Development and Comparative Evaluation of Two Enzyme-Based Amperometric Biosensor Designs for Alanine Aminotransferase Determination in Biological Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Amperometric Equipment
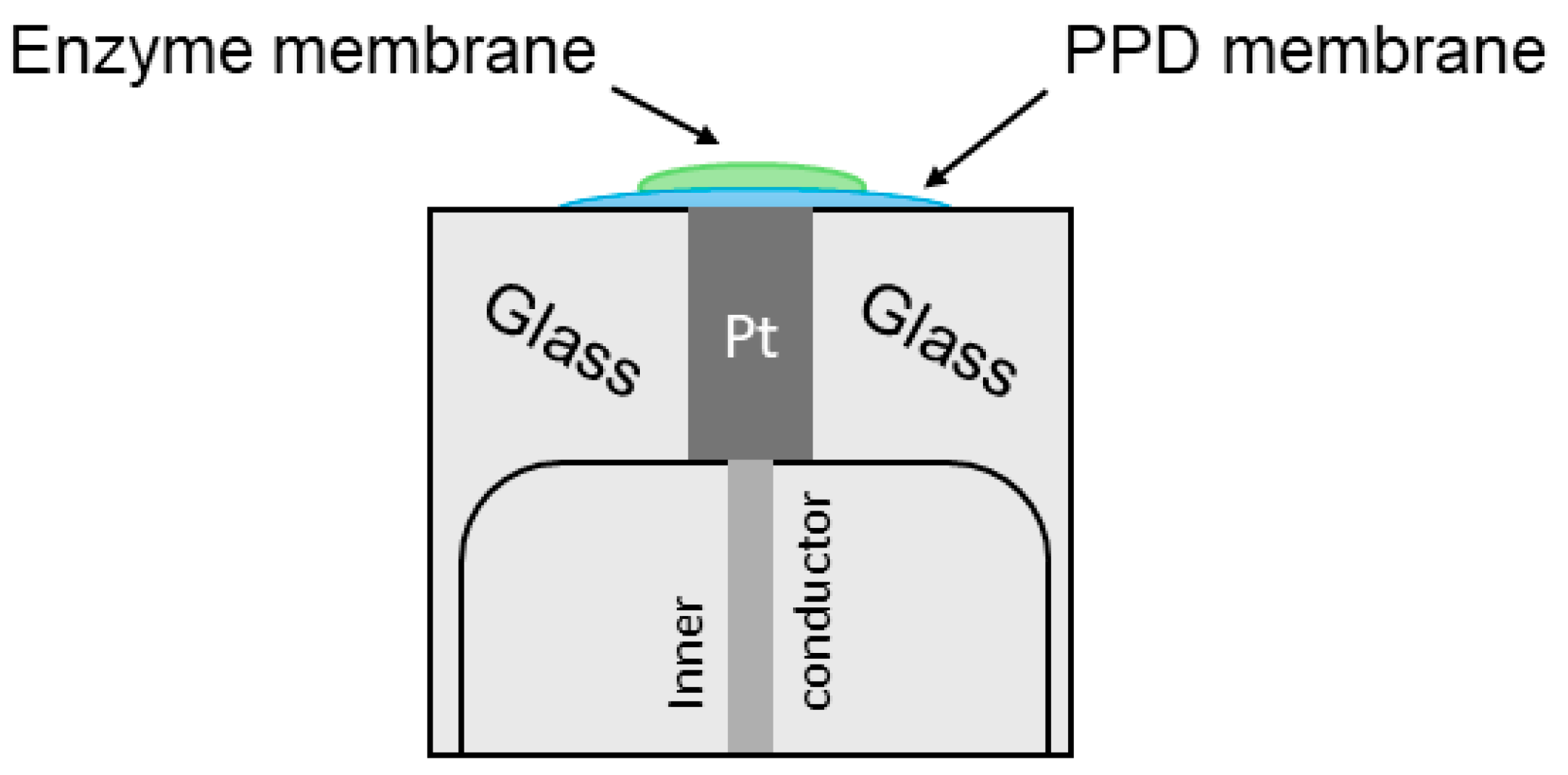
2.3. PPD Membrane
2.4. Methods of Manufacturing Bioselective Membranes
2.5. Methodology for Biosensor Measurement of ALT
2.6. Statistical Treatment
3. Results
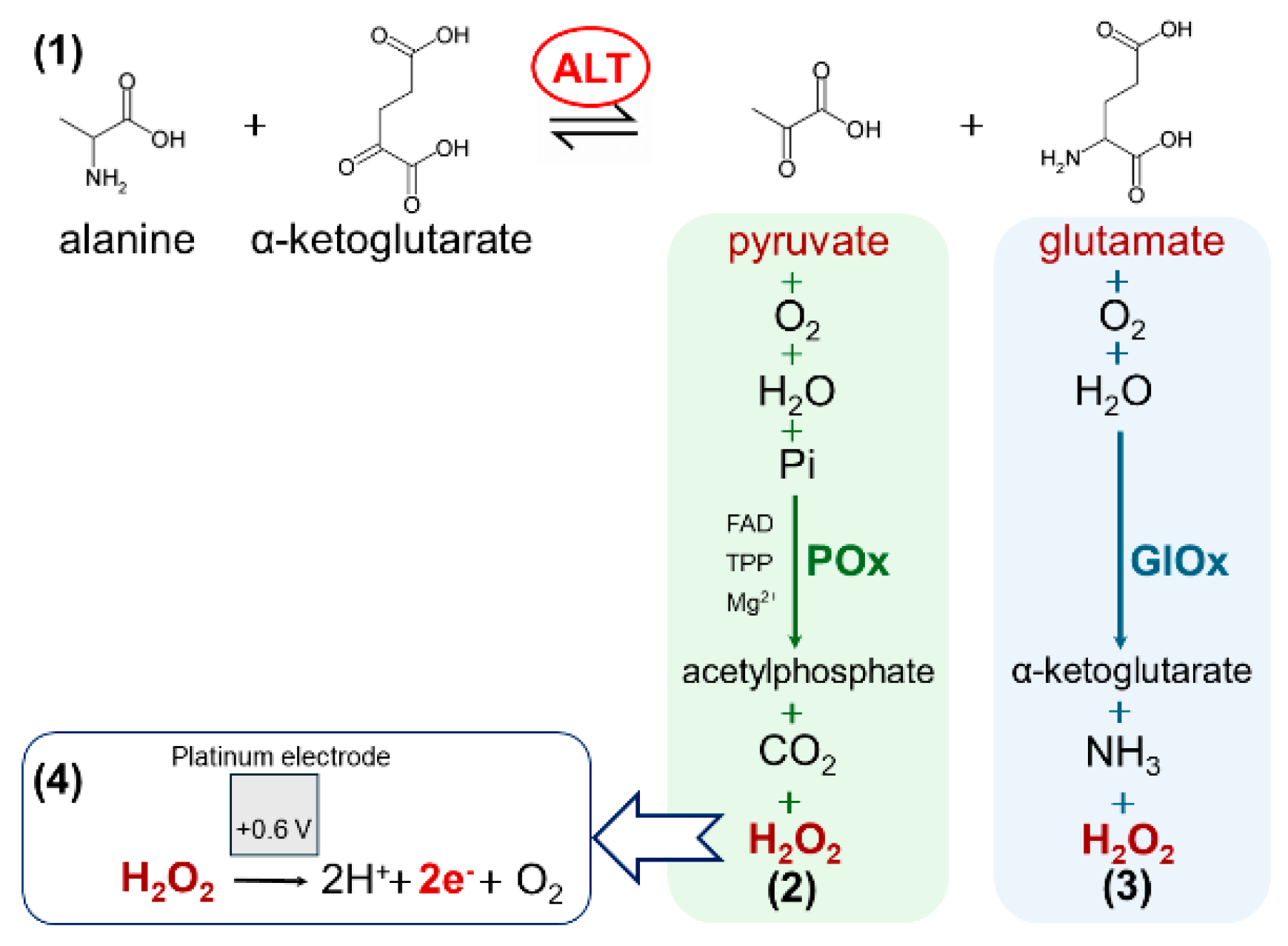
3.1. Principle of Detection
- Electrodes without enzyme-containing membranes showed no response to the presence of all reagents (including ALT) in the working buffer, maintaining a stable baseline current.
