Destruxin B Suppresses Drug-Resistant Colon Tumorigenesis and Stemness Is Associated with the Upregulation of miR-214 and Downregulation of mTOR/β-Catenin Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Stemness Assays
2.3. In Vitro Cell Viability and Drug Test
2.4. Western Blot Analysis
2.5. Colony-Forming and Tumor Sphere-Forming Assays
2.6. Animal Study
2.7. Statistical Analysis
3. Results
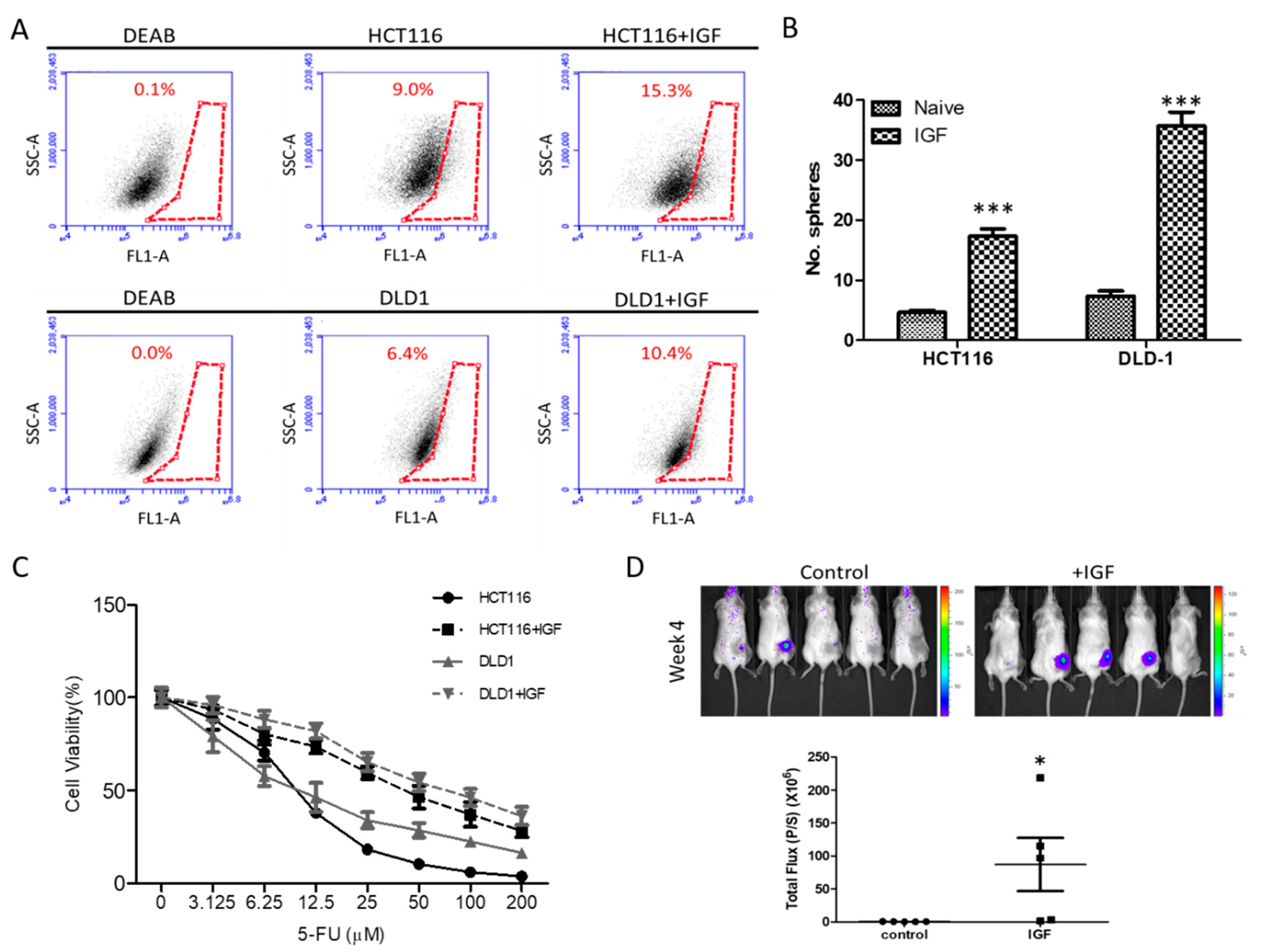
3.1. Exogenous IGF1 Enriched Cancer Stem-Like Colon Cancer Cells and Induced 5-FU Resistance
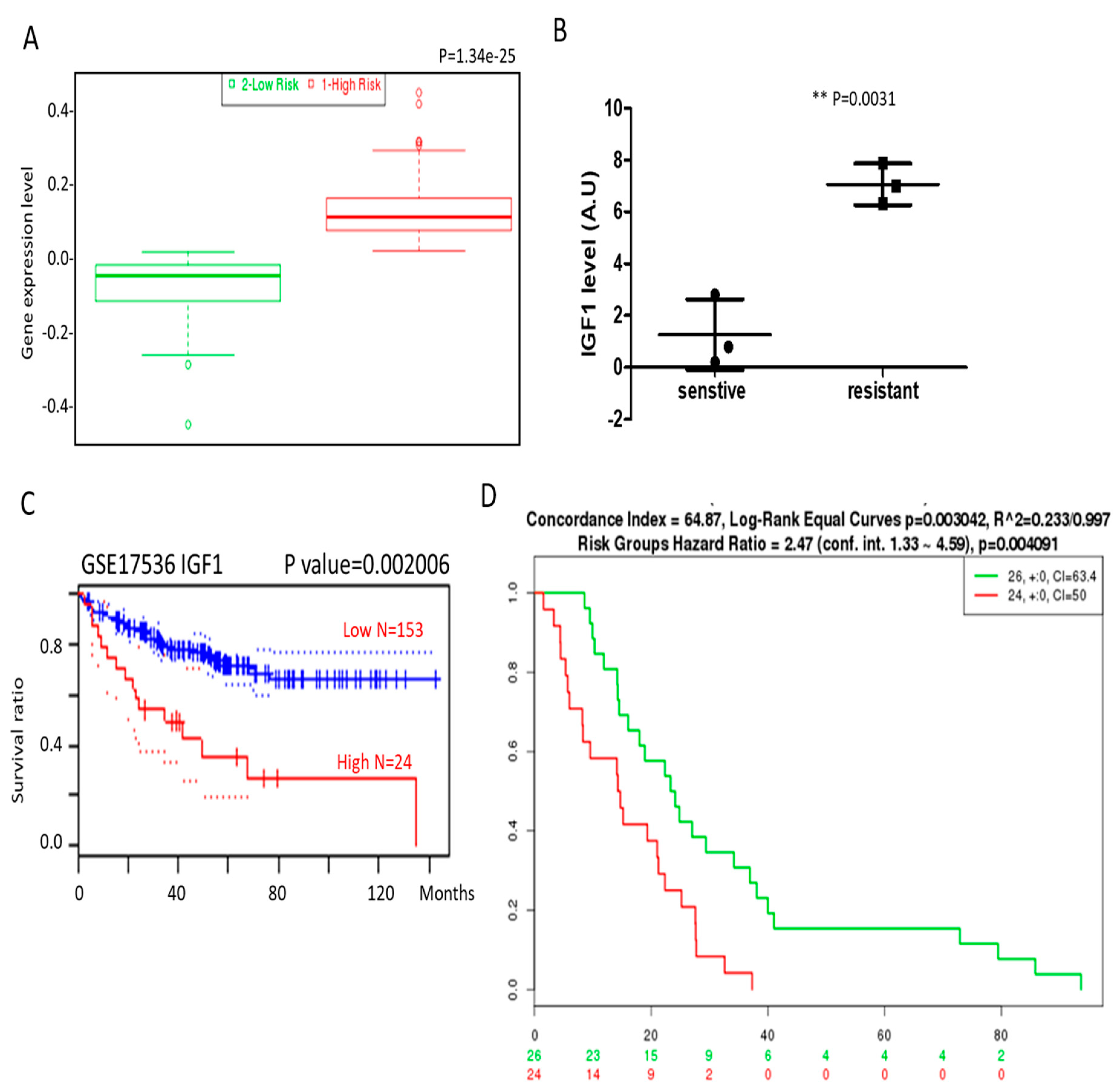
3.2. IGF1 and β-Catenin Expression Is Associated with Drug Resistance and Poor Prognosis in Colon Cancer Patients
3.3. DB Treatment Suppressed Drug-Resistant Colon Tumorigenesis and Stemness
3.4. DB and 5-FU Synergistically Suppresses Viability of Colon Cancer Cells
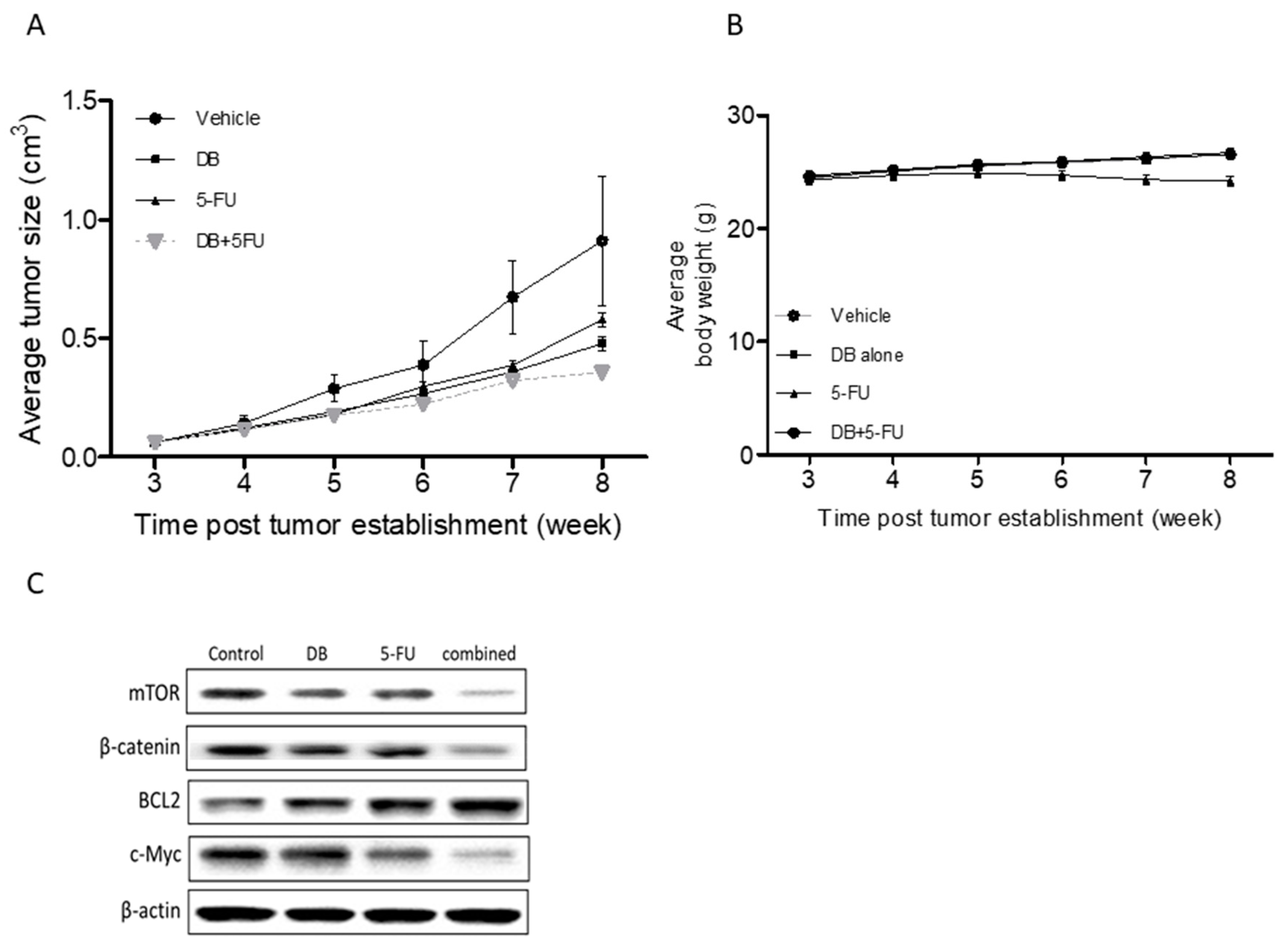
3.5. In Vivo Demonstration of DB Treatment Enhanced 5-FU Efficacy
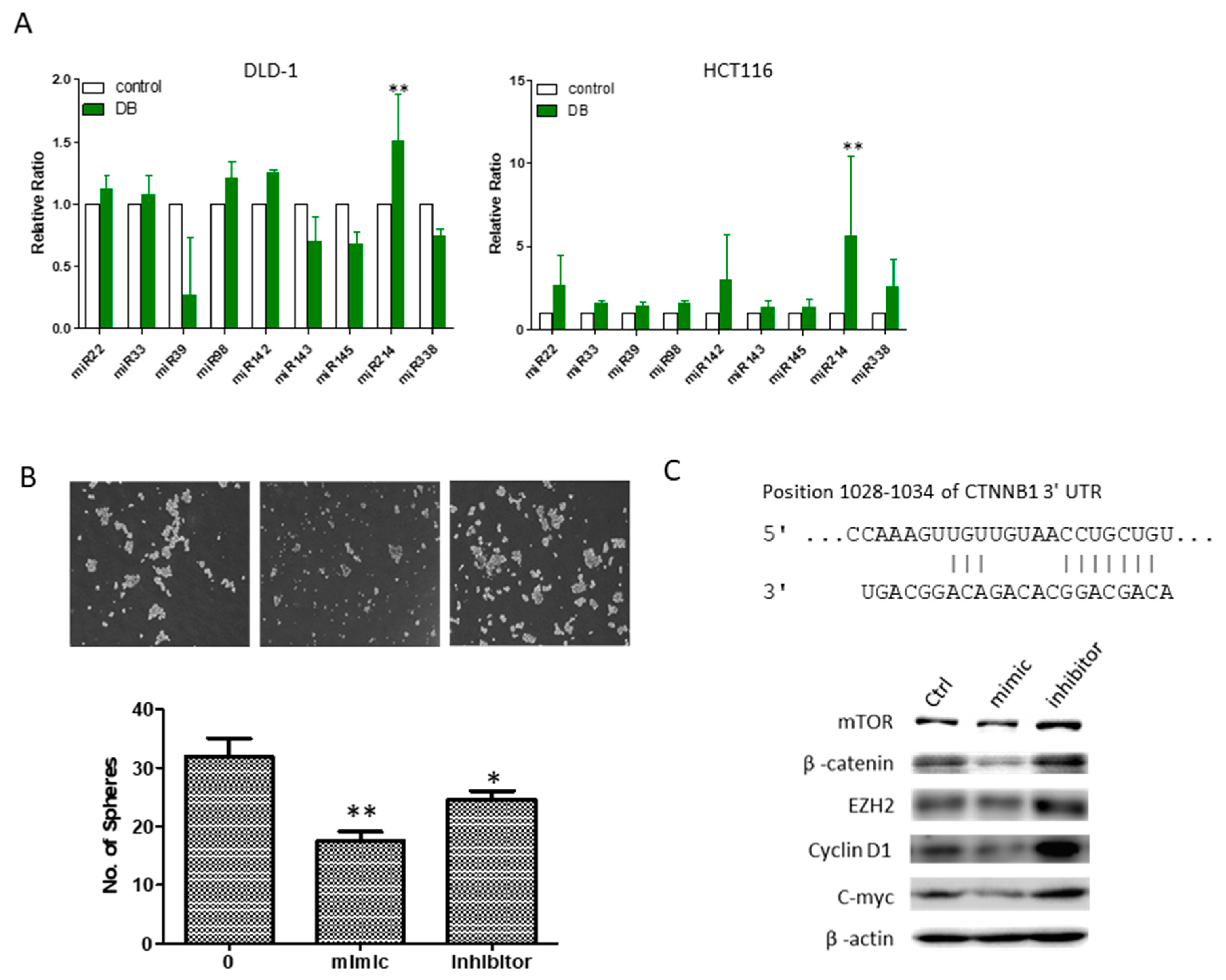
3.6. An Increased MicroRNA-214 Level Was Associated with DB Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DB | Destruxin B |
| CSCs | Cancer stem-like cells |
| 5-FU | Fluorouracil |
| SP | Side population |
| IGF1 | Insulin-likegrowth factor 1 |
| mTOR | The mammalian target of rapamycin |
References
- Boyle, P.; Langman, J.S. ABC of colorectal cancer: Epidemiology. BMJ 2000, 321, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Boushey, R.P. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.H.; Chen, M.C.; Baskaran, R.; Lin, Y.M.; Day, C.H.; Lin, Y.J.; Tu, C.C.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell. Physiol. 