Continuous Recirculation of Microdroplets in a Closed Loop Tailored for Screening of Bacteria Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
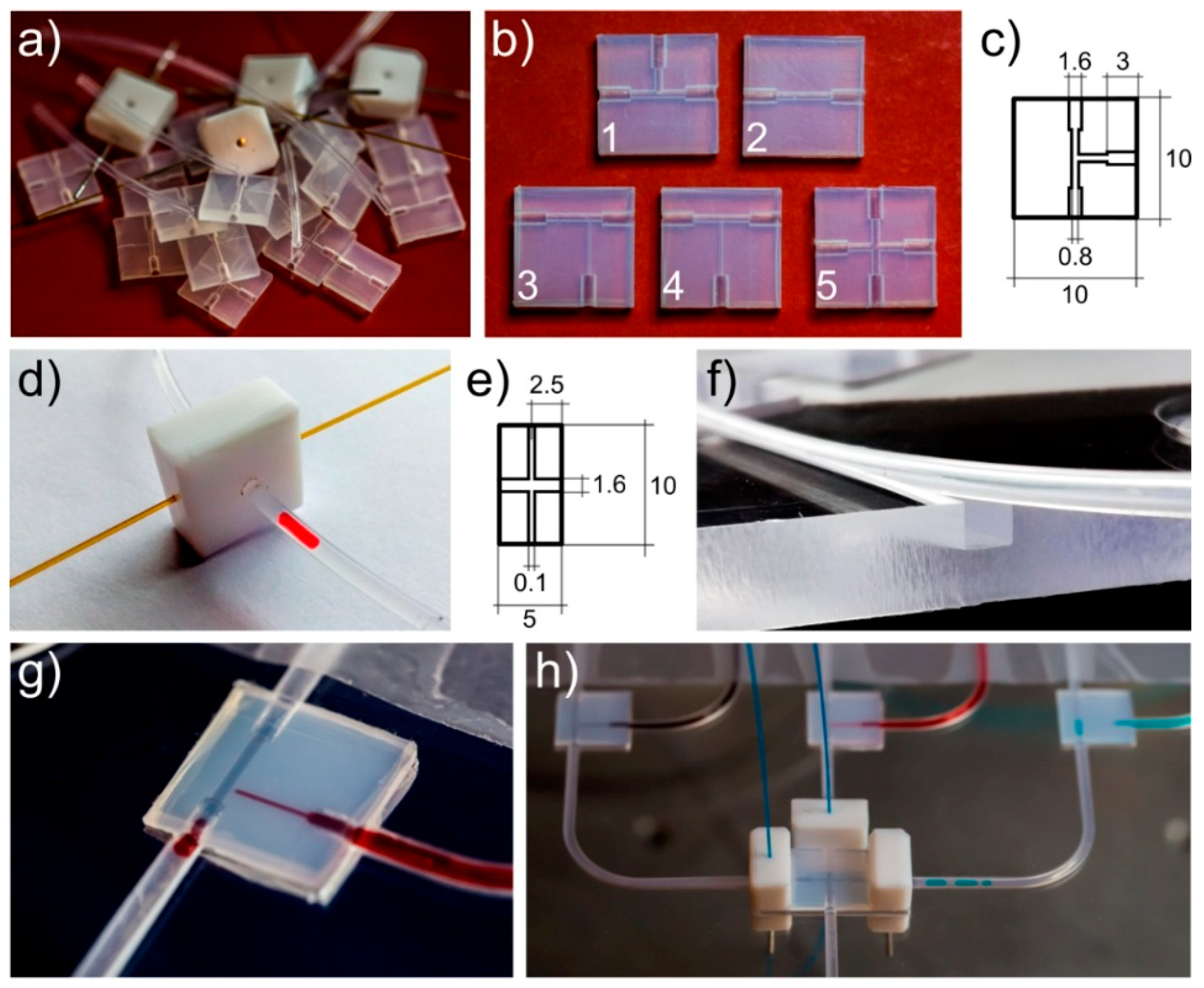
2.2. Microchip Fabrication
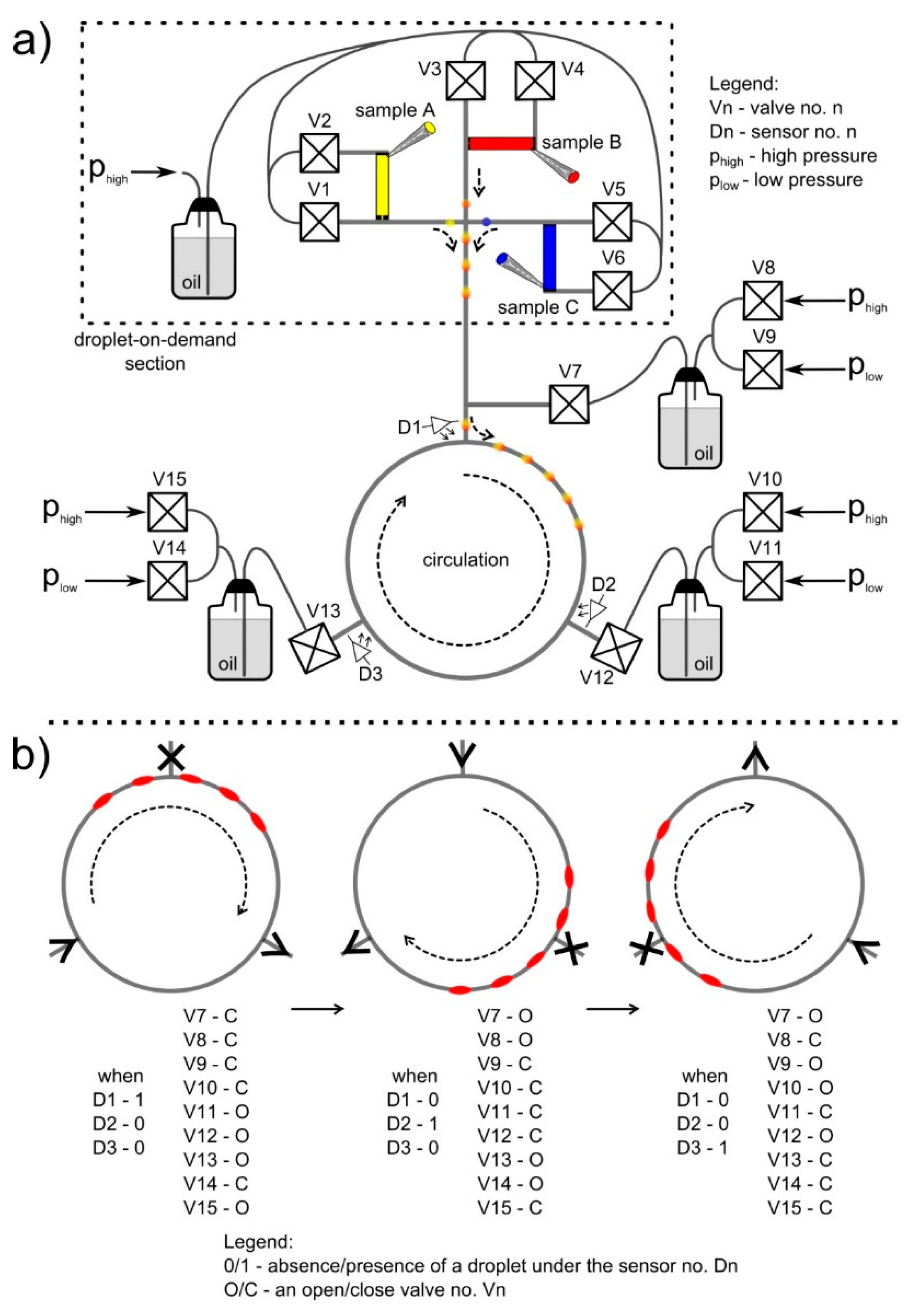
2.3. Automation
3. Results and Discussion
3.1. Motion of Fluids in the Loop
3.2. Time Evolution of Droplets
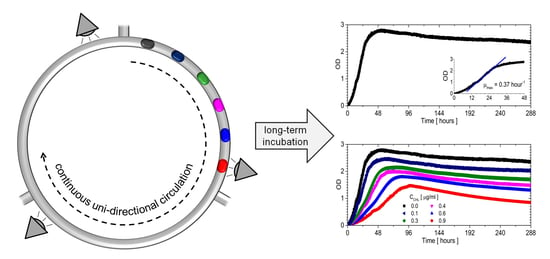
3.3. The Bacterial Growth in Closed Loop Circulation
3.4. Influence of Chloramphenicol on Bacterial Culture
3.5. Verifying the Lack of Cross-Contamination of Tubing Walls during the Continuous Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.R.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Jakiela, S.; Kaminski, T.S.; Cybulski, O.; Weibel, D.B.; Garstecki, P. Bacterial Growth and Adaptation in Microdroplet Chemostats. Angew. Chem. Int. Ed. 2013, 52, 8908–8911. [Google Scholar] [CrossRef] [PubMed]
- Korczyk, P.M.; Dolega, M.E.; Jakiela, S.; Jankowski, P.; Makulska, S.; Garstecki, P. Scaling up the Throughput of Synthesis and Extraction in Droplet Microfluidic Reactors. J. Flow Chem. 2015, 5, 110–118. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Tian, H.; Li, X.; Jin, D.; Li, X.; Kong, J.; Yang, C.; Yang, X.; Lu, Y.; Luo, Y.; et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS ONE 2017, 12, e0175050. [Google Scholar] [CrossRef] [PubMed]
- Oh, K. Multidisciplinary Role of Microfluidics for Biomedical and Diagnostic Applications: Biomedical Microfluidic Devices. Micromachines 2017, 8, 343. [Google Scholar] [CrossRef]
- Yuen, P.K.; Bliss, J.T.; Thompson, C.C.; Peterson, R.C. Multidimensional modular microfluidic system. Lab Chip 2009, 9, 3303–3305. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-F.; Yang, A.-S.; Chen, J.-W.; Liao, S.-K.; Su, T.-W.; Yeh, S.-H.; Chen, P.-J.; Chen, P.-H. A Lego®-like swappable fluidic module for bio-chem applications. Sens. Actuators B Chem. 2014, 204, 489–496. [Google Scholar] [CrossRef]