- Biosensors in the absence of ALT also exhibited no response to the complete reagent mixture, retaining a stable baseline current.
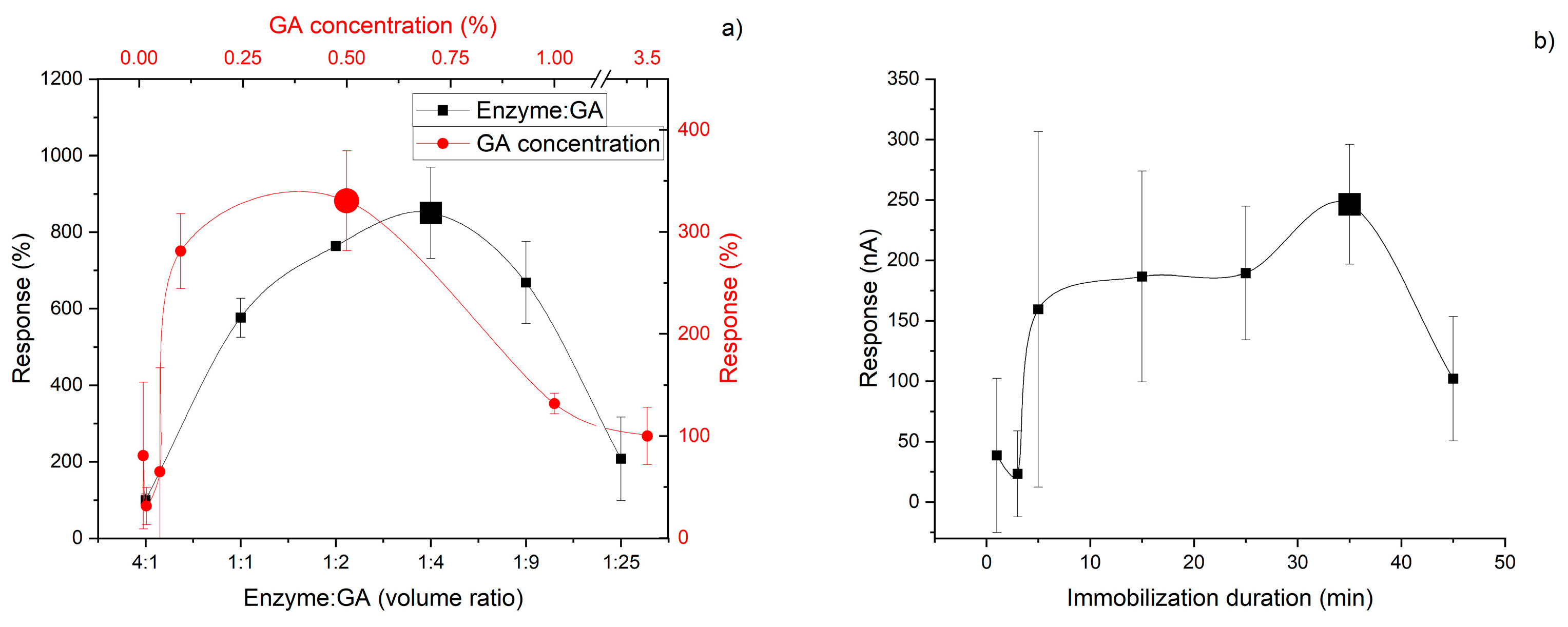
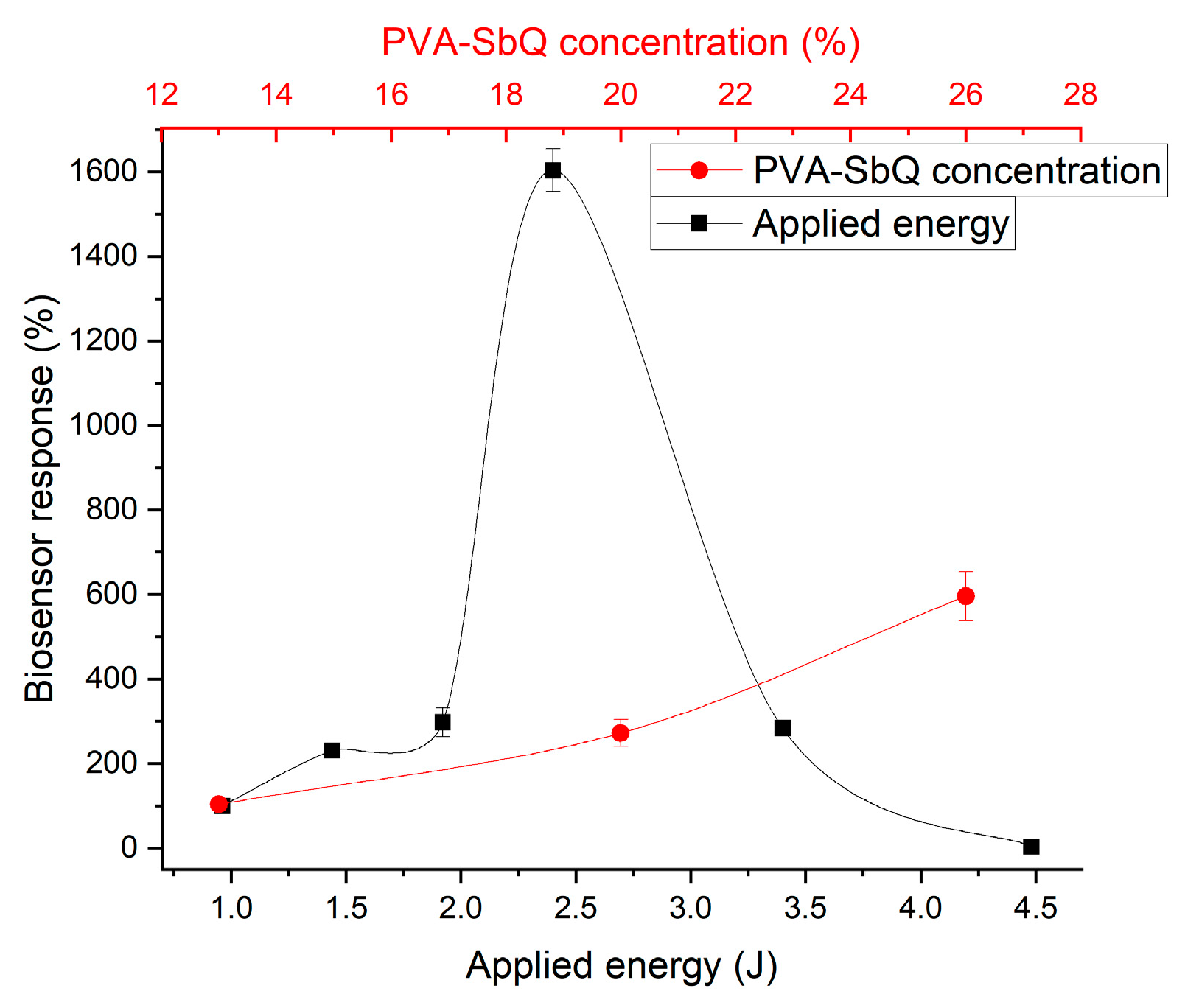
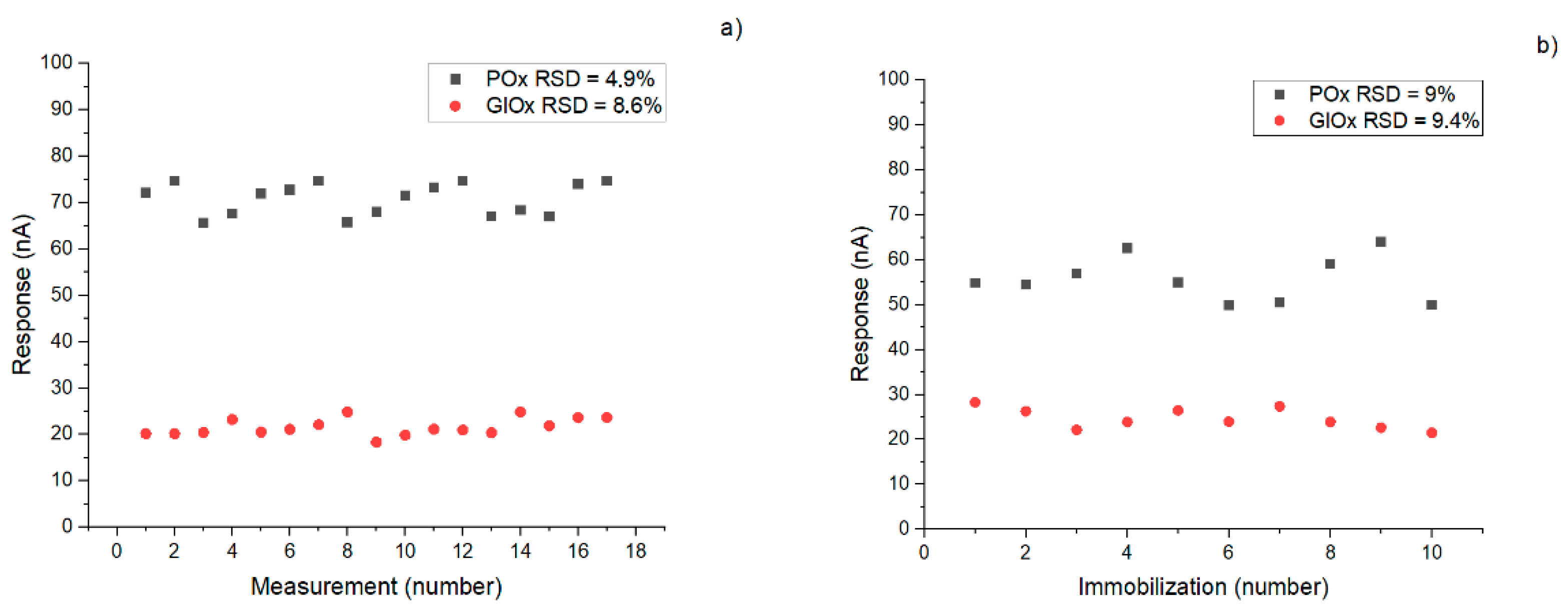
3.2. Immobilization Processes
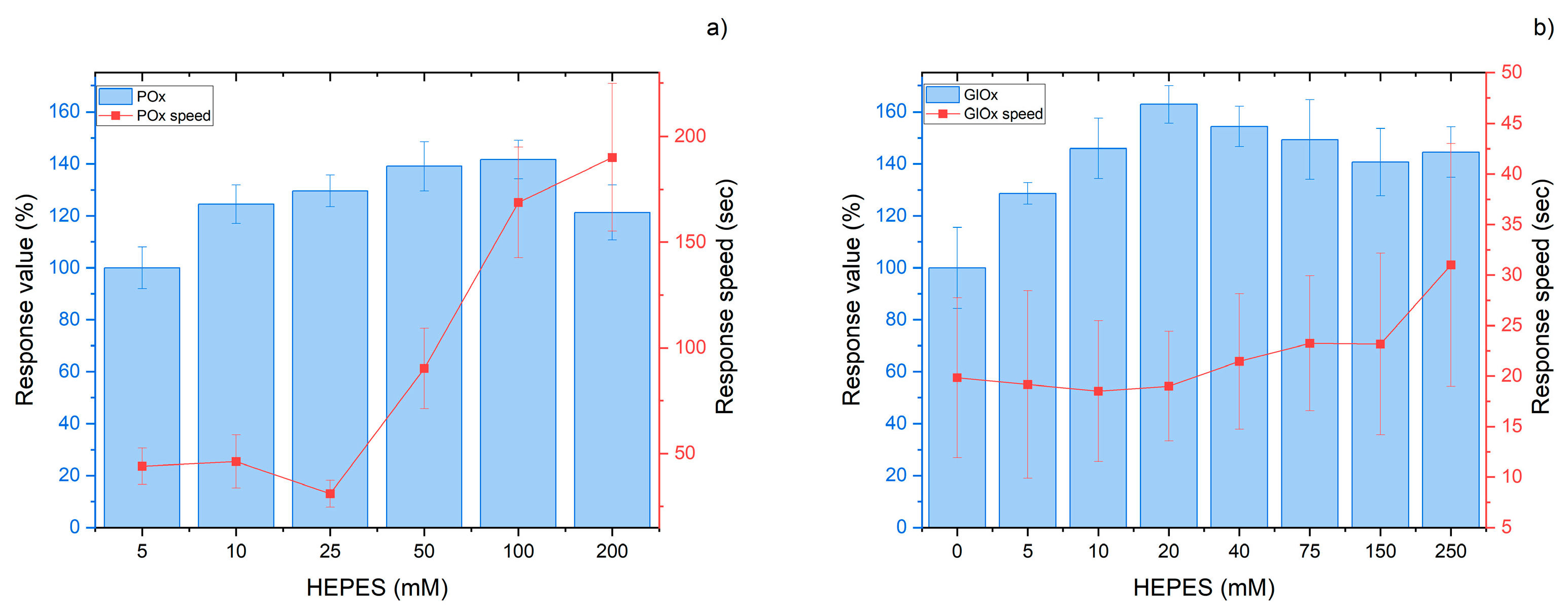
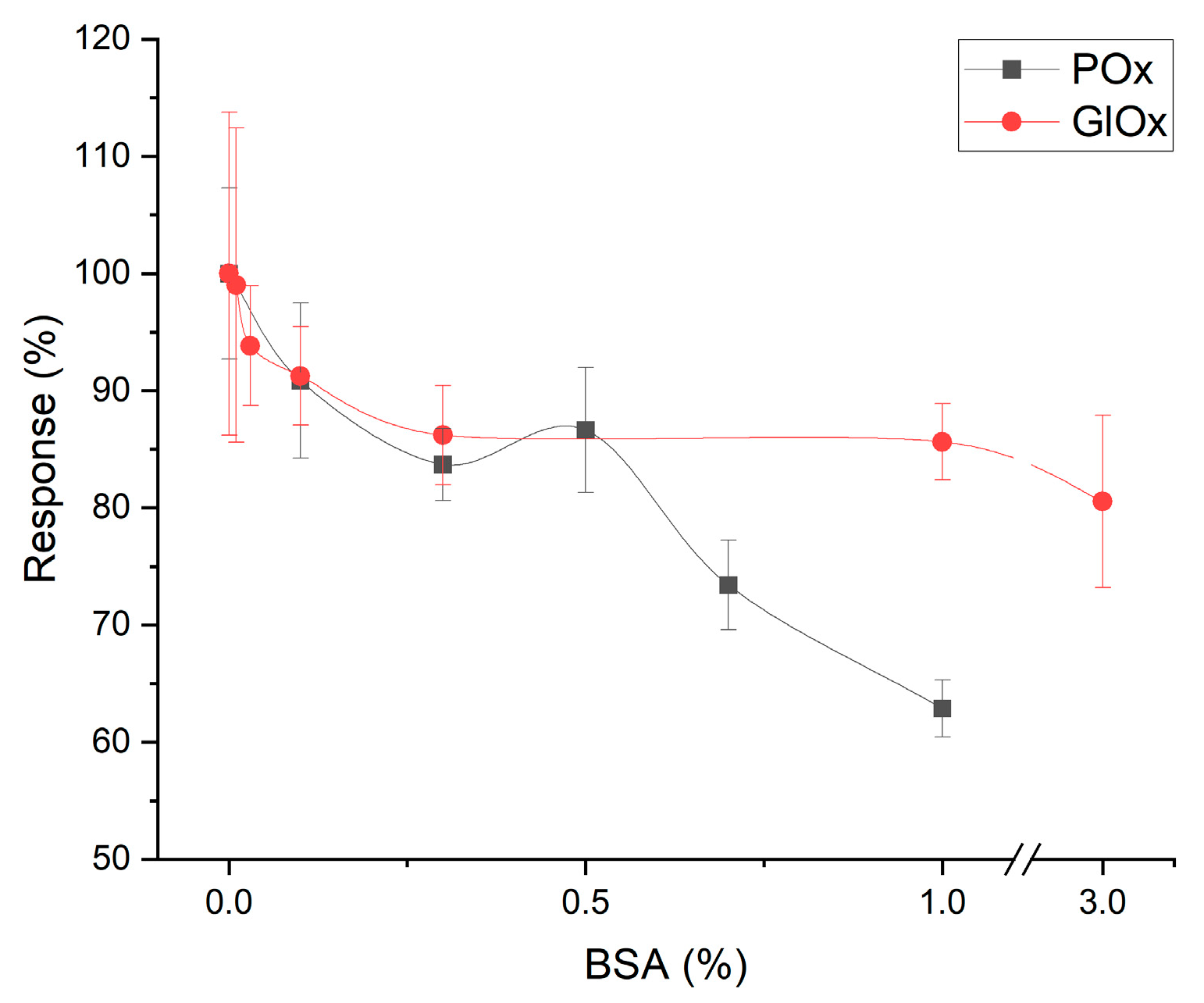
3.3. Media Requirements
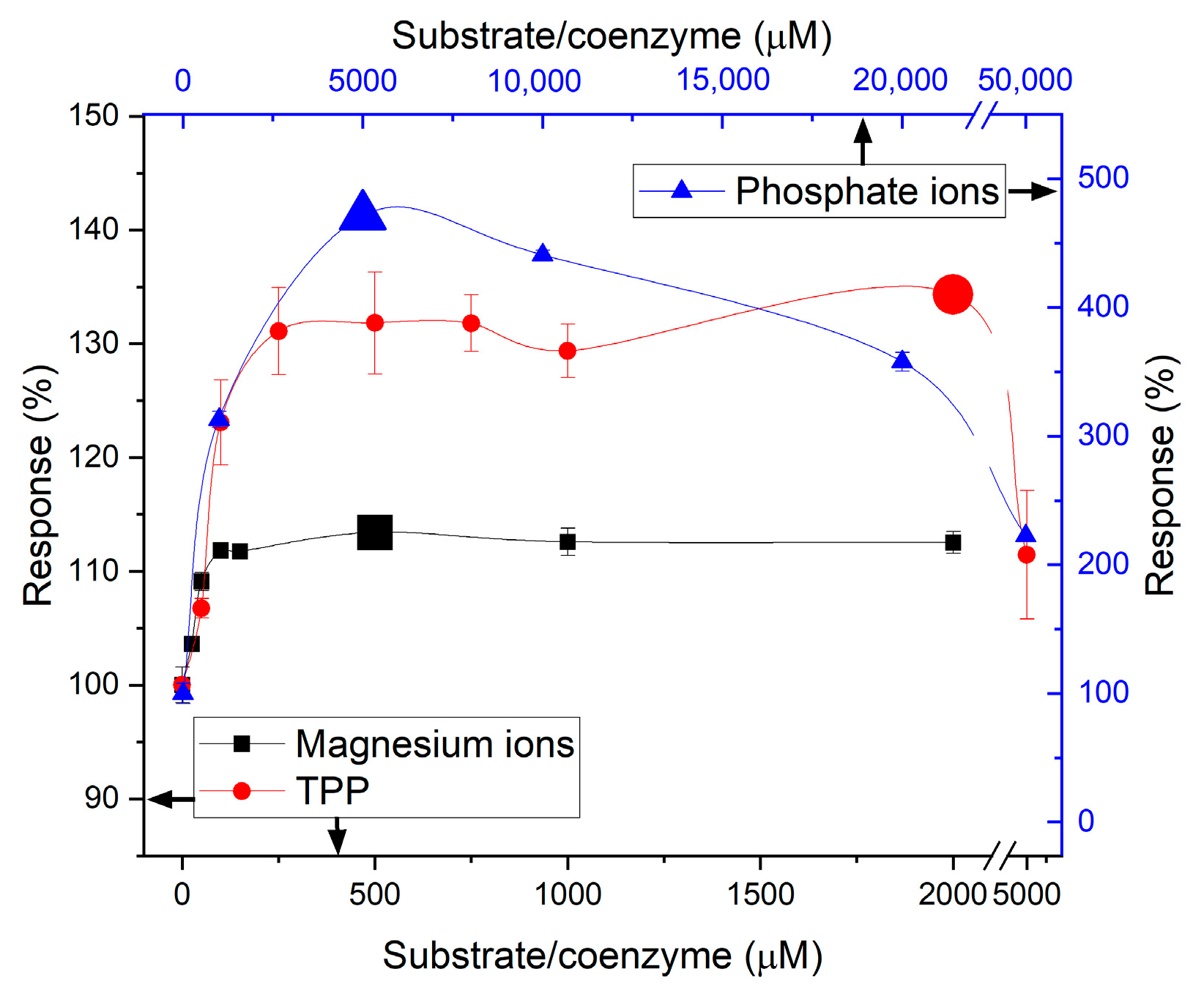
3.4. ALT Substrates and Coenzyme Content
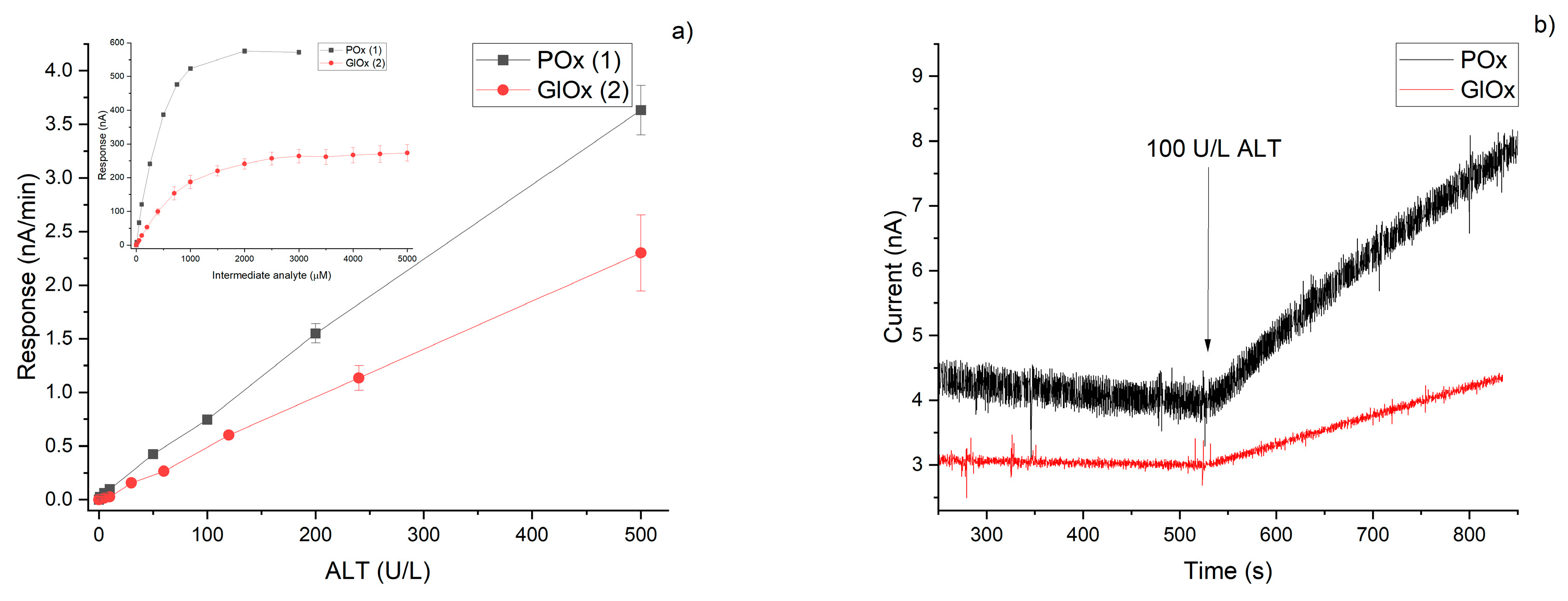
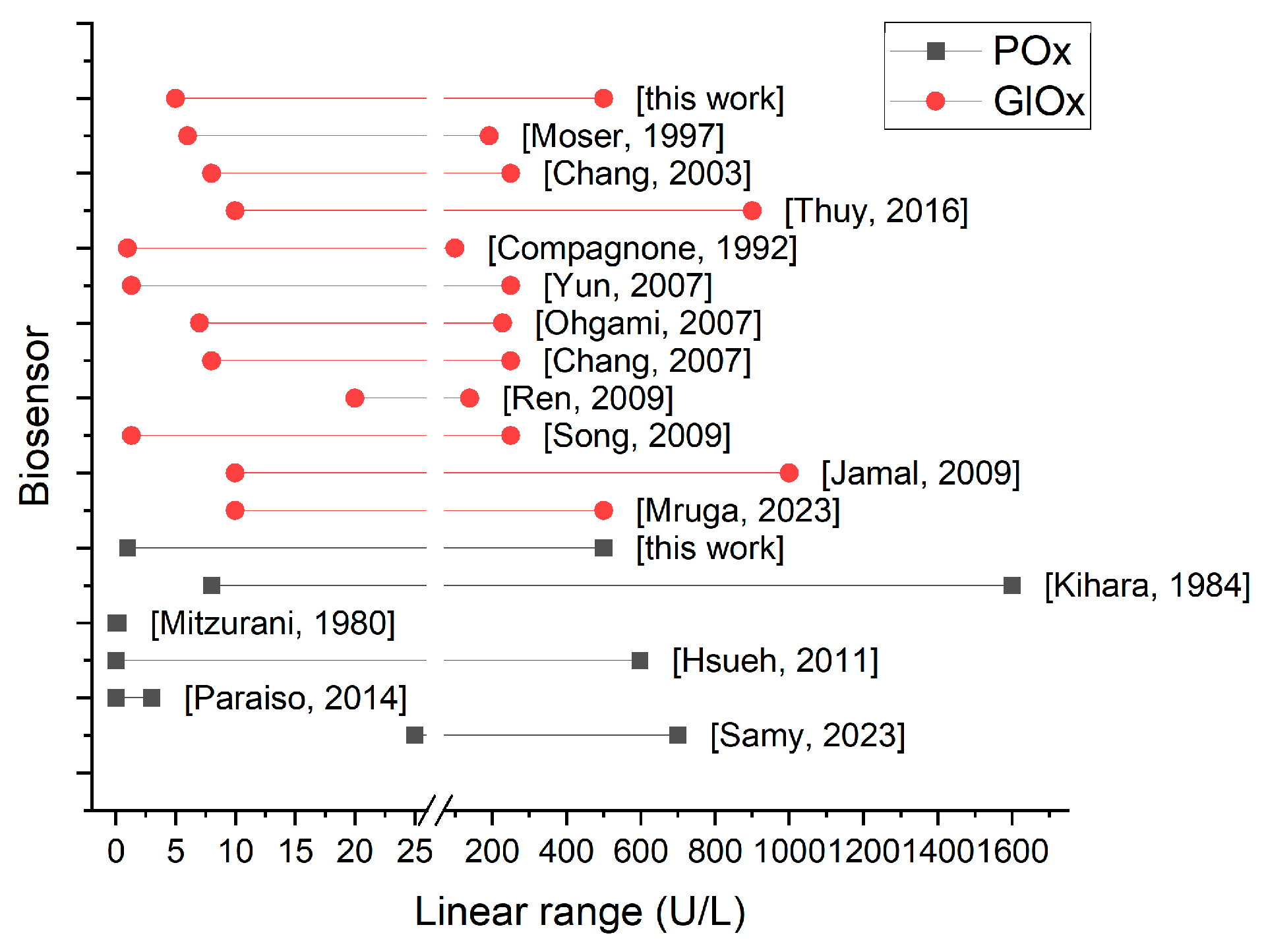
3.5. Comparison of Analytical Characteristics
4. Discussion
- The response times of POx-based sensors were generally faster (20–240 s) compared with GlOx-based sensors (one reported 5 s, one 10 s, while the majority ranged from 100 to 900 s).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| POx | Pyruvate oxidase |
| GlOx | Glutamate oxidase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| PVA-SbQ | Poly(vinyl alcohol)-quarternized stilbazole |
| BSA | Bovine serum albumin |
| LOD | Limit of detection |
| PLP | Pyridoxal phosphate |
| TPP | Thiamine pyrophosphate |
| HEPES | N-[2-Hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] |
| RT | Room temperature |
References
- Sherman, K.E. Alanine aminotransferase in clinical practice: A review. Arch. Intern. Med. 1991, 151, 260–265. [Google Scholar] [CrossRef]
- Borkum, J.M. The Tricarboxylic Acid Cycle as a Central Regulator of the Rate of Aging: Implications for Metabolic Interventions. Adv. Biol. 2023, 7, 2300095. [Google Scholar] [CrossRef]
- Son, J.; Lyssiotis, S.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.; Ferrone, C.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine supports pancreatic cancer growth through a Kras-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin. Chem. 2000, 46, 2027–2049. [Google Scholar] [CrossRef]