2017, 233, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.L.; Schumacher, D.; Staudte, S.; Steffen, A.; Haybaeck, J.; Keilholz, U.; Schweiger, C.; Golob-Schwarzl, N.; Mumberg, D.; Henderson, D.; et al. Non-Canonical Hedgehog Signaling Is a Positive Regulator of the WNT Pathway and Is Required for the Survival of Colon Cancer Stem Cells. Cell Rep. 2017, 21, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.S.; Pelagalli, A.; Passaro, N.; Zannetti, A. Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget 2017, 8, 73296–73311. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, C.; Kramer, N.; Unterleuthner, D.; Scherzer, M.; Burian, A.; Rudisch, A.; Stadler, M.; Schlederer, M.; Lenhardt, D.; Riedl, A.; et al. Stromal-derived IGF2 promotes colon cancer progression via paracrine and autocrine mechanisms. Oncogene 2017, 36, 5341–5355. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, W.; Li, M.; Wen, J.; Zhu, M.; Xu, S. Insulin-Like Growth Factor-1 Modulates Polycomb Cbx8 Expression and Inhibits Colon Cancer Cell Apoptosis. Cell Biochem. Biophys. 2015, 71, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Bagheri, N.; Rafieian-Kopaei, M.; Shirzad, H. Inhibition of Th1 and Th17 Cells by Medicinal Plants and Their Derivatives: A Systematic Review. Phytother. Res. PTR 2017, 31, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Mahadevappa, R.; Kwok, H.F. Phytochemicals—A Novel and Prominent Source of Anti-cancer Drugs Against Colorectal Cancer. Comb. Chem. High Throughput Screen. 2017, 20, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. The protective role of plant biophenols in mechanisms of Alzheimer’s disease. J. Nutr. Biochem. 