- Cybulski, O.; Jakiela, S.; Garstecki, P. Whole Teflon valves for handling droplets. Lab Chip 2016, 16, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.E.; Hart, A.J. High-precision modular microfluidics by micromilling of interlocking injection-molded blocks. Lab Chip 2018, 18, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Thompson, B.; Tembhekar, A.; Malmstadt, N.; Bhargava, K.C.; Thompson, B.; Tembhekar, A.; Malmstadt, N. Temperature Sensing in Modular Microfluidic Architectures. Micromachines 2016, 7, 11. [Google Scholar] [CrossRef]
- Vittayarukskul, K.; Lee, A.P. A truly Lego®-like modular microfluidics platform. J. Micromech. Microeng. 2017, 27, 035004. [Google Scholar] [CrossRef]
- Bhargava, K.C.; Thompson, B.; Malmstadt, N. Discrete elements for 3D microfluidics. Proc. Natl. Acad. Sci. USA 2014, 111, 15013–15018. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Gao, Q.; Qiu, J.; Sun, M.; Liu, A.; Shao, L.; Fu, J.; Zhao, P.; He, Y. 3D printed Lego®-like modular microfluidic devices based on capillary driving. Biofabrication 2018, 10, 035001. [Google Scholar] [CrossRef] [PubMed]
- Joensson, H.N.; Svahn, H.A. Droplet Microfluidics—A Tool for Single-Cell Analysis. Angew. Chem. Int. Ed. 2012, 51, 12176–12192. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet based microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef] [PubMed]
- Streets, A.M.; Huang, Y. Chip in a lab: Microfluidics for next generation life science research. Biomicrofluidics 2013, 7, 011302. [Google Scholar] [CrossRef] [PubMed]
- Churski, K.; Kaminski, T.S.; Jakiela, S.; Kamysz, W.; Baranska-Rybak, W.; Weibel, D.B.; Garstecki, P. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip 2012, 12, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Ramji, R.; Xiang, A.C.; Ying, N.J.; Teck, L.C.; Hung, C.C. Microfluidic Single Mammalian Cell Lysis in Picolitre Droplets. J. Biosens. Bioelectron. 2013, S12, 1–4. [Google Scholar] [CrossRef]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; deMello, A.J. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; deMello, A.J. Droplet-based microfluidics for artificial cell generation: A brief review. Interface Focus 2016, 6, 20160011. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Morita, M.; Sugiura, H.; Fujiwara, K.; Onoe, H.; Takinoue, M. Generation of monodisperse cell-sized microdroplets using a centrifuge-based axisymmetric co-flowing microfluidic device. J. Biosci. Bioeng. 2015, 119, 492–495. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Edgar, J.S.; Jeffries, G.D.M.; Lorenz, R.M.; Shelby, J.P.; Chiu, D.T. Selective Encapsulation of Single Cells and Subcellular Organelles into Picoliter- and Femtoliter-Volume Droplets. Anal. Chem. 2005, 77, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Jung, J.-H.; Rhee, Y.W.; Kim, D.-P.; Shim, S.-E.; Lee, C.-S. Generation of monodisperse alginate microbeads and in situ encapsulation of cell in microfluidic device. Biomed. Microdevices 2007, 9, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Liu, P.; Chen, P.; Wu, L.; Wang, Y.; Feng, X.; Liu, B.-F. Encapsulation of single cells into monodisperse droplets by fluorescence-activated droplet formation on a microfluidic chip. Talanta 2016, 153, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Bratton, D.; Whyte, G.; Yang, M.; Demello, A.J.; Abell, C.; Hollfelder, F. Static microdroplet arrays: A microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab Chip 2009, 9, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, O.; Garstecki, P. Dynamic memory in a microfluidic system of droplets traveling through a simple network of microchannels. Lab Chip 2010, 10, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Maddala, J.; Vanapalli, S.A.; Rengaswamy, R. Origin of periodic and chaotic dynamics due to drops moving in a microfluidic loop device. Phys. Rev. E 2014, 89, 023015. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Jung, J.-H.; Jeong, S.