- Prati, D.; Taioli, E.; Zanella, A.; Torre, E.D.; Butelli, S.; Del Vecchio, E.; Vianello, L.; Zanuso, F.; Mozzi, F.; Milani, S.; et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann. Intern. Med. 2002, 137, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase—Old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925–935. [Google Scholar] [CrossRef]
- Huang, X.J.; Choi, Y.K.; Im, H.S.; Yarimaga, O.; Yoon, E.; Kim, H.S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Lagarde, F.; Jaffrezic-Renault, N.; Dzyadevych, S.V.; Soldatkin, A.P. Determination of total creatine kinase activity in blood serum using an amperometric biosensor based on glucose oxidase and hexokinase. Talanta 2015, 144, 604–611. [Google Scholar] [CrossRef]
- Wei, L.Y.; Lin, W.; Leo, B.F.; Kiew, L.V.; Chang, C.C.; Yuan, C.J. Development of the sensing platform for protein tyrosine kinase activity. Biosensors 2021, 11, 240. [Google Scholar] [CrossRef]
- Mruga, D.; Dzyadevych, S.; Soldatkin, O. Development and optimisation of the biosensor for aspartate aminotransferase blood level determination. Anal. Bioanal. Chem. 2025, 417, 721–731. [Google Scholar]
- Moed, S.; Zaman, M. A Quantitative Electrochemical Assay for Liver Injury. Biosens. Bioelectron. 2019, 131, 204–209. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Yang, F.; Yue, W. Self-template sacrifice and in situ oxidation of a constructed hollow MnO2 nanozymes for smartphone-assisted colorimetric detection of liver function biomarkers. Anal. Chim. Acta 2023, 1278, 341744. [Google Scholar] [CrossRef]
- Quan, C.; Quan, L.; Wen, Q.; Yang, M.; Li, T. Alanine aminotransferase electrochemical sensor based on graphene@MXene composite nanomaterials. Microchim. Acta 2024, 191, 45. [Google Scholar] [CrossRef]
- Hsueh, C.J.; Wang, J.H.; Dai, L.; Liu, C.C. Determination of alanine aminotransferase with an electrochemical nano Ir-C biosensor for the screening of liver diseases. Biosensors 2011, 1, 107–117. [Google Scholar] [CrossRef]