2017, 47, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dornetshuber-Fleiss, R.; Heffeter, P.; Mohr, T.; Hazemi, P.; Kryeziu, K.; Seger, C.; Berger, W.; Lemmens-Gruber, R. Destruxins: Fungal-derived cyclohexadepsipeptides with multifaceted anticancer and antiangiogenic activities. Biochem. Pharmacol. 2013, 86, 361–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang Liu, R.; Chen, S.P.; Lu, T.M.; Tsai, W.Y.; Tsai, C.H.; Yang, C.C.; Tzeng, Y.M. Selective apoptotic cell death effects of oral cancer cells treated with destruxin B. BMC Complement. Altern. Med. 2014, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; Rao, Y.K.; Lee, W.H.; Chen, H.A.; Le, T.D.; Tzeng, D.T.; Wang, L.S.; Wu, A.T.; Lin, Y.F.; Tzeng, Y.M.; et al. Destruxin B inhibits hepatocellular carcinoma cell growth through modulation of the Wnt/beta-catenin signaling pathway and epithelial-mesenchymal transition. Toxicol. In Vitro 2014, 28, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Rao, Y.K.; Ye, M.; Wu, W.S.; Chang, T.C.; Wang, L.S.; Wu, C.H.; Wu, A.T.; Tzeng, Y.M. Preclinical evaluation of destruxin B as a novel Wnt signaling target suppressing proliferation and metastasis of colorectal cancer using non-invasive bioluminescence imaging. Toxicol. Appl. Pharmacol. 2012, 261, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Prieur, A.; Cappellini, M.; Habif, G.; Lefranc, M.P.; Mazard, T.; Morency, E.; Pascussi, J.M.; Flaceliere, M.; Cahuzac, N.; Vire, B.; et al. Targeting the Wnt Pathway and Cancer Stem Cells with Anti-progastrin Humanized Antibodies as a Potential Treatment for K-RAS-Mutated Colorectal Cancer. Clin. Cancer Res. 2017, 23, 5267–5280. [Google Scholar] [CrossRef] [PubMed]