-G.; Kim, J.; Park, T.J.; Lee, C.-S. Microfluidic dual loops reactor for conducting a multistep reaction. Front. Chem. Sci. Eng. 2018, 12, 239–246. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Sha, J.; Zhang, Z.; Tu, Q.; Chen, P.; Wang, J. High-throughput microfluidic system for long-term bacterial colony monitoring and antibiotic testing in zero-flow environments. Biosens. Bioelectron. 2011, 26, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.S.; Scheler, O.; Garstecki, P. Droplet microfluidics for microbiology: Techniques, applications and challenges. Lab Chip 2016, 16, 2168–2187. [Google Scholar] [CrossRef] [PubMed]
- Battino, R.; Rettich, T.R.; Tominaga, T. The Solubility of Oxygen and Ozone in Liquids. J. Phys. Chem. Ref. Data 1983, 12, 163–178. [Google Scholar] [CrossRef]
- Xu, J.; Attinger, D. Drop on demand in a microfluidic chip. J. Micromech. Microeng. 2008, 18, 065020. [Google Scholar] [CrossRef]
- Khalil, A.; Puel, F.; Chevalier, Y.; Galvan, J.-M.; Rivoire, A.; Klein, J.-P. Study of droplet size distribution during an emulsification process using in situ video probe coupled with an automatic image analysis. Chem. Eng. J. 2010, 165, 946–957. [Google Scholar] [CrossRef]
- Jakiela, S. Measurement of the hydrodynamic resistance of microdroplets. Lab Chip 2016, 16, 3695–3699. [Google Scholar] [CrossRef] [PubMed]
- Baraban, L.; Bertholle, F.; Salverda, M.L.M.; Bremond, N.; Panizza, P.; Baudry, J.; de Visser, J.A.G.M.; Bibette, J. Millifluidic droplet analyser for microbiology. Lab Chip 2011, 11, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Hodges, S.R.; Jensen, O.E.; Rallison, J.M. The motion of a viscous drop through a cylindrical tube. J. Fluid Mech. 2004, 501, 279–301. [Google Scholar] [CrossRef]
- Lac, E.; Sherwood, J.D. Motion of a drop along the centreline of a capillary in a pressure-driven flow. J. Fluid Mech. 2009, 640, 27–54. [Google Scholar] [CrossRef]