- Jamal, M.; Worsfold, O.; McCormac, T.; Dempsey, E. A stable and selective electrochemical biosensor for the liver enzyme alanine aminotransferase (ALT). Biosens. Bioelectron. 2009, 24, 2926–2930. [Google Scholar] [CrossRef]
- Thuy, T.N.T.; Tseng, T.T.C. A micro-platinum wire biosensor for fast and selective detection of alanine aminotransferase. Sensors 2016, 16, 767. [Google Scholar] [CrossRef]
- Mruga, D.; Berketa, K.; Dzyadevych, S.; Soldatkin, O. Biosensors for transaminase detection: Their diversity and trends–a comprehensive review. TrAC Trends Anal. Chem. 2025, 193, 118460. [Google Scholar] [CrossRef]
- Mruga, D.; Soldatkin, O.; Paliienko, K.; Topcheva, A.; Krisanova, N.; Kucherenko, D.; Borisova, T.; Dzyadevych, S.; Soldatkin, A. Optimization of the design and operating conditions of an amperometric biosensor for glutamate concentration measurements in the blood plasma. Electroanalysis 2021, 33, 1299–1307. [Google Scholar] [CrossRef]
- Soldatkina, O.V.; Kucherenko, I.S.; Pyeshkova, V.M.; Alekseev, S.A.; Soldatkin, O.O.; Dzyadevych, S.V. Improvement of amperometric transducer selectivity using nanosized phenylenediamine films. Nanoscale Res. Lett. 2017, 12, 594. [Google Scholar] [CrossRef]
- Hameed, H.A.D.; Ali, E.H. Extraction and purification of extracellular L-glutamate oxidase from Streptomyces. Arch. Razi Inst. 2021, 76, 769–779. [Google Scholar]
- Wachiratianchai, S.; Bhumiratana, A.; Udomsopagit, S. Isolation, purification, and characterization of L-glutamate oxidase from Streptomyces sp. 18G. Electron. J. Biotechnol. 2004, 7, 9–10. [Google Scholar] [CrossRef][Green Version]
- Sekisui Diagnostics. Pyruvate Oxidase. Available online: https://sekisuidiagnostics.com/product/pyruvate-oxidase/ (accessed on 25 September 2025).[Green Version]
- Katsos, N.E.; Labrou, N.E.; Clonis, Y.D. Interaction of L-glutamate oxidase with triazine dyes: Selection of ligands for affinity chromatography. J. Chromatogr. B 2004, 807, 277–285. [Google Scholar] [CrossRef]