- Young, M.A.; Daly, C.S.; Taylor, E.; James, R.; Clarke, A.R.; Reed, K.R. Subtle Deregulation of the Wnt-Signaling Pathway Through Loss of Apc2 Reduces the Fitness of Intestinal Stem Cells. Stem Cells 2018, 36, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.K.; Kanojia, D.; Liu, X.; Singh, U.P.; Berger, F.G.; Wang, Q.; Chen, H. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene 2012, 31, 2614–2626. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, P.G.; Tirro, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Ma, K.; Zhao, Y.; Liu, X.; Wang, Y.; Liu, M.; Liang, S.; Zhu, H.; Xu, N. Polarization of macrophages in the tumor microenvironment is influenced by EGFR signaling within colon cancer cells. Oncotarget 2016, 7, 75366–75378. [Google Scholar] [CrossRef] [PubMed]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L. Colon cancer: Cancer stem cells markers, drug resistance and treatment. Biomed. Pharmacother. 2014, 68, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Sodani, K.; Dai, C.L.; Ashby, C.R., Jr.; Chen, Z.S. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Nebozhyn, M.V.; Watters, J.W.; Buser, C.A.; Shaw, P.M.; Huang, P.S.; Van’t Veer, L.; Tollenaar, R.A.; Jackson, D.B.; Agrawal, D.; et al. EMT is the dominant program in human colon cancer. BMC Med. Genom. 2011, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Mencia, N.; Selga, E.; Noe, V.; Ciudad, C.J. Underexpression of miR-224 in methotrexate resistant human colon cancer cells. Biochem. Pharmacol. 