epstein-Barr virus-related lymphomas. J. Infect. Dis. 2009, 199, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- McMaster, M.L.; Sun, C.; Landi, M.T.; Savage, S.A.; Rotunno, M.; Yang, X.R.; Jones, K.; Vogt, A.; Hutchinson, A.; Zhu, B.; et al. Germline mutations in Protection of Telomeres 1 in two families with Hodgkin Lymphoma. Br. J. Haematol. 2018, 181, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Al Jawhari, M.; Cuceu, C.; Frenzel, M.; Stimmer, L.; Junker, S.; Bosq, J.; Carde, P.; Mehrling, T.; Sabatier, L.; Pflumio, F.; et al. In vitro and in vivo preclinical activity of EDO-S101 in Hodgkin lymphoma. In Proceedings of the Haematologica, 10th International Symposium on Hodgkin Lymphoma, Cologne, Germany, 20 October 2016; Volume 101, pp. 6–7. [Google Scholar]
- Cuceu, C.; Hempel, W.M.; Sabatier, L.; Bosq, J.; Carde, P.; M’kacher, R. Chromosomal Instability in Hodgkin Lymphoma: An In-Depth Review and Perspectives. Cancers 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Maalouf, E.E.; Ricoul, M.; Heidingsfelder, L.; Laplagne, E.; Cuceu, C.; Hempel, W.M.; Colicchio, B.; Dieterlen, A.; Sabatier, L. New tool for biological dosimetry: Reevaluation and automation of the gold standard method following telomere and centromere staining. Mutat. Res. 2014, 770, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, A.; Colicchio, B.; Buron, D.; El Maalouf, E.; Laplagne, E.; Borie, C.; Ricoul, M.; Lenain, A.; Hempel, W.M.; Morat, L.; et al. Transmission of Induced Chromosomal Aberrations through Successive Mitotic Divisions in Human Lymphocytes after In Vitro and In Vivo Radiation. Sci Rep. 2017, 7, 3291. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | N° of Patients | % |
|---|---|---|
| (N = 50) | ||
| Male | 31 | 62 |
| Age | 35 y | |
| <45 | 40 | 80 |
| >45 | 10 | 20 |
| Stage | ||
| Stage I | 10 | 20 |
| Stage II | 35 | 70 |
| Stage II | 5 | 10 |
| Treatment | ||
| Chemotherapy only | 0 | 0 |
| Combined modality | 50 | 100 |
| Histological sub-type | ||
| Nodular Sclerosis | 36 | 94.7 |
| Mixed cellularity | 6 | 2.6 |
| Classic lymphocyte rich | 4 | 2.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’kacher, R.; Frenzel, M.; Al Jawhari, M.; Junker, S.; Cuceu, C.; Morat, L.; Bauchet, A.-L.; Stimmer, L.; Lenain, A.; Dechamps, N.; et al. Establishment and Characterization of a Reliable Xenograft Model of Hodgkin Lymphoma Suitable for the Study of Tumor Origin and the Design of New Therapies. Cancers 2018, 10, 414. https://doi.org/10.3390/cancers10110414
M’kacher R, Frenzel M, Al Jawhari M, Junker S, Cuceu C, Morat L, Bauchet A-L, Stimmer L, Lenain A, Dechamps N, et al. Establishment and Characterization of a Reliable Xenograft Model of Hodgkin Lymphoma Suitable for the Study of Tumor Origin and the Design of New Therapies. Cancers. 2018; 10(11):414. https://doi.org/10.3390/cancers10110414
Chicago/Turabian StyleM’kacher, Radhia, Monika Frenzel, Mustafa Al Jawhari, Steffen Junker, Corina Cuceu, Luc Morat, Anne-Laure Bauchet, Lev Stimmer, Aude Lenain, Nathalie Dechamps, and et al. 2018. "Establishment and Characterization of a Reliable Xenograft Model of Hodgkin Lymphoma Suitable for the Study of Tumor Origin and the Design of New Therapies" Cancers 10, no. 11: 414. https://doi.org/10.3390/cancers10110414
APA StyleM’kacher, R., Frenzel, M., Al Jawhari, M., Junker, S., Cuceu, C., Morat, L., Bauchet, A.-L., Stimmer, L., Lenain, A., Dechamps, N., Hempel, W. M., Pottier, G., Heidingsfelder, L., Laplagne, E., Borie, C., Oudrhiri, N., Jouni, D., Bennaceur-Griscelli, A., Colicchio, B., ... Carde, P. (2018). Establishment and Characterization of a Reliable Xenograft Model of Hodgkin Lymphoma Suitable for the Study of Tumor Origin and the Design of New Therapies. Cancers, 10(11), 414. https://doi.org/10.3390/cancers10110414