- Schreiner, M.E.; Eikmanns, B.J. Pyruvate: Quinone oxidoreductase from Corynebacterium glutamicum: Purification and biochemical characterization. J. Bacteriol. 2005, 187, 862–871. [Google Scholar] [CrossRef]
- Juan, E.C.M.; Hoque, M.M.; Hossain, M.T.; Yamamoto, T.; Imamura, S.; Suzuki, K.; Takénaka, A. The structures of pyruvate oxidase from Aerococcus viridans with cofactors and with a reaction intermediate reveal the flexibility of the active-site tunnel for catalysis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007, 63, 900–907. [Google Scholar] [CrossRef]
- Bonsembiante, F.; Giordano, A.; Gili, C.; Mazzariol, S.; Berlanda, M.; Guglielmini, C.; Bedin, S.; Gelain, M.E. Serum Protein Concentration and Serum Protein Fractions in Bottlenose Dolphins (Tursiops truncatus) under Human Care Using Agarose Gel Electrophoresis. Animals 2023, 13, 1745. [Google Scholar] [CrossRef] [PubMed]
- Sedewitz, B.; Schleifer, K.H.; Götz, F. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J. Bacteriol. 1984, 160, 273–278. [Google Scholar] [CrossRef]
- Zhang, G.; Dai, J.; Lu, Z.; Dunaway-Mariano, D. The Phosphonopyruvate Decarboxylase from Bacteroides fragilis. J. Biol. Chem. 2003, 278, 41302–41308. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2012, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Dherbassy, Q.; Mayer, R.J.; Muchowska, K.B.; Moran, J. Metal-Pyridoxal cooperativity in nonenzymatic transamination. J. ACS. 2023, 145, 13357–13370. [Google Scholar] [CrossRef]
- Toney, M.D. Aspartate aminotransferase: An old dog teaches new tricks. Arch. Biochem. Biophys. 2014, 544, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Gruber, W.; Möllering, H.; Perras, L. Isolation, pH-Optima and Apparent Michaelis Constants of Highly Purified Enzymes from Human and Animal Sources. Comparison of Enzymes of Human and Animal Origin, I. CCLM 1977, 15, 565–573. [Google Scholar] [CrossRef]
- Case, D.R.; Zubieta, J.; Doyle, R.P. The coordination chemistry of Bio-Relevant ligands and their magnesium complexes. Molecules 2020, 25, 3172. [Google Scholar] [CrossRef]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal liver enzymes: A review for clinicians. World J. Hepatol. 2021, 13, 1688–1698. [Google Scholar] [CrossRef]