2011, 82, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Deane, N.G.; Wu, F.; Merchant, N.B.; Zhang, B.; Jiang, A.; Lu, P.; Johnson, J.C.; Schmidt, C.; Bailey, C.E.; et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology 2010, 138, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Jorissen, R.N.; Gibbs, P.; Christie, M.; Prakash, S.; Lipton, L.; Desai, J.; Kerr, D.; Aaltonen, L.A.; Arango, D.; Kruhoffer, M.; et al. Metastasis-Associated Gene Expression Changes Predict Poor Outcomes in Patients with Dukes Stage B and C Colorectal Cancer. Clin. Cancer Res. 2009, 15, 7642–7651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.P.; Wang, C.W.; Liao, W.C.; Yang, C.R.; Yeh, C.T.; Tsai, C.H.; Yang, C.C.; Tzeng, Y.M. In vitro and in vivo anticancer effects of destruxin B on human colorectal cancer. Anticancer Res. 2012, 32, 2735–2745. [Google Scholar] [PubMed]
- Wu, C.C.; Chen, T.H.; Liu, B.L.; Wu, L.C.; Chen, Y.C.; Tzeng, Y.M.; Hsu, S.L. Destruxin B Isolated from Entomopathogenic Fungus Metarhizium anisopliae Induces Apoptosis via a Bcl-2 Family-Dependent Mitochondrial Pathway in Human Nonsmall Cell Lung Cancer Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 548929. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Stoica, V.; Radulian, G. Stem cells and colorectal carcinogenesis. J. Med. Life 2016, 9, 6–11. [Google Scholar] [PubMed]