- Horka, M.; Sun, S.; Ruszczak, A.; Garstecki, P.; Mayr, T. Lifetime of Phosphorescence from Nanoparticles Yields Accurate Measurement of Concentration of Oxygen in Microdroplets, Allowing One to Monitor the Metabolism of Bacteria. Anal. Chem. 2016, 88, 12006–12012. [Google Scholar] [CrossRef] [PubMed]
- Sklodowska, K.; Jakiela, S. Enhancement of bacterial growth with the help of immiscible oxygenated oils. RSC Adv. 2017, 7, 40990–40995. [Google Scholar] [CrossRef]
- Bhuta, P.; Chung, H.L.; Hwang, J.-S.; Zemlicka, J. Analogs of chloramphenicol: Circular dichroism spectra, inhibition of ribosomal peptidyltransferase, and possible mechanism of action. J. Med. Chem. 1980, 23, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.-Q.; Li, Z.-Y.; Zhang, X.-Y.; Jiang, L.; Ren, N.-Q.; Sun, K. Advance in Bacteria Chemotaxis on Microfluidic Devices. Chin. J. Anal. Chem. 2017, 45, 1734–1744. [Google Scholar] [CrossRef]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debski, P.R.; Sklodowska, K.; Michalski, J.A.; Korczyk, P.M.; Dolata, M.; Jakiela, S. Continuous Recirculation of Microdroplets in a Closed Loop Tailored for Screening of Bacteria Cultures. Micromachines 2018, 9, 469. https://doi.org/10.3390/mi9090469
Debski PR, Sklodowska K, Michalski JA, Korczyk PM, Dolata M, Jakiela S. Continuous Recirculation of Microdroplets in a Closed Loop Tailored for Screening of Bacteria Cultures. Micromachines. 2018; 9(9):469. https://doi.org/10.3390/mi9090469
Chicago/Turabian StyleDebski, Pawel R., Karolina Sklodowska, Jacek A. Michalski, Piotr M. Korczyk, Miroslaw Dolata, and Slawomir Jakiela. 2018. "Continuous Recirculation of Microdroplets in a Closed Loop Tailored for Screening of Bacteria Cultures" Micromachines 9, no. 9: 469. https://doi.org/10.3390/mi9090469
APA StyleDebski, P. R., Sklodowska, K., Michalski, J. A., Korczyk, P. M., Dolata, M., & Jakiela, S. (2018). Continuous Recirculation of Microdroplets in a Closed Loop Tailored for Screening of Bacteria Cultures. Micromachines, 9(9), 469. https://doi.org/10.3390/mi9090469