- Samy, M.A.; Abdel-Tawab, M.A.; Abdel-Ghani, N.T.; Nashar, R.M. Application of molecularly imprinted microelectrode as a promising point-of-care biosensor for alanine aminotransferase enzyme. Chemosensors 2023, 11, 262. [Google Scholar] [CrossRef]
- Paraiso, L.; Paula, L.; Franco, D.; Madurro, J.; Madurro, A. Bioelectrochemical detection of alanine aminotransferase for molecular diagnostic of the liver disease. Int. J. Electrochem. Sci. 2014, 9, 1286–1297. [Google Scholar] [CrossRef]
- Kihara, K.; Yasukawa, E.; Hayashi, M.; Hirose, S. Determination of glutamate-pyruvate transaminase activity in blood serum with a pyruvate oxidase/poly(vinyl chloride) membrane sensor. Anal. Chim. Acta 1984, 159, 81–86. [Google Scholar] [CrossRef]
- Mizutani, F.; Tsuda, K.; Kaeube, I.; Suzuki, S.; Matsumoto, K. Determination of glutamate pyruvate transaminase and pyruvate with an amperometric pyruvate oxidase sensor. Anal. Chim. Acta 1980, 118, 65–71. [Google Scholar] [CrossRef]
- Kihara, K.; Yasukawa, E.; Hirose, S. Sequential determination of glutamate-oxalacetate transaminase and glutamate-pyruvate transaminase activities in serum using an immobilized bienzyme-poly(vinyl chloride) membrane electrode. Anal. Chem. 1984, 56, 1876–1880. [Google Scholar] [CrossRef]
- Song, M.-J.; Yun, D.-H.; Hong, S.-I. An electrochemical biosensor array for rapid detection of alanine aminotransferase and aspartate aminotransferase. Biosci. Biotechnol. Biochem. 2009, 73, 474–478. [Google Scholar] [CrossRef]
- Ren, H.; Huang, X.; Kim, J.; Choi, Y.; Gu, N. Pt/Au bimetallic hierarchical structure with micro/nano-array via photolithography and electrochemical synthesis: From design to GOT and GPT biosensors. Talanta 2009, 78, 1371–1377. [Google Scholar] [CrossRef]