- Han, L.; Zhang, G.F.; Cheng, Y.H.; Zhao, Q.C. Correlations of insulin-like growth factor I and insulin-like growth factor I receptor with the clinicopathological features and prognosis of patients with colon cancer. Jpn. J. Clin. Oncol. 2016, 46, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Pischon, T. Obesity Biomarkers, Metabolism and Risk of Cancer: An Epidemiological Perspective. Recent Results Cancer Res. 2016, 208, 199–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, N.; Oshima, T.; Yoshihara, K.; Aoyama, T.; Hayashi, T.; Yamada, T.; Sato, T.; Shiozawa, M.; Yoshikawa, T.; Morinaga, S.; et al. Clinicopathological significance and impact on outcomes of the gene expression levels of IGF-1, IGF-2 and IGF-1R, IGFBP-3 in patients with colorectal cancer: Overexpression of the IGFBP-3 gene is an effective predictor of outcomes in patients with colorectal cancer. Oncol. Lett. 2017, 13, 3958–3966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, G.; Singh, G.; Dar, M.S.; Singh, P.; Bano, N.; Syed, S.H.; Sandhu, P.; Akhter, Y.; Monga, S.P.; Dar, M.J. Identification of a unique loss-of-function mutation in IGF1R and a crosstalk between IGF1R and Wnt/beta-catenin signaling pathways. Biochim. Biophys. Acta 2018, 1865, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Zhang, W.; Rengarajan, T. Vicenin-2 inhibits Wnt/beta-catenin signaling and induces apoptosis in HT-29 human colon cancer cell line. Drug Des. Dev. Ther. 2018, 12, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Benthani, F.A.; Herrmann, D.; Tran, P.N.; Pangon, L.; Lucas, M.C.; Allam, A.H.; Currey, N.; Al-Sohaily, S.; Giry-Laterriere, M.; Warusavitarne, J.; et al. ‘MCC’ protein interacts with E-cadherin and beta-catenin strengthening cell-cell adhesion of HCT116 colon cancer cells. Oncogene 2018, 37, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, K.; Zhang, N.; Zhu, B.; Feng, G.; Fan, Q. Colon cancers carrying BRAF V600E and beta-catenin T41A activating mutations are resistant to numerous common anticancer drugs. Oncol. Lett. 2018, 15, 4471–4476. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-Y.; Huang, Y.-J.; Tzeng, Y.-M.; Huang, C.-Y.F.; Hsiao, M.; Wu, A.T.H.; Huang, T.-H. Destruxin B Suppresses Drug-Resistant Colon Tumorigenesis and Stemness Is Associated with the Upregulation of miR-214 and Downregulation of mTOR/β-Catenin Pathway. Cancers 2018, 10, 353. https://doi.org/10.3390/cancers10100353
Wu S-Y, Huang Y-J, Tzeng Y-M, Huang C-YF, Hsiao M, Wu ATH, Huang T-H. Destruxin B Suppresses Drug-Resistant Colon Tumorigenesis and Stemness Is Associated with the Upregulation of miR-214 and Downregulation of mTOR/β-Catenin Pathway. Cancers. 2018; 10(10):353. https://doi.org/10.3390/cancers10100353
Chicago/Turabian StyleWu, Szu-Yuan, Yan-Jiun Huang, Yew-Min Tzeng, Chi-Ying F. Huang, Michael Hsiao, Alexander T.H. Wu, and Tse-Hung Huang. 2018. "Destruxin B Suppresses Drug-Resistant Colon Tumorigenesis and Stemness Is Associated with the Upregulation of miR-214 and Downregulation of mTOR/β-Catenin Pathway" Cancers 10, no. 10: 353. https://doi.org/10.3390/cancers10100353
APA StyleWu, S.-Y., Huang, Y.-J., Tzeng, Y.-M., Huang, C.-Y. F., Hsiao, M., Wu, A. T. H., & Huang, T.-H. (2018). Destruxin B Suppresses Drug-Resistant Colon Tumorigenesis and Stemness Is Associated with the Upregulation of miR-214 and Downregulation of mTOR/β-Catenin Pathway. Cancers, 10(10), 353. https://doi.org/10.3390/cancers10100353