- Chang, K.; Chang, C.; Chou, S.; Chen, C. Sequential measurement of aminotransferase activities by amperometric biosensors. Biosens. Bioelectron. 2007, 22, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, N.; Upadhyay, S.; Kabata, A.; Morimoto, K.; Kusakabe, H.; Suzuki, H. Determination of the activities of glutamic oxaloacetic transaminase and glutamic pyruvic transaminase in a microfluidic system. Biosens. Bioelectron. 2007, 22, 1330–1336. [Google Scholar] [CrossRef]
- Yun, M.-J.; Min, N.K.; Hong, S.-I. Electrochemical biosensor array for liver diagnosis using silanization technique on nanoporous silicon electrode. J. Biosci. Bioeng. 2007, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, D.; Federici, G.; Massoud, R.; Santoro, L.; Anichini, M.; Palleschi, G. Analysis for transaminases in serum with an amperometric glutamate electrode. Clin. Chem. 1992, 38, 2306–2310. [Google Scholar] [CrossRef]
- Mruga, D.; Dzyadevych, S.; Soldatkin, O. High-selective alanine transaminase-sensitive biosensor based on nanosize semipermeable poly-meta-phenylenediamine membrane. Appl. Nanosci. 2023, 13, 6939–6949. [Google Scholar] [CrossRef]
- Chang, K.-S.; Hsu, W.L.; Chen, H.Y.; Chang, C.K.; Chen, C.Y. Determination of glutamate pyruvate transaminase activity in clinical specimens using a biosensor composed of immobilized L-glutamate oxidase in a photo-crosslinkable polymer membrane on a palladium-deposited screen-printed carbon electrode. Anal. Chim. Acta 2003, 481, 199–208. [Google Scholar] [CrossRef]
- Moser, I.; Jobst, G.; Svasek, P.; Varahram, M.; Urban, G. Rapid liver enzyme assay with miniaturized liquid handling system comprising thin film biosensor array. Sens. Actuators B Chem. 1997, 44, 377–380. [Google Scholar] [CrossRef]
- Cooper, J.M.; McNeil, C.J.; Spoors, J.A. Amperometric enzyme electrode for the determination of aspartate aminotransferase and alanine aminotransferase in serum. Anal. Chim. Acta 1991, 245, 57–62. [Google Scholar] [CrossRef]

| Parameter | POx-Based Biosensor | GlOx-Based Biosensor | |
|---|---|---|---|
| Intermediate analyte | Sensitivity, nA/mM | 573 ± 8 | 186 ± 19 |
| Linear range, μM | 10–750 | 10–700 | |
| LOD, μM | 0.25 ± 0.1 | 1.39 ± 0.2 | |
| Dynamic range, μM | 1–2000 | 1–3000 | |
| Immobilization reproducibility error, % | 9 | 9.4 | |
| Response reproducibility error, % | 4.9 | 8.6 | |
| Baseline current, nA | 4.1 ± 1.8 | 3.18 ± 0.05 | |
| Baseline noise, nA | 0.20 ± 0.12 | 0.17 ± 0.04 | |
| Response noise, nA | 0.61 ± 0.28 | 0.19 ± 0.04 | |
| Response time, s | 55 ± 11 | 19 ± 5 | |
| Analysis duration, s | 700 | 700 | |
| ALT | Sensitivity, nA/min at 100 U/L | 0.75 | 0.49 |
| Linear range, μM | 1–500 | 5–500 | |
| LOD, μM | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mruga, D.; Vakhovskyi, Y.; Bakhmat, V.; Pyeshkova, V.; Dzyadevych, S.; Soldatkin, O. Development and Comparative Evaluation of Two Enzyme-Based Amperometric Biosensor Designs for Alanine Aminotransferase Determination in Biological Fluids. Micromachines 2025, 16, 1168. https://doi.org/10.3390/mi16101168
Mruga D, Vakhovskyi Y, Bakhmat V, Pyeshkova V, Dzyadevych S, Soldatkin O. Development and Comparative Evaluation of Two Enzyme-Based Amperometric Biosensor Designs for Alanine Aminotransferase Determination in Biological Fluids. Micromachines. 2025; 16(10):1168. https://doi.org/10.3390/mi16101168
Chicago/Turabian StyleMruga, Daryna, Yevhen Vakhovskyi, Veronika Bakhmat, Viktoriya Pyeshkova, Sergii Dzyadevych, and Oleksandr Soldatkin. 2025. "Development and Comparative Evaluation of Two Enzyme-Based Amperometric Biosensor Designs for Alanine Aminotransferase Determination in Biological Fluids" Micromachines 16, no. 10: 1168. https://doi.org/10.3390/mi16101168
APA StyleMruga, D., Vakhovskyi, Y., Bakhmat, V., Pyeshkova, V., Dzyadevych, S., & Soldatkin, O. (2025). Development and Comparative Evaluation of Two Enzyme-Based Amperometric Biosensor Designs for Alanine Aminotransferase Determination in Biological Fluids. Micromachines, 16(10), 1168. https://doi.org/10.3390/mi16